Abstract
We reported that the combination of ROS scavengers MnTBAP, catalase, and glutathione (MCG) given before 2 h cold cardiac ischemia better protected cardiac mitochondria against cold ischemia-induced damage and warm reperfusion (IR) than did MnTBAP alone. Here we hypothesized that high K+ cardioplegia (CP) plus MCG would improve mitochondrial bioenergetics and provide added cardiac functional protection against IR injury. We used fluorescence spectrophotometry to monitor redox balance (NADH/FAD), O2•−, and mitochondrial Ca2+ (m[Ca2+]) in the left ventricular wall. Guinea pig isolated hearts were perfused with either Krebs Ringer’s (KR) solution (control), CP, or CP+MCG, before and during 27°C perfusion followed immediately by 2 h of global ischemia at 27°C. Drugs were washed out with KR at the onset of 2 h 37°C reperfusion. After 120 min reperfusion myocardial infarction was lowest in the CP+MCG group and highest in the KR group. Developed left ventricular pressure recovery was similar in CP and CP+MCG groups and was better than in the KR group. O2•−, m[Ca2+ ], NADH/FAD were significantly different between the treatment and KR groups. O2•− was lower in the CP+MCG group than in the CP group. This study suggests that CP and ROS scavengers act in parallel to improve mitochondrial bioenergetics and to provide functional protection against IR injury at 27°C.
Keywords: ROS scavengers, mitochondrial redox state, hypothermia, ischemia
INTRODUCTION
Hypothermia is commonly used to protect hearts against subsequent ischemia and reperfusion (IR) injury. The lower the temperature, the more protective is hypothermia against cardiac IR injury so ischemic time can be prolonged. Hypothermia, however, is a two-edged sword because of the intrinsic deleterious effects that become increasingly greater the lower the temperature. For example, hypothermia causes mitochondrial (m) Ca2+ loading1 secondary to reduced Na+/K+ ATPase activity and cytosolic Na+ and Ca2+ loading, and is coupled to slowed mitochondrial enzyme function as evidenced by increased NADH.1, 2 These factors lead to impaired mitochondrial electron transport chain (ETC) activity which may allow electron leak and augments superoxide (O2•−) generation as well as other reactive oxygen species (ROS). With inefficient scavenging of ROS by temperature sensitive scavengers such as superoxide dismutase (SOD) and catalase, this could further enhance ROS emission (generation – scavenging) during cold perfusion.
ROS generation and mCa2+ loading during hypothermia may together reduce the potentially optimal protective effect of hypothermia due to ROS-induced cell injury. Many cold cardioplegic solutions (CP) contain ROS scavengers,3-6 and it is thought these ROS scavengers are useful because they generally scavenge the “bad” ROS on warm reperfusion after cold storage. However, ROS is also generated during ischemia.7, 8 Moreover, we reported that hypothermic perfusion, per se, without concomitant ischemia also results in ROS emission that is inversely proportional to temperatures below 37°C largely because of reduced ROS scavenging capability.9
In a previous study2 we examined the scavenging of O2•− and other oxidants produced during cold perfusion with normal K+ Krebs Ringer’s solution before cold ischemia. We used a cocktail of scavengers, i.e. Mn(III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), a mitochondrial SOD2 mimetic, catalase, an enzyme that catalyzes H2O2 to H2O, and glutathione, a tripeptide that in its reduced form scavenges ROS, and found that the combination (MCG) provided better mitochondrial preservation as shown by a more normalized redox state, less mCa2+ accumulation, and less ROS emission compared to control hearts after 2 h cold ischemia. This preservation of the mitochondrial bioenergetic state was associated with improved cardiac function and reduced infarct size. Interestingly, we also observed that scavenging O2•− with MnTBAP alone resulted in a more oxidized mitochondrial redox state as evidenced by lower NADH and higher FAD, greater mCa2+ loading, more ROS emission, and worse cardiac function compared to the control and the combination of MnTBAP, catalase and glutathione (MCG).2 The implication of the prior study was that O2•− and its downstream products are present in higher concentrations during cold perfusion and so are important factors to be scavenged during cold storage.
Although our prior study2 showed an additive effect of ROS scavengers when added to hypothermic normal K+ perfusion in improving cardiac recovery after IR, in the clinical setting of coronary artery bypass surgery hypothermia is applied during perfusion with a CP solution. CP plus hypothermia may better protect against IR injury than hypothermia or CP alone.3, 5, 6 Thus our aim was to determine if perfusion with a cold CP solution enhances cardiac recovery after IR more than hypothermia alone, and if adding the cocktail of ROS scavengers (MCG) used in our previous study has an additive effect with cold CP solution in protecting the heart against IR injury. Because mitochondrial function and ROS emission are integral factors in IR injury, we also investigated if there is an additive effect of ROS scavengers with cold CP solution on improving mitochondrial function as assessed continuously on line by measuring mitochondrial redox state (NADH, FAD), ROS, and [Ca2+].
MATERIALS AND METHODS
Langendorff heart preparation
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the NIH (Publication No. 85-23, 1996) and was approved by the institutional animal care committee. Our methods have been described previously in detail.9-14 Guinea pigs (n=73) were anesthetized with ketamine (50 mg/kg, IP), and heparin (5000 U, IP) was administered to prevent clotting. Following decapitation and thoracotomy, hearts were removed and perfused at 55 mmHg via the aortic root with a Krebs-Ringer’s (KR) solution containing (mM): 138 Na+, 4.5 K+, 1.2 Mg2+, 2.5 Ca2+, 134 Cl−, 14.5 HCO3−, 1.2 H2PO4− , 11.5 glucose, 2 pyruvate, 16 mannitol, 0.1 probenecid, 0.05 EDTA, and 5 U/l insulin gassed with ~97% O2 and ~3% CO2 (pH 7.4±0.01) at 37°C. Left ventricular pressure (LVP) was measured with a saline-filled latex balloon inserted into the left ventricle. Spontaneous heart rate was monitored with bipolar electrodes placed in the right atrial and ventricular walls. Coronary flow was measured by an ultrasonic flowmeter (Transonic T106X, Ithaca, NY) placed directly into the aortic inflow line. Cardiac O2 consumption (MVO2) was calculated as coronary flow • heart weight−1 • (PaO2 – PvO2) • 24 μl O2 / ml (37°C) at 760 mmHg, and cardiac efficiency as developed LVP • heart rate / MVO2, and %O2 extraction as 100 • (PaO2 – PvO2) / PaO2 (where PaO2 and PvO2 are arterial and venous PO2, respectively).
Online assessment of mitochondrial bioenergetic function
All fluorescence signals were detected in photons/second. All FAD and most NADH are confined to mitochondria.15-17 Most of the O2•− during cardiac IR injury likely originates from mitochondria while only a small amount is derived from non-mitochondrial sources.9 Non-myocyte sources such as endothelial and vascular cells also contribute to the total O2•− generated. However, the overall contribution of this source of ROS is minimal considering that mitochondrial content of endothelial cells ranges between 2-5%18 while mitochondria constitute 22-37% of myocyte volume.19, 20 These observations indicate that most of the O2•− generated in hearts subject to IR injury originates in cardiac myocyte mitochondria.
NADH and FAD, m[Ca2+], and O2•− emission were measured continuously at the left ventricle (LV) free wall using one of four excitation and emission fluorescence spectra9-14 in different subsets of hearts. A trifurcated fiberoptic probe was placed against the LV to excite and record light signals at specific wavelengths using spectrophotofluorometers (SLM Instruments Inc, Urbana, IL; or PTI, London, Ontario).
In a subset of hearts, as described previously,11, 12, 21 10 μM dihydroethidium (DHE, Molecular Probes, Eugene, OR), a fluorescent probe used to detect the O2•− radical,8, 22, 23 was loaded for 20 min and washed out; the LV free wall was excited at 540 nm, and light emission was recorded at 590 nm. O2•− non-enzymatically converts DHE to 2-hydroxyethidium (2-OH-E+) or a labile precursor that appears to be rapidly made and fluoresces at a slightly shorter wavelength than the heme-peroxidase oxidation product ethidium that can intercalate with DNA.22, 23 In other hearts NADH and FAD autofluorescence was assessed at 350 nm excitation and 450 and 390 nm emissions, and at 480 nm excitation and 540 nm emission, respectively.1, 7, 13, 17
Alternatively, other hearts were loaded with 6 μM indo 1 AM (Molecular Probes, Eugene, OR) for 30 min to measure Ca2+ transients at an excitation of 350 nm and emissions were recorded at 390 and 450 nm. After initially observing cytosolic Ca2+ transients, hearts were perfused for 15 min with 100 μM MnCl2 to quench the cytosolic indo 1 signal;24, 25 this was followed by a 15 min washout. To estimate actual m[Ca2+], NADH autofluorescence was subtracted from the underlying changes in mCa2+ fluorescent signals for each group.2, 7, 26 pH in the range of 6.2-8.0 does not alter the Ca2+ fluorescence signal in our model.11 This excludes the possibility of lactic acidosis during ischemia from having a significant effect on fluorescence signals.
Protocol
Hearts were randomly divided into three experimental groups: control (CON; KR 4 mM K+), high K+ cardioplegia (CP; KR with 16 mM K+), and CP plus a combination of ROS scavengers consists of 10 μM MnTBAP (A.G. Scientific Inc, San Diego, CA), 50 U/ml catalase (Sigma-Aldrich, St. Louis, MO), and 500 μM glutathione (Sigma-Aldrich, St. Louis, MO) (CP+MCG). Each heart initially underwent a stabilization period to attain a steady rhythm and level of contractility; this was followed by loading and washout of unbound dye to measure either m[Ca2+] or O2•−. NADH and FAD autofluorescence were assessed simultaneously. All hearts undergoing ischemia were perfused with either KR alone (CON), CP, or CP+MCG, first for 10 min at 37°C, and then continued during an additional 30 min at 27°C. This was followed by 120 min of global 27°C ischemia and 120 min reperfusion at 37°C. At the end of each experiment the ventricles were cut into 4-5 transverse sections of approximately 3 mm and incubated in buffered 0.1% 2,3,5-triphenyltetrazolium chloride to distinguish viable tissue (stained) from necrotic tissue (unstained) for estimating infarct size.27
Statistical analysis
All data are expressed as means ± SEM. Among-group data were compared by ANOVA to determine significance (Super ANOVA 1.11 software; Abacus Concepts, Berkeley, CA) at specific times. If F values were significant (P < 0.05), post hoc comparisons of means tests (Student-Newman-Keuls) were used to compare the groups within each subset. Differences among means were considered statistically significant when P < 0.05 (two tailed).
RESULTS
Baseline values were not different among treatment groups. Similar to our previous study,28 values for all measurements did not change throughout the duration of the time control experiments (data not shown), which indicates stability of the preparation. Figures 1A and 1B show timeline changes in systolic minus diastolic LVP (developed LVP) and diastolic LVP (diaLVP), respectively. All treated hearts were arrested (due to the high [K+] in CP) during the 10 min perfusion at 37°C and the 30 min perfusion at 27°C before ischemia. By the end of 60 min reperfusion developed LVP was higher in CP and CP+MCG groups than in the CON group, and diaLVP was lower in the CP and CP+MCG groups compared to the CON group. Values at 120 min reperfusion were similar to those at 60 min. Cardiac contraction, relaxation, and metabolic data were compared for all groups at 60 min reperfusion (Table 1). On reperfusion, each index was more improved in the treatment groups compared to the CON group. Although some indices in Table 1 are different between CP+MCG and CP alone, these were not statistically significant.
Figure 1.
Time course of changes in developed LVP (A) and diastolic LVP (B) at baseline, during treatment, hypothermia, cold ischemia, and warm reperfusion for control (n=15), CP (n=16), and CP+MCG (n=14) groups. The arrow indicates where the data point was recorded during treatment at 37°C. The solid horizontal bar indicates the period of treatment at 27°C. The period between the two unbroken vertical lines refers to ischemia. *P < 0.05 values vs. baseline within each group; #P < 0.05 each treatment vs. the control group; ¶P < 0.05, CP+MCG vs. CP alone.
Table 1.
Changes in heart rate, maximal rates of contractility (dP/dt max) and relaxation (dP/dt min), O2 consumption, % O2 extraction, and cardiac efficiency at baseline and 60 min reperfusion.
| Baseline | Reperfusion 60 min | |
|---|---|---|
| Heart Rate (beats/min) | ||
| Control | 276±9 | 269±6 |
| CP | 269±6 | 262±7 |
| CP+MCG | 269±15 | 279±8 |
| dP/dt max (mmHg/s) | ||
| Control | 2024±175 | 735±142 |
| CP | 1909±193 | 1221±141# |
| CP+MCG | 2012±168 | 1040±67# |
| dP/dt min (mmHg/s) | ||
| Control | −1502±124 | −516±101 |
| CP | −1542±206 | −904±112# |
| CP+MCG | −1577±171 | −716±54# |
| O2 consumption (μl O2/g/min) | ||
| Control | 105.1±7.8 | 57.6±6.8 |
| CP | 102.2±7.0 | 81.4±6.8# |
| CP+MCG | 121.8±8.7 | 87.3±5.6# |
| % O2 extraction | ||
| Control | 72±4 | 76±5 |
| CP | 76±2 | 85±2# |
| CP+MCG | 73±3 | 81±4 |
| Cardiac efficiency (mmHg • beat • 0.1 μL O2 / g) | ||
| Control | 23±3 | 14±2 |
| CP | 23±1 | 16±1 |
| CP+MCG | 18±1 | 14±2 |
Values are mean ± SEM
Abbreviations: CP, high K+ cardioplegia; CP+MCG, high K+ cardioplegia with MnTBAP, catalase, and glutathione.
P < 0.05 each treatment vs. the control group
P < 0.05, CP+MCG vs. CP
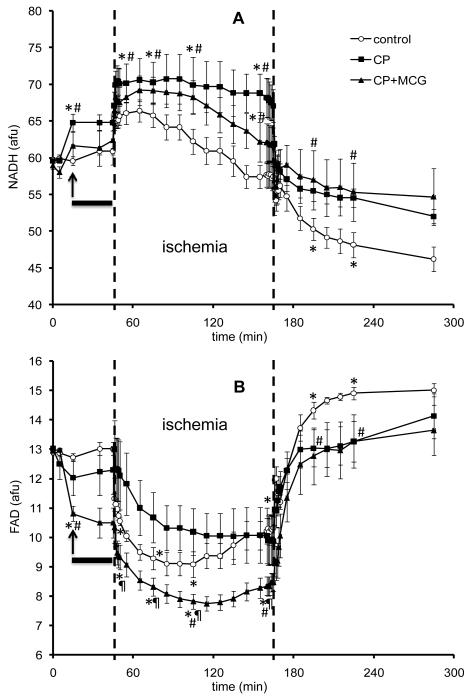
Figures 2A and 2B, respectively, show timeline changes in NADH and FAD. An increase in NADH and a decrease in FAD indicate a more reduced mitochondrial redox state.7, 26 NADH increased during treatment with CP and CP+MCG before hypothermia and this was followed by an additional increase during the first 5 min of ischemia. Conversely, FAD decreased during treatment with CP and more so during ischemia with CP+MCG. On reperfusion, mitochondrial NADH and FAD were more preserved in the CP and CP+MCG groups than in the CON group.
Figure 2.
Time course of changes in NADH autofluorescence (A) and FAD autofluorescence (B) at baseline, during treatment, hypothermia, cold ischemia, and warm reperfusion in control (n=5), CP (n=5), and CP+MCG (n=5) groups. The arrow indicates where the data point was recorded during treatment at 37°C. The solid horizontal bar indicates the period of treatment at 27°C. The period between the two unbroken vertical lines refers to ischemia. *P < 0.05 values vs. baseline within each group; #P < 0.05 each treatment vs. the control group; ¶P < 0.05, CP+MCG vs. CP alone.
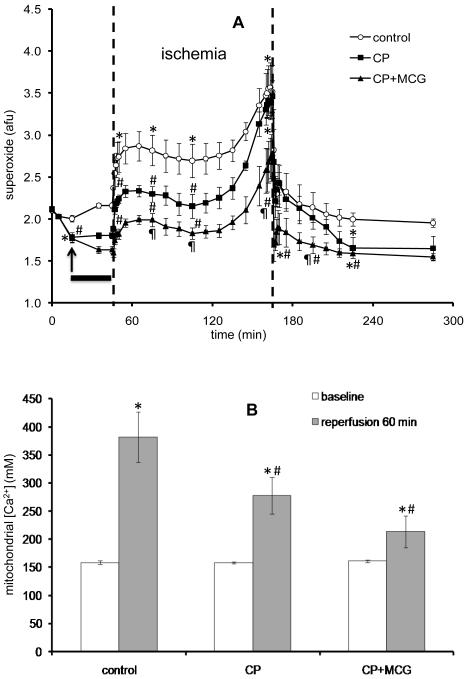
Figure 3A shows timeline changes in O2•− emission in the CON and treatment groups. CP perfusion alone caused a decrease in O2•− emission and cold perfusion increased the O2•− emission slightly in the CON group. During initial ischemia, O2•− emission increased in all groups and surged during the last 20 min of ischemia to reach a significantly higher value in the CON group compared to the treatment groups, with CP+MCG having the lowest O2•− emission throughout ischemia. On reperfusion, the O2•− signal declined in all groups and by the end of reperfusion, O2•− emission was more elevated in the CON group than in the CP or CP+MCG groups. Figure 3B shows m[Ca2+] at baseline and at 60 min reperfusion. m[Ca2+] was not different among groups during ischemia (data not shown). At 60 min reperfusion, CP and CP+MCG treatments resulted in less mCa2+ loading compared to CON with no significant difference between CP and CP+MCG groups by the end of reperfusion.
Figure 3.
(A). Time course of changes in superoxide (O2•−) signal at baseline, during treatment, hypothermia, cold ischemia, and warm reperfusion in control (n=4), CP (n=4), and CP+MCG (n=4) groups. The arrow indicates where the data point was recorded during treatment at 37°C. The solid horizontal bar indicates the period of treatment at 27°C. The period between the two unbroken vertical lines refers to ischemia. Figure 3(B). Mitochondrial Ca2+ levels in control (n=6), CP (n=7), and CP+MCG (n=5) groups at baseline and 60 min of reperfusion. *P < 0.05 values vs. baseline within each group; #P < 0.05 each treatment vs. the control group; ¶P < 0.05, CP+MCG vs. CP alone.
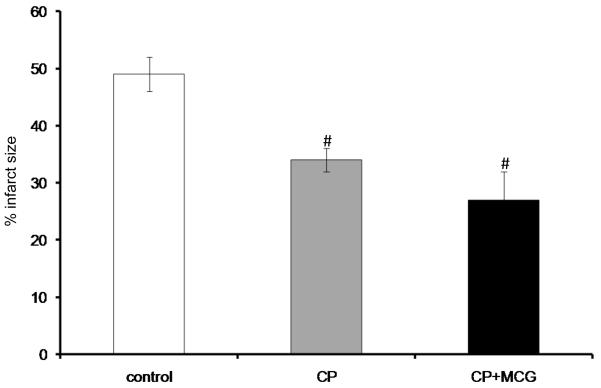
Figure 4 shows that CP and CP+MCG groups had smaller infarct sizes compared to the CON group; CP+MCG group showed the smallest infarct sizes. Table 2 shows cardiac functional recovery and changes in mitochondrial bioenergetics for two additional treatment groups: CP+MnTBAP and CP+catalase+glutathione. There were no significant differences in cardiac function and infarct size among these groups when compared to CP alone.
Figure 4.
Infarct size as a percentage of total ventricular weight measured after 120 min reperfusion from random hearts in control (n=10), CP (n=8), and CP+MCG (n=6). #P < 0.05 each treatment vs. the control group; ¶P < 0.05, CP+MCG vs. CP alone.
Table 2.
Changes in developed left ventricular pressure, diastolic left ventricular pressure, and mitochondrial bioenergetics (NADH, FAD, superoxide, mitochondrial Ca2+) at baseline and 60 min reperfusion in two additional treatment groups: CP+M, CP+CG
| Baseline | Reperfusion 60 minutes | |
|---|---|---|
| Developed left ventricular pressure (mmHg) | ||
| CP+M (n=14) | 86±3 | 43±5# |
| CP+CG (n=14) | 90±1 | 42±3# |
| Diastolic left ventricular pressure (mmHg) | ||
| CP+M (n=14) | 0.6±0.4 | 12.4±3#¶ |
| CP+CG (n=14) | 0.4±0.2 | 10.3±4.2#¶ |
| NADH (afu) | ||
| CP+M (n=5) | 59.7±0.1 | 44.9±1.6¶ |
| CP+CG (n=5) | 60.1±0.3 | 47.6±2.1¶ |
| FAD (afu) | ||
| CP+M (n=5) | 12.9±0.1 | 13.8±0.6 |
| CP+CG (n=5) | 13.1±0.1 | 14.8±0.3 |
| Superoxide (afu) | ||
| CP+M (n=5) | 2.12±0.01 | 1.76±0.09# |
| CP+CG (n=6) | 2.1±0.02 | 1.89±0.13# |
| Mitochondrial Ca2+ (nM) | ||
| CP+M (n=4) | 184±19 | 207±53# |
| CP+CG (n=3) | 155±1 | 186±47# |
| Infarct Size % | ||
| CP+M (n=6) | - | 39±4# |
| CP+CG (n=6) | - | 31±5# |
Values are mean ± SEM
Abbreviations: CP+M, high K+ cardioplegia with MnTbap; CP+CG, high K+ cardioplegia with catalase+glutathione
P < 0.05 each treatment vs. the control group
P < 0.05, CP+M or CP+CG vs. CP
DISCUSSION
The major findings of this study are that exposure to hypothermic (27°C) CP before and during ischemia protects hearts, preserves mitochondrial redox state (NADH/FAD), lowers m[Ca2+] during reperfusion, and reduces O2•− emission during IR more than does hypothermia alone. Adding a combination of ROS scavengers to CP did not further enhance protection against myocardial dysfunction during IR, but did provide modest improvement against infarction when MCG was added to CP. Mitochondrial dysfunction was reduced equivalently with CP treatment with or without the ROS scavengers compared to the non-treated, control hearts. However, O2•− emission was lower during IR, and FAD remained more reduced during ischemia when the CP+MCG group was compared to the CP group.
Cold CP is widely used for cardiac preservation because it reduces the energy demand which helps to rapidly regenerate ATP on reperfusion.2 Hypothermia decreases the rate at which intracellular enzymes degrade essential cellular components for organ viability during ischemia.29 Although hypothermia is the most effective method to preserve hearts during ischemic storage, hypothermia itself has deleterious effects on contractile element and endothelial cell function as the cooling becomes more severe. These defects include cytosolic and mCa2+ loading due to impaired ion pumps.1, 25 Moreover, hypothermia even without concomitant ischemia increases ROS levels likely by increasing ROS generation and/or reducing ROS scavenging,9 which results in mitochondrial and cellular damage proportional to the degree and duration of hypothermia. In a previous study,2 we showed that the SOD mimetic, MnTBAP, used alone without CP, did not protect against ischemic damage due to increased O2•− downstream products like H2O2 and OH•. But when combined with catalase and glutathione (MCG), cardiac protection was characterized by better normalization of the mitochondrial redox state (NADH and FAD), decreased O2•− levels and m[Ca2+] loading during and after ischemia, greater contractile function on reperfusion, and reduced infarct size. That study indicated clearly that cardioprotection obtained by mild hypothermia alone during ischemia can be enhanced by selected ROS scavengers administered during the period of cold perfusion before ischemia.
In clinical settings, hypothermic perfusion is seldom used alone. It is most often combined with a variety of CP solutions to achieve cardiac arrest during ischemia and to optimize post ischemic recovery.30-34 Our rationale for using the ROS scavenger cocktail MCG with cold CP perfusion was to reduce ROS emission induced by hypothermia as we showed earlier.2 Hence in the present study we wished to determine if addition of ROS scavengers to cold CP before ischemia would have an additive effect on cardiac protection, and more importantly, if this additive protection is associated with a reduction in mitochondrial ROS, m[Ca2+], and/or improved redox state. Indeed, our data show there was equivalent cardiac functional recovery in the two treatment groups after 60 min of reperfusion and this was associated with similar preservation of mitochondrial redox state (NADH/FAD). Although adding MCG to CP did not further improve cardiac recovery after 2 h cold ischemia more than did CP alone, it appeared to decrease infarct size, though not significantly. However, the CP+MCG group exhibited reduced O2•− emission during ischemia compared to both the control and CP groups, and at 30 min reperfusion O2•− emission remained lower in the CP+MCG group than in the control group.
It is unclear how CP alone is effective in decreasing ROS. It is possible that electron flow through the ETC is diminished due to less ATP demand in CP treated hearts, which could result in less electron leak and lower O2•− levels. Another possibility could be simply that there is more effective ROS scavenging by the endogenous glutathione in the CP group. Glutathione is highly regulated by the cytosolic redox state and is rapidly taken up from the cytosol via the decarboxylate and 2-oxoglutarate transporters.35-37 In this way it effectively links changes in the cellular redox state.36, 37 Indeed, one defense against IR-induced ROS accumulation and damage may involve preservation of the NAD(P)H pool. The NADH/NAD+ level through the NADH kinase and transhydrogenase38, 39-dependent mechanism maintains the mitochondrial NAD(P)H pool required to maintain the redox status necessary for effective scavenging. Thus an increase in NADH, as was observed in the CP group, would correlate with increased NAD(P)H dependent redox scavenging by glutathione.
It is unlikely that CP reduced the ROS level indirectly by reducing m[Ca2+] since m[Ca2+] was not different between the CP and CON groups during ischemia (data not shown). Although high m[Ca2+] during ischemia is believed to be an instigator of IR injury, it seems that the most damaging Ca2+ influx occurs on reperfusion.40 All together, it appears that addition of MCG to CP, although it decreased ROS even more than did CP alone during ischemia and during early reperfusion, did not markedly enhance the protection afforded by CP alone, which may indicate that not all the ROS generated during ischemia is damaging.
Results from our previous study2 show that hearts treated with MnTBAP alone had a worse outcome compared to hearts treated with the combination MCG or control hearts. In the present study, when CP was combined only with MnTBAP, functional recovery was improved, but this improvement was not significantly different from CP plus the scavenging cocktail MCG (Table 2). This could be attributed to the finding that CP alone reduced O2•− available for scavenging and resulted in decreased downstream products of O2*− associated with better cardiac recovery.
Our results showing that addition of MCG to CP did not significantly improve cardiac recovery compared to CP alone are inconsistent with several studies3, 41-47 that showed improvement in cardiac function when ROS scavengers were included. It seems that since the production of ROS by cardiac mitochondria in our model during cold ischemia is substantially reduced by CP alone, the added benefit of ROS scavengers is small. It is clear that in the absence of CP during hypothermic protection, the scavenging cocktail provides good protection.2 Although we observed reduced O2•− emission during ischemia with MCG (Figure 3), superoxide dismutase and catalase may not be as effective during ischemia because ROS scavenging enzymes are reported to provide more benefit when O2 is restored to previously ischemic tissue due to regeneration of reduced scavenging systems.48
In summary, our data show that hypothermic CP perfusion alone before 2 h cold ischemia preserved mitochondrial redox potential (NADH, FAD), lowered ROS during subsequent cold IR, and lowered m[Ca2+] during reperfusion. Adding ROS scavengers to the cold CP perfusate provided limited added cardiac and mitochondria protection, but did decrease O2•− emission during ischemia and early reperfusion.
ACKNOWLEDGEMENTS
The authors wish to thank Anita Tredeau and Steven Contney for their valuable assistance.
This work was done in the department of Anesthesiology and Anesthesia Research at the Medical College of Wisconsin and was supported in part by the National Institutes of Health [K01 HL73246 to A.K.S. Camara, R01 HL089514 to D.F. Stowe]; and the Veterans Administration [VA Merit 8204-05P to D.F. Stowe].
Footnotes
Conflict of interest: None declared.
REFERENCES
- 1.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc Res. 2004;61(3):580–590. doi: 10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Camara AK, Aldakkak M, Heisner JS, et al. ROS scavenging before 27°C ischemia protects hearts and reduces mitochondrial ROS, Ca2+ overload, and changes in redox state. Am J Physiol Cell Physiol. 2007;292(6):C2021–2031. doi: 10.1152/ajpcell.00231.2006. [DOI] [PubMed] [Google Scholar]
- 3.Menasche P, Grousset C, Gauduel Y, Mouas C, Piwnica A. [Protective effects of inhibitors of oxygen free radicals on the ischemic and reperfused heart. Applications to cardioplegia] Archives des maladies du coeur et des vaisseaux. 1986;79(13):1918–1923. [PubMed] [Google Scholar]
- 4.Tossios P, Bloch W, Huebner A, et al. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126(5):1513–1520. doi: 10.1016/s0022-5223(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 5.Vento AE, Nemlander A, Aittomaki J, Salo J, Karhunen J, Ramo OJ. N-acetylcysteine as an additive to crystalloid cardioplegia increased oxidative stress capacity in CABG patients. Scand Cardiovasc J. 2003;37(6):349–355. doi: 10.1080/14017430310015406. [DOI] [PubMed] [Google Scholar]
- 6.Ytrehus K, Gunnes S, Myklebust R, Mjos OD. Protection by superoxide dismutase and catalase in the isolated rat heart reperfused after prolonged cardioplegia: a combined study of metabolic, functional, and morphometric ultrastructural variables. Cardiovasc Res. 1987;21(7):492–499. doi: 10.1093/cvr/21.7.492. [DOI] [PubMed] [Google Scholar]
- 7.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77(2):406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29(9):2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 9.Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF. Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiol Heart Circ Physiol. 2004;286(4):H1289–1299. doi: 10.1152/ajpheart.00811.2003. [DOI] [PubMed] [Google Scholar]
- 10.An J, Varadarajan SG, Camara A, et al. Blocking Na+/H+ exchange reduces [Na+]i and [Ca2+]i load after ischemia and improves function in intact hearts. Am J Physiol Heart Circ Physiol. 2001;281(6):H2398–2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 11.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284(2):H566–574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 12.Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003;96(4):949–955. doi: 10.1213/01.ANE.0000052515.25465.35. [DOI] [PubMed] [Google Scholar]
- 13.Riess ML, Camara AK, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283(1):H53–60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 14.Riess ML, Camara AK, Novalija E, Chen Q, Rhodes SS, Stowe DF. Anesthetic preconditioning attenuates mitochondrial Ca2+ overload during ischemia in Guinea pig intact hearts: reversal by 5-hydroxydecanoic acid. Anesth Analg. 2002;95(6):1540–1546. doi: 10.1097/00000539-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J. 1996;71(2):1024–1035. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chance B, Williamson JR, Jamieson D, Schoenner B. Properties and kinetics of reduced pyridine nucleotide fluorescence of the isolated and in vivo rat heart. Biochem Z. 1965;341:357–377. [Google Scholar]
- 17.Nuutinen EM. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol. 1984;79(1):49–58. doi: 10.1007/BF01935806. [DOI] [PubMed] [Google Scholar]
- 18.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1(5):409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 19.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24(7):669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 20.Vinnakota KC, Bassingthwaighte JB. Myocardial density and composition: a basis for calculating intracellular metabolite concentrations. Am J Physiol Heart Circ Physiol. 2004;286(5):H1742–1749. doi: 10.1152/ajpheart.00478.2003. [DOI] [PubMed] [Google Scholar]
- 21.Camara AK, Chen Q, An J, et al. Comparison of hyperkalemic cardioplegia with altered [CaCl2] and [MgCl2] on [Ca2+]i transients and function after warm global ischemia in isolated hearts. J Cardiovasc Surg (Torino) 2004;45(1):1–13. [PubMed] [Google Scholar]
- 22.Zhao H, Joseph J, Fales HM, et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102(16):5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Kalivendi S, Zhang H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34(11):1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 24.Brandes R, Figueredo VM, Camacho SA, Baker AJ, Weiner MW. Investigation of factors affecting fluorometric quantitation of cytosolic [Ca2+] in perfused hearts. Biophys J. 1993;65(5):1983–1993. doi: 10.1016/S0006-3495(93)81275-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stowe DF, Fujita S, An J, Paulsen RA, Varadarajan SG, Smart SC. Modulation of myocardial function and [Ca2+] sensitivity by moderate hypothermia in guinea pig isolated hearts. Am J Physiol. 1999;277(6 Pt 2):H2321–2332. doi: 10.1152/ajpheart.1999.277.6.H2321. [DOI] [PubMed] [Google Scholar]
- 26.Aldakkak M, Stowe DF, Heisner JS, Spence M, Camara AK. Enhanced Na+/H+ exchange during ischemia and reperfusion impairs mitochondrial bioenergetics and myocardial function. J Cardiovasc Pharmacol. 2008;52(3):236–244. doi: 10.1097/FJC.0b013e3181831337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riess ML, Rhodes SS, Stowe DF, Aldakkak M, Camara AK. Comparison of cumulative planimetry versus manual dissection to assess experimental infarct size in isolated hearts. Journal of pharmacological and toxicological methods. 2009;60(3):275–280. doi: 10.1016/j.vascn.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An J, Camara AK, Riess ML, Rhodes SS, Varadarajan SG, Stowe DF. Improved mitochondrial bioenergetics by anesthetic preconditioning during and after 2 hours of 27°C ischemia in isolated hearts. J Cardiovasc Pharmacol. 2005;46(3):280–287. doi: 10.1097/01.fjc.0000175238.18702.40. [DOI] [PubMed] [Google Scholar]
- 29.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45(4):673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Stowe DF, Camara AK, Heisner JS, Aldakkak M, Harder DR. Ten-hour preservation of guinea pig isolated hearts perfused at low flow with air-saturated Lifor solution at 26°C: comparison to ViaSpan solution. Am J Physiol Heart Circ Physiol. 2007;293(1):H895–901. doi: 10.1152/ajpheart.00149.2007. [DOI] [PubMed] [Google Scholar]
- 31.Stowe DF, Camara AK, Heisner JS, Aldakkak M, Harder DR. Low-flow perfusion of guinea pig isolated hearts with 26°C air-saturated Lifor solution for 20 hours preserves function and metabolism. J Heart Lung Transplant. 2008;27(9):1008–1015. doi: 10.1016/j.healun.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camara AK, An J, Chen Q, et al. Na+/H+ exchange inhibition with cardioplegia reduces cytosolic [Ca2+] and myocardial damage after cold ischemia. J Cardiovasc Pharmacol. 2003;41(5):686–698. doi: 10.1097/00005344-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Ross JD, Ripper R, Law WR, et al. Adding bupivacaine to high-potassium cardioplegia improves function and reduces cellular damage of rat isolated hearts after prolonged, cold storage. Anesthesiology. 2006;105(4):746–752. doi: 10.1097/00000542-200610000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Stowe DF, Heisner JS, An J, et al. Inhibition of Na+/H+ isoform-1 exchange protects hearts perfused after 6-hour cardioplegic cold storage. J Heart Lung Transplant. 2002;21(3):374–382. doi: 10.1016/s1053-2498(01)00383-7. [DOI] [PubMed] [Google Scholar]
- 35.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 36.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282(30):21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camara AK, Lesnefsky EJ, Stowe DF. Potential Therapeutic Benefits of Strategies Directed to Mitochondria. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanukoglu I, Rapoport R. Routes and regulation of NADPH production in steroidogenic mitochondria. Endocrine research. 1995;21(1-2):231–241. doi: 10.3109/07435809509030439. [DOI] [PubMed] [Google Scholar]
- 39.Outten CE, Culotta VC. A novel NADH kinase is the mitochondrial source of NADPH in Saccharomyces cerevisiae. The EMBO journal. 2003;22(9):2015–2024. doi: 10.1093/emboj/cdg211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen DG, Xiao XH. Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc Res. 2003;57(4):934–941. doi: 10.1016/s0008-6363(02)00836-2. [DOI] [PubMed] [Google Scholar]
- 41.Bernard M, Menasche P, Pietri S, Grousset C, Piwnica A, Cozzone PJ. Cardioplegic arrest superimposed on evolving myocardial ischemia. Improved recovery after inhibition of hydroxyl radical generation by peroxidase or deferoxamine. A 31P nuclear resonance study. Circulation. 1988;78(5 Pt 2):III164–172. [PubMed] [Google Scholar]
- 42.Menasche P, Grousset C, Gauduel Y, Mouas C, Piwnica A. Enhancement of cardioplegic protection with the free-radical scavenger peroxidase. Circulation. 1986;74(5 Pt 2):III138–144. [PubMed] [Google Scholar]
- 43.Menasche P, Grousset C, Gauduel Y, Mouas C, Piwnica A. Prevention of hydroxyl radical formation: a critical concept for improving cardioplegia. Protective effects of deferoxamine. Circulation. 1987;76(5 Pt 2):V180–185. [PubMed] [Google Scholar]
- 44.Menasche P, Grousset C, Gauduel Y, Mouas C, Piwnica A. [A new concept of cardioplegic protection in cardiac surgery: iron chelation] Archives des maladies du coeur et des vaisseaux. 1988;81(6):811–816. [PubMed] [Google Scholar]
- 45.Menasche P, Grousset C, Gauduel Y, Piwnica A. A comparative study of free radical scavengers in cardioplegic solutions. Improved protection with peroxidase. J Thorac Cardiovasc Surg. 1986;92(2):264–271. [PubMed] [Google Scholar]
- 46.Myers CL, Weiss SJ, Kirsh MM, Shepard BM, Shlafer M. Effects of supplementing hypothermic crystalloid cardioplegic solution with catalase, superoxide dismutase, allopurinol, or deferoxamine on functional recovery of globally ischemic and reperfused isolated hearts. J Thorac Cardiovasc Surg. 1986;91(2):281–289. [PubMed] [Google Scholar]
- 47.Shlafer M, Kane PF, Kirsh MM. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg. 1982;83(6):830–839. [PubMed] [Google Scholar]
- 48.Gallagher KP, Buda AJ, Pace D, Gerren RA, Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986;73(5):1065–1076. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]