Abstract
Objectives
FoxO proteins are transcription factors involved in varieties of cellular processes, including immune cell homeostasis, cytokine production, anti-oxidative stress, and cell proliferation and differentiation. Although these processes are implicated in the development of atherosclerosis, very little is known about the role of FoxO proteins in the context of atherosclerosis. Our objectives were to determine whether and how inactivation of Foxo4, a member of the FoxO family, in vivo promotes atherosclerosis.
Methods and Results
Apolipoprotein E-deficient (apoE−/−) mice were crossbred with animals lacking Foxo4 (Foxo4−/−). After 10 weeks on a high fat diet (HFD), Foxo4−/−apoE−/− mice showed elevated atherosclerosis and increased amount of macrophages and T cells in the plaque compared to apoE−/− mice. Bone marrow transplantations of chimeric C57B/6 mice reconstituted with either wild-type or Foxo4−/− bone marrows indicate that Foxo4-deficiency in bone marrow derived cells sufficiently promoted atherosclerosis. Foxo4-null macrophages produced elevated inflammatory cytokine IL-6 and levels of reactive oxygen species (ROS) in response to lipopolysaccharides in vitro. Serum levels of IL-6 were upregulated in HFD-fed Foxo4−/−apoE−/− mice compared to those of apoE−/− mice.
Conclusions
FoxO4 inhibits atherosclerosis through bone marrow derived cells, possibly by inhibition of ROS and inflammatory cytokines that promote monocyte recruitment and/or retention.
Keywords: FoxO, macrophage, bone marrow, inflammation
Introduction
Atherosclerosis is the most common pathological process that underlies adverse cardiovascular events, including coronary artery disease, stroke, abdominal aortic aneurisms, and ischemic gangrene (Lloyd-Jones et al., 2009). Clinical complications arising from atherosclerosis, i.e. myocardial infarction and stroke are responsible for most of the cardiovascular morbidity and mortality in the world. Although statin therapy has been proven to significantly reduce cardiovascular mortality in hypercholesterolemic patients, there remains an unmet need for non-statin drugs for patients with normal levels of LDL-cholesterol and/or who are maximized on current therapy or are intolerant to statins (Davison, 2009; Klingerber et al., 2009; Sadowitz et al, 2010).
Interplay among factors, including hyperlipidemia, modified lipoproteins, resident endothelial cells (ECs) and smooth muscle cells (SMCs), and infiltrating monocyte and T cells, plays important roles in the development of atherosclerosis (Libby et al., 2010). Atherosclerosis is initiated by focal stimulation. As a result, activated ECs express adhesion molecules and chemokines that promote recruitment of monocyte and T cells into the intima. Macrophages and T cells in the intima produce inflammatory cytokines that can promote VSMCs from the media to proliferate and migrate into the intima, forming more advanced, complex lesions (Shin et al., 2003; Raines & Ferri, 2005). The accumulation of immune cells and inflamed VSMCs in the lesion leads to increased expression of cytokines and proteases that enhance a further influx of monocytes and T cells, promoting lesion progression. Atherosclerosis can potentially be inhibited at any point during disease progression, including production of ROS, recruitment of immune cells, VSMC proliferation and migration, and expression of inflammatory cytokines. Identification of factors involved at various stages and understanding their mechanisms of action are of paramount importance to developing successful strategies to prevent, treat, or even reverse atherosclerosis.
FoxO4 is a member of the forkhead transcription factor O subfamily that also includes FoxO1, FoxO3, and FoxO6. FoxO proteins are ubiquitously expressed and often perform highly specialized and non-redundant functions through interactions with other transcriptional (co)factors in a context and cell type dependent manner (Ronnebaum and Patterson, 2010; Van der Vos and Coffer, 2010). FoxO proteins are involved in many of the cellular processes that are implicated in the development of atherosclerosis. FoxO proteins promote apoptosis of insulin-resistant macrophages in response to cholesterol-induced endoplasmic reticulum stress (Senokuchi et al., 2008). FoxO proteins can act as a sensor, mediator, and regulator of redox signaling and control ROS level by transcriptional regulation of ROS scavenger enzymes (Keizer et al., 2011). FoxO proteins are also involved in homeostasis of the immune system (Dejean et al., 2011). FoxO3 regulates production of IL-6, TNFα, and MCP-1 in dendritic cells (DCs). Upon viral infection, Foxo3-null DCs have elevated production of IL-6, leading to enhanced T cell survival. Although the precise molecular mechanism remains elusive, FoxO1 has been shown to activate transcription factor Klf2 that regulates L-selectin and CCR7, which drives recruitment and migration of T cells into the T cell zone of secondary lymphoid organs (Fabre et al., 2008; Dengler et al., 2008). Previously, we have shown that FoxO4 inhibits SMC differentiation and promotes dedifferentiation and proliferation of intima SMCs in response to a mechanical injury (Liu et al., 2005; Li et al, 2007). We also found that FoxO4 inhibits the expression of NF-κB-activated inflammatory cytokines, including CCL5, CXCL9, TNFα, IFNγ, IL-1β, and IL-6, and plays important role in mucosal innate immunity (Zhou et al., 2009). The role of FoxO4 in atherosclerosis remains to be determined; it can be either protective or pathological depending on the relative contribution of the SMCs and immune cells to the development of atherosclerosis. Therefore, it is necessary to investigate the role of FoxO4 in the context of atherosclerosis.
In the present study, we investigated the effect of inactivation of Foxo4 in vivo on the development of atherosclerosis using a mouse model of atherosclerosis. We generated Foxo4−/−apoE−/− mice and tested the effect of Foxo4-deficiency on the high fat diet-induced atherosclerosis in comparison to apoE−/− mice. Our studies show that Foxo4-deficiency enhances the atherosclerotic burden in the apoE−/− background and increases the amount of macrophages and T cells in the lesion. Bone marrow transplantations of C57B/6 mice reconstituted with either wild-type or Foxo4-deficient bone marrows indicate that inactivation of Foxo4 in bone marrow derived cells is sufficient to promote atherosclerosis and upregulate the macrophage content in the lesion. We also show that Foxo4-null macrophages have altered ability to produce higher amount of inflammatory cytokine IL-6 and levels of reactive oxygen species (ROS) in response to lipopolysaccharides (LPS) in vitro. Serum levels of IL-6 were upregulated in HFD-fed Foxo4−/−apoE−/− mice compared to that of apoE−/− mice. Taken together, our studies indicate that FoxO4 is an anti-atherogenic factor and inhibits atherosclerosis likely through its function in bone marrow derived monocytes.
Methods
Generation of Foxo4−/−apoE−/− double knockout (DKO), Foxo4+/+apoE−/− (apoE SKO), and Foxo4−/−apoE+/+ (Foxo4 SKO) mice
Foxo4−/− mice in the FVB/N background (Paik et al., 2007) were backcrossed >8 generations into the C57B/6 background. The resulting Foxo4−/− mice were then intercrossed with apoE−/− mice in the same genetic background to yield F1 apoE+/−Foxo4+/− offspring. The F1 compound heterozygous mice were mated to each other to generate F2 mice. Because a large number of mice were needed for the experiments, we took the advantage that the Foxo4 gene is X-chromosome linked and used F2 mating pairs to generate F3 apoE/Foxo4 double knockout (DKO), apoE single KO (SKO) and Foxo4 SKO mice, respectively. Age-matched mice from these three groups were used in the following experiments.
Induction of atherosclerosis, morphological analysis, and quantification of lesions
Foxo4apoE DKO and apoE SKO mice of both sexes at age of 8 weeks were fed with a high-fat rodent diet (21% milk fat, 1.25% cholesterol, 0.5% Cholic acid) for 10 weeks. Quantification of lesion size was carried out with en face (Daugherty & Rateri, 2005) and cross-sectional (Baglione & Smith, 2006) analysis, and performed without prior knowledge of the genotypes. In en face analysis, the aorta was removed in its entirety, fixed in 10% buffered formalin, and opened longitudinally. The arteries were pinned on a surface and stained with oil red O. Areas that were stained positive for oil red O was outlined manually, quantified by Image J software (NIH), and determined as a percentage of total aortic surface area. For quantification of lesion size at the aortic root areas, hearts containing the aortic root were embedded in OCT, and sequential 8-μm frozen sections were made. Serial sections of the entire length of aortic roots were obtained. The section with the largest cross sectional lesion from each individual mouse was stained with Oil red O and photographed. Cross-sectional plaque areas were then traced manually, and quantified using Image J software.
Bone marrow transplantation
To induce bone marrow aplasia, female C57B/6 mice (age 8 to 10 weeks old) were irradiated with two doses of 4.75 Gy within an interval of 4 h, giving a total of 9.5 Gy, one day before transplantation. Bone marrow cell suspensions were isolated by flushing the femurs and tibias from male wild type or Foxo4−/− mice in the C57B/6 background with PBS. 2×106 bone marrow cells were injected into Irradiated recipient mice by intravenous injection via the tail vein. After transplantation, mice were placed on the regular chow diet for 4 weeks to recover and then on the HFD for 4 months. Successful reconstitution of recipients with cells of donor origin after bone marrow transplantation was established at time of sacrifice. Male-female chimerism of the transplanted mouse was assessed using genomic DNA isolated from the peripheral blood of transplanted chimeric mice for Foxo4 and Sry, a marker for the Y chromosome.
IL-6 Elisa assay, Immunohistochemistry (IHC), immunofluorescence, ROS staining, cell culture, plasmid, and reporter assay
Serum IL-6 levels were determined using a standard IL-6 ELISA assay following the manufacturer’s protocol (BD Bioscience). IHCs were performed on frozen sections of hearts containing the aortic root area with antibody against CD68 (BD Pharmingen) and CD4 (AbD Serotec) following the manufacturer’s protocol. Immunofluorescences were performed on frozen sections with the antibody against smooth muscle α-actin (Sigma). For ROS staining, peritoneal macrophages were seeded in to 6 well plates with coverslips. After treated with or without LPS (100 ng/ml, overnight), cells were washed twice with 1×PBS, incubated with 10 uM DHE (dihydroethidium, Sigma) for 30 min at 37°C incubator with 5% CO2, quickly washed twice with 1×PBS and mounted with anti-fading mounting media. IL-6-luciferase reporter was constructed by subcloning the PCR-amplified insert (0.5 kb) corresponding to the promoter sequence of IL-6 from human genomic DNA (Mori et al., 1994) into the pGL3-basic vector (Promega). Macrophage RAW264 cells were cultured in complete DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, and penicillin/streptomycin. Cells were transfected with combination of plasmids indicated for each experiment using Fugene 6 (Roche). Cell lysates were assayed for luciferase expression using a luciferase assay kit (Promega). Promoter activities were normalized against co-transfected β-galactosidase activities in the cell and expressed relative to control the vector transfected and vehicle treated cells.
Statistical analysis
Data are expressed as mean ± SEM. All statistical analysis was performed using Graphpad Prism software (San Diego, CA). The two-tailed t-test was used for comparisons between experimental groups. Differences were considered statistically significant at p < 0.05.
Results
Foxo4-deficiency enhances high fat diet-induced atherosclerosis
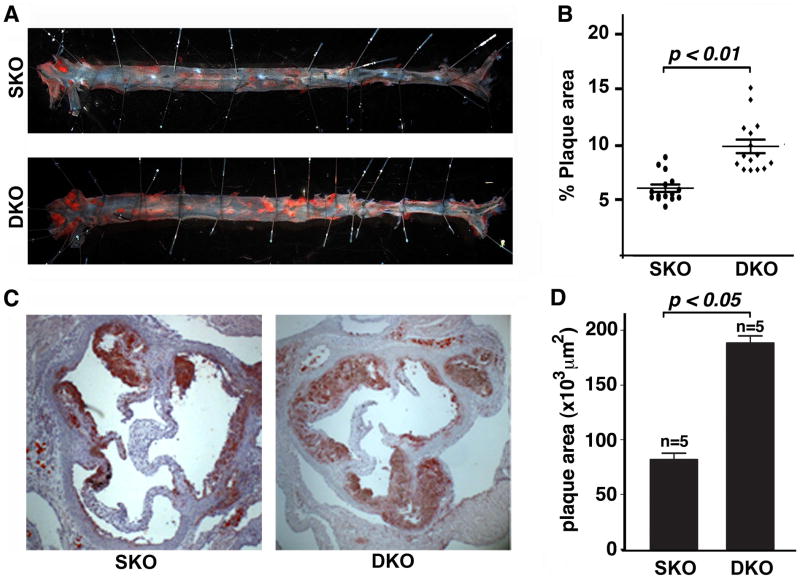
To study the role of Foxo4 in atherosclerosis, we used the apoE−/− mouse, a well-known mouse model of atherosclerosis (Nakashima et al., 1994). We generated Foxo4−/−apoE−/− (double knockout, DKO) mice and fed them, along with control apoE−/− (single KO, SKO) mice, with a high fat diet (HFD) for 10 weeks. At the time of sacrifice, no significant differences in body weight and total serum cholesterol levels between Foxo4/apoE DKO and apoE SKO mice were observed (data not shown). However, the atherosclerosis in the aorta of Foxo4/apoE DKO mice was significantly elevated compared to that of apoE SKO as shown by an en face analysis (Figure 1, A & B, aortic coverage of 9.6 ± 0.6% in DKO vs 6.0 ± 0.3% in apoE SKO mice). We also analyzed the atherosclerotic development in the aortic root in another cohort of mice. As anticipated, we observed a similar significant increase of atherosclerosis in Foxo4/apoE DKO compared to that of apoE SKO mice (Figure 1, C & D).
Figure 1. Inactivation of Foxo4 promotes atherosclerosis formation.
(A)en face analysis of atherosclerotic lesions of apoE SKO and Foxo4/apoE DKO mice after 10 weeks of HFD. The aorta was removed in its entirety and opened longitudinally. The artery was pinned on a surface and stained with oil red O. (B) The area of intima that is covered by lesions was outlined and determined as a percentage of total intimal surface area. Foxo4/apoE DKO mice have significantly higher amounts of atherosclerotic lesions than apoE SKO mice (n=15, mean ± SEM, p < 0.01). (C) Representative photomicrographs of atherosclerotic plaques at the aortic root of a Foxo4/apoE DKO and apoE SKO mouse. Frozen cross-sections of the aortic root were obtained and stained with oil red O. (D) Cross-sectional plaque areas were traced manually and measured morphometrically. Foxo4/apoE DKO mice have significantly higher amounts of atherosclerotic lesions than apoE SKO (n=5, mean ± SEM, p < 0.05).
Foxo4/apoE DKO mice have elevated levels of macrophages and T cells in the plaque
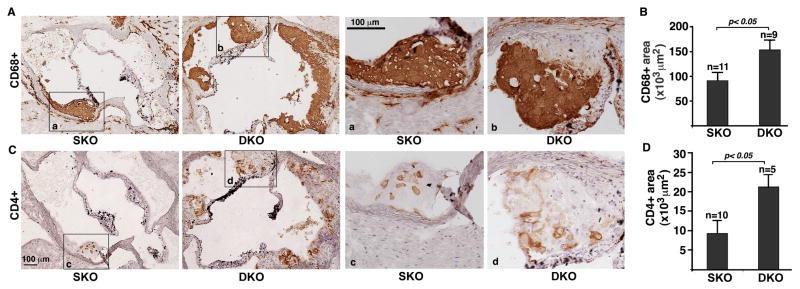
To identify the cellular mechanism(s) responsible for the enhanced atherosclerosis in Foxo4/apoE DKO mice, we analyzed the amount of T cells, macrophages, and SMCs in the atherosclerotic lesions of HFD-fed apoE/Foxo4 DKO and apoE SKO mice with antibodies against CD68 (macrophage), CD4 (T cells), and smooth muscle α-actin (SMCs), respectively. A significant increase in the macrophage and T-cell contents was observed in the lesions of Foxo4/apoE DKO mice when compared to those of apoE SKO mice (Figure 2). The number of SMCs was not significantly different between Foxo4/apoE DKO and apoE SKO mice (supplemental Figure 1).
Figure 2. Macrophages and T cells are upregulated in the atherosclerotic plaques of Foxo4/apoE DKO mice.
Immunohistochemistry was performed on sections of the aortic root of HFD-fed Foxo4/apoE DKO and apoE SKO mice using antibodies against CD68 (A and B) ad CD4 (C and D). (A) Representative of photomicrograph of sections stained with anti-CD68 antibody. High-magnification images of the insets (a and b) on the left panels are shown on the right panels. (B) CD68+ stains in DKO mice were upregulated compared to that of control SKO mice. Values are mean ± SEM, p < 0.05. High-magnification images of the insets (c and d) on the left panels are shown in the right panels. (C) Representative of photomicrograph of sections stained with anti-CD4 antibody. (D) CD4+ T cells in the lesions of DKO were significantly upregulated compared to control SKO mice. Values are mean ± SEM, p < 0.05.
Inactivation of Foxo4 in bone marrow–derived cells in mice leads to increased formation of atherosclerotic lesions
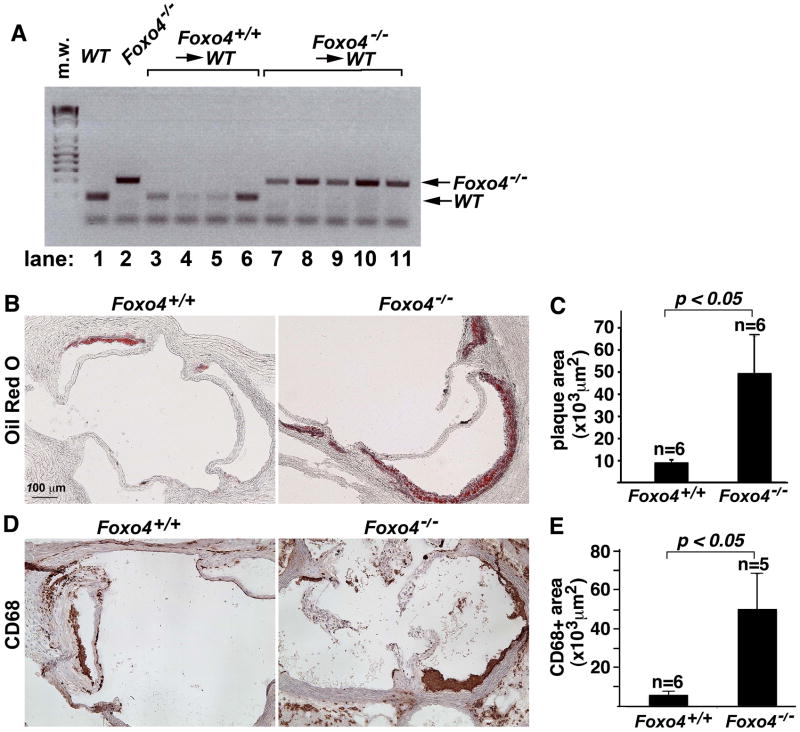
Macrophages in atherosclerotic lesions are derived from circulating blood monocytes that are mainly of bone marrow origin (Lessner et al., 2002; Aparicio-Vergara et al., 2010). Given that macrophages were upregulated in the atherosclerotic lesion of HFD-fed Foxo4/apoE DKO mice, we postulated that inactivation of Foxo4 in bone marrow-derived cells may promote atherosclerosis. To test this hypothesis, we employed a sex-mismatched bone marrow transplant approach and generated female chimeric C57B/6 mice with either Foxo4+/+ (wild type) or Foxo4−/− male bone morrows. In this experiment, we used WT C57B/6 mice rather than apoE−/− mice as recipients to address the effect of Foxo4 by itself. Four weeks after bone marrow transplantation, chimeric mice were fed with a HFD for 4 months. PCR genotyping indicate that >95% of the bone marrow cells from Foxo4−/− chimeras were of donor origin (Figure 3A), indicating successful bone marrow transplantation. No visible atherosclerotic plaques in the aorta were detected in chimeric mice reconstituted with either Foxo4+/+ or Foxo4−/− bone marrows (data not shown). However, when we analyzed the atherosclerotic development in the aortic root, we found that chimeric C57B/6 mice transplanted with Foxo4−/− bone marrows have significantly higher atherosclerotic lesion burdens than mice received with Foxo4+/+ bone marrows (Figure 3, B & C).
Fig. 3. Foxo4-deficient BMs are sufficient to promote enhanced atherosclerosis.
Female WT C57B/6 mice were irradiated one day before the BMT. The BM suspension (2×106) from either control WT or Foxo4−/− mice was injected intravenously via the tail vein. After 4 weeks recovery, the chimeric mice were fed HFD for four months and sacrificed. (A) PCR genotyping of bone marrows of wild type (lane 1), Foxo4-null (lane 2), and chimeric mice received with Foxo4+/+ bone marrows (lanes, 3–6), and Foxo4−/− bone marrows (lanes, 7–11). The peripheral blood DNA from Foxo4−/−→WT mice has the presence of donor Foxo4−/− genotype with negligible host DNA remaining. (B) Representative photographs of plaques in the aortic root of chimeric mice received with Foxo4-null (left) and wild type bone marrows. (C) The atherosclerotic lesion in chimeric mice reconstituted with Foxo4−/− BMs is significantly larger than that of mice received with wild type BMs. Values are mean ± SEM, p <0.05. (D) Macrophage contents in the lesions of chimeric mice were quantified using anti-CD68 antibody. (E) Chimeric mice transplanted with Foxo4-null bone marrows have higher number of macrophages than those received with wild type bone marrows after fed with HFD for 4 months. Values are mean ± SEM, p < 0.05.
Morphometric analysis of the composition of the lesion showed that the macrophage is the predominant cell type in the lesion and is significantly upregulated in chimeric mice transplanted with Foxo4−/− bone marrows as compared to mice received with wild type bone marrows (Figure 3, D & E).
Inactivation of Foxo4 results in an elevated expression of IL-6 and ROS in macrophages upon stimulation by LPS
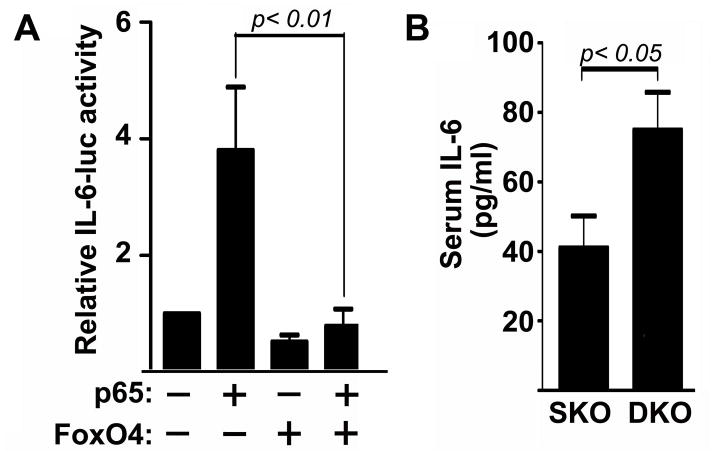
Previously, we found that FoxO4 inhibits NF-κB-activated transcription of inflammatory cytokines and Foxo4-deficient macrophages produce higher amount of IL-6 in response to LPS compared to wild-type macrophages (Zhou et al., 2009). We cloned the IL-6 promoter and constructed an IL-6-luciferase reporter. The promoter analysis indicates that FoxO4 can inhibit NF-κB-activated IL-6 transcription in macrophages (Figure 4A). We also measured serum levels of IL-6 in mice and observed significant upregulation of IL-6 in HFD-fed Foxo4/apoE DKO mice compared to that of apoE SKO mice (Figure 4B).
Figure 4. FoxO4 inhibits IL-6 expression.
(A) The IL-6-luciferase reporter was transfected into macrophages in the presence of the plasmids indicated. Luciferase activities were measured 24 hrs after transfection and normalized against co-transfected β-galactosidase. FoxO4 inhibited NF-κB activated IL-6-luc activity (n=4, mean ± SEM). (B) The serum levels of IL-6 in HFD-fed Foxo4/apoE DKO and apoE SKO mice were quantified with a standard IL-6 ELISA assay (n=15, mean ± SEM).
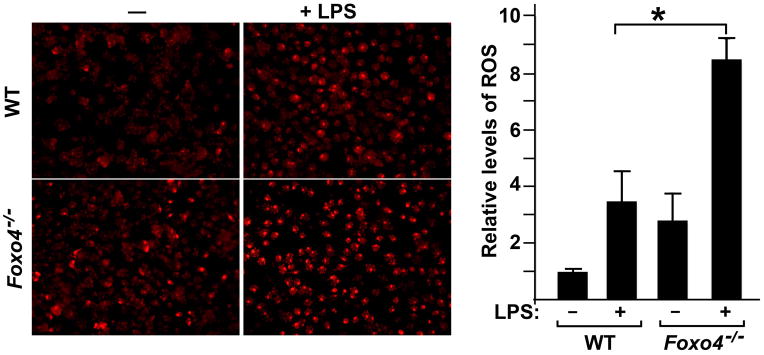
FoxO proteins are known to activate the transcription of several ROS scavenger enzymes. To test whether inactivation of Foxo4 in macrophages could upregulate levels of ROS, we stained the peritoneal macrophages with DHE in the presence or absence of LPS stimulation. DHE is a dye that specifically reacts with intracellular ROS, and is oxidized to ethidium, which then intercalates into double-stranded DNA and fluoresces under ultraviolet light (Zao et al., 2003). As expected, we observed a significant increase in the ROS level in Foxo4-null macrophages in response to LPS stimuli compared to that of WT counterparts (Figure 5).
Figure 5. Inactivation of Foxo4 in macrophages leads to enhanced production of ROS in response to LPS.
Equal numbers of peritoneal macrophages from wild type and Foxo4-null mice were plated onto 6-well plates in triplicates, stimulated with or without LPS. Cells were stained with DHE. Levels of ROS were measured as the product of the intensity and area of DHE-positive cells and expressed relative to the value of WT cells without LPS stimulation. n=4±SEM, *, p < 0.05.
Discussions
The major finding of this study is that Foxo4-deficiency in vivo enhances atherosclerotic lesion burden in a mouse model of atherosclerosis (Figure 1). Our studies also suggest that the cellular mechanism by which FoxO4 inhibits atherosclerosis is likely through its function in monocyte from bone marrows, as we have shown that Foxo4-inactivation in bone marrow derived cells is sufficient to promote the development of atherosclerosis and to upregulate the macrophage content in the lesion (Figure 3). How does FoxO4 inhibit atherosclerosis through its function in monocytes/macrophages?
Macrophages in the atherosclerotic lesion are mainly derived from circulating blood monocytes that have undergone differentiation upon activation by modified lipids and/or lipoproteins. The upregulation of macrophages in lesions of HFD-fed Foxo4-null mice could result from the increased recruitment of monocytes. FoxO proteins are known to activate the transcription of several ROS scavenge enzymes, including manganese superoxide dismutase, catalase, and peroxiredoxin 3 (Chiribau et al., 2008, Keizer al., 2010). ROS are essential for normal metabolism, but they are potentially destructive if not tightly controlled (Harrison et al., 2003). Excessive ROS have been shown to be involved in several atherosclerotic processes, including LDL oxidation, alteration of endothelial cell function, macrophage trapping in the lesion, and the stimulation of VSMC proliferation and migration (Souza et al., 2003; Park et al., 2009a, Tabas, 2010). Inactivation of Foxo4 could result in excess of ROS that promotes EC dysfunction, which in turn enhances monocyte recruitment. To test this hypothesis, we measured the ROS level of WT and Foxo4-null macrophages treated with and without LPS, and tested the adhesion of activated/non-activated WT and Foxo4-null macrophages to HUVECs. Although Foxo4-null macrophages produced higher level of ROS upon stimulation by LPS when compared to wild type macrophages (Figure 5), activated Foxo4-null macrophage adhesion to HUVECs is similar to that of WT macrophages (data not shown), suggesting that increased production of ROS from Foxo4-null macrophage alone may not be sufficient to promote its recruitment by ECs.
FoxO4 could also inhibit atherosclerosis through its action on immune cell homeostasis. Previously, we found that FoxO4 plays an important role in mucosal innate immunity through regulating expression of NF-κB-activated inflammatory cytokines, including CCL5, CXCL9, TNFα, IFNγ, IL-1β, and IL-6 (Zhou et al., 2009). As NF-κB plays a central role in the process of vascular inflammation (Brasier, 2010; Gareus et al., 2008; Van der Heiden et al., 2010), it is possible that FoxO4-regulated immune response may play a role in atherosclerosis. Indeed, atherosclerotic lesions in HFD-fed Foxo4-deficient mice, either Foxo4/apoE DKO mice or chimeric mice with Foxo4-null bone marrows, have increased amount of immune cells, indicating an escalation of local inflammation. Furthermore, circulating IL-6 levels in HFD-fed Foxo4/apoE DKO mice were elevated compared to those of apoE SKO mice, suggesting an elevation of systemic inflammation. Chemokines and inflammatory cytokines secreted by Foxo4-null immune cells could promote dedifferentiation and proliferation of residence smooth muscle cells and dysfunction of endothelial cells, which could in turn enhance further recruitment of immune cells and perpetuate the inflammation.
The atherogenic diet we used in this study contains cholic acid. The presence of cholic acid has been shown to aid cholesterol and fat absorption, and to suppress conversion of cholesterol to bile acids (Ando et al., 2005), which reduces removal of cholesterol and promotes early formation of atherosclerosis in susceptible mouse strains such as C57BL/6 (Nishina et al., 1990). Cholic acid has also been shown to influence transcription factors in transcriptional regulation of genes involved in lipid metabolism and inflammation (Ander et al., 2005). Whether cholic acid is involved in FoxO4-regulated gene transcription remains to be determined.
In this study, we found that chimeric C57B/6 mice reconstituted with Foxo4-null bone marrows have enhanced atherosclerosis in the aortic root than mice received with wild-type bone marrows. This finding is significant as it shows that inaction of Foxo4 alone in bone marrow derived cells is sufficient to promote regional atherosclerosis even in the absence of a defect in lipid metabolism such as inactivation of apoE, demonstrating the critical importance of the immune component of atherogenesis. Nonetheless, a reciprocal experiment with the Foxo4-null mouse as the recipient and WT bone marrow as the donor should further clarify the role of Foxo4-null bone marrow in atherogenesis.
It is also interesting to note that the lesions were found only in the aortic root in C57B/6 mice after feeding with HFD. No lesions were observed in the descending aorta. This is expected, as it was known that flow characteristics is a risk factor for development of atherosclerosis and the aortic root area is prone to atherosclerosis due to its disturbed flow (Traub & Berk, 1998; Haidari et al., 2010). Consistent with the observation that only localized atherosclerotic plaques are upregulated in the chimeric mice with Foxo4-null bone marrows compared with chimeric mice with WT bone marrows, markers of systemic inflammation such as serum IL-6 were not significantly upregulated in these mice either (data not shown), suggesting that inactivation of Foxo4 in bone marrows may not be sufficient to cause systemic inflammation. These results also suggest the importance of non-immune cell components such as ECs and SMCs in the vascular wall in the initiation of atherogenesis. At present, it is not clear which cell type in the vascular wall is the main target for FoxO4-induced vascular inflammation. Further studies are needed to identify the vascular cell types that mediate the effect of FoxO4 on atherosclerosis.
Previously, we showed that FoxO4 promotes dedifferentiation and proliferation of arterial SMCs upon mechanical injury, as inactivation of Foxo4 led to a decreased neointimal formation upon carotid artery ligation (Li et al., 2007). The amount of SMCs in HFD-fed Foxo4/apoE DKO was not significantly upregulated compared to that of apoE SKO mice (data not shown), suggesting that the effect of FoxO4 on SMC proliferation may be context-dependent. This is not surprising, since the signals that act upon vascular SMCs in atherosclerosis are ROS and cytokines whereas those in the carotid artery ligation model are characteristics of flow-induced mechanical forces.
In the current study, we also observed increased amount of T-cells in atherosclerotic lesions of Foxo4/apoE DKO mice compared to apoE SKO mice. This is significant as T-cell mediated adaptive immunity is known to drive the progression of atherosclerosis (Hansson & Jonasson, 2009; Anderson et al., 2010). It will be worthy to determine the mechanism(s) by which Foxo4-deficiency leads to the upregulation of T-cells in the atherosclerotic lesion of Foxo4/apoE DKO mice. IL-6 is a T-cell survival factor and promotes expansion of effect/memory T-cells (Dienz & Rincon, 2009). The increased amount of CD4+ T cells in the lesions of HFD-fed DKO mice could be the consequence of increased production of IL-6 in the lesion. Alternatively, FoxO4 could have T-cell autonomous functions similar to that of FoxO1 (Fabre et al., 2008; Dengler et al., 2008). A mouse model with Foxo4-inactivated in the T-cell compartment could facilitate our understanding of the role of FoxO4 in T-cell mediated atherosclerosis in the future.
In conclusion, our studies indicate that FoxO4 is an anti-atherogenic factor and inhibits atherosclerosis through its function in bone marrow derived monocyte/macrophages. FoxO proteins are major downstream effectors of insulin-activated PI3K/Akt signaling pathway. Inactivation of the PI3K/Akt signaling pathway activates FoxO proteins and promotes longevity in worms (Kenyon, 2010) and flies (Puig & Tijin 2006; Lee et al., 2009). Our data suggest that activation of FoxO proteins through the insulin/Akt pathway could also be beneficial in inhibition of atherosclerosis and maintaining a long and healthy life-span in humans.
Supplementary Material
Acknowledgments
This study was supported by a Scientist-development-grant from the American Heart Association and RO1 HL085749 from the National Heart, Lung, and Blood Institute to Z.P. Liu. Min Zhu is supported by a Postdoctoral Fellowship Award from the American Heart Association. Foxo4−/− mice in FVB/N background were provided by Dr. Ronald A. DePinho (Harvard Medical School).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Ando H, Tsuruoka S, Yamamoto H, Takamura T, Kaneko S, Fujimura A. Regulation of cholesterol 7alpha-hydroxylase mRNA expression in C57BL/6 mice fed an atherogenic diet. Atherosclerosis. 2005;178:265–269. doi: 10.1016/j.atherosclerosis.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Aparicio-Vergara M, Shiri-Sverdlov R, de Haan G, Hofker MH. Bone marrow transplantation in mice as a tool for studying the role of hematopoietic cells in metabolic and cardiovascular diseases. Atherosclerosis. 2010 doi: 10.1016/j.atherosclerosis.2010.05.030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Baglione J, Smith JD. Quantitative assay for mouse atherosclerosis in the aortic root. Methods Mol Med. 2006;129:83–95. doi: 10.1385/1-59745-213-0:83. [DOI] [PubMed] [Google Scholar]
- 5.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–8. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calkin AC, Tontonoz P. Genome-wide association studies identify new targets in cardiovascular disease. Sci Transl Med. 2010;2:48ps46. doi: 10.1126/scitranslmed.3001557. [DOI] [PubMed] [Google Scholar]
- 7.Chiribau CB, Cheng L, Cucoranu IC, Yu YS, Clempus RE, Sorescu D. FOXO3A regulates peroxiredoxin III expression in human cardiac fibroblasts. J Biol Chem. 2008;283:8211–7. doi: 10.1074/jbc.M710610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson MH. Novel nonstatin strategies to lower low-density lipoprotein cholesterol. Curr Atheroscler Rep. 2009;11:67–70. doi: 10.1007/s11883-009-0011-0. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Rateri DL. Development of experimental designs for atherosclerosis studies in mice. Methods. 2005;36:129–38. doi: 10.1016/j.ymeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388–98. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dejean AS, Hedrick SM, Kerdiles YM. Highly specialized role of Foxo transcription factors in the immune system. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3414. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–9. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 14.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–83. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29:1714–7. doi: 10.1161/ATVBAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- 16.Haidari M, Ali M, Gangehei L, Chen M, Zhang W, Cybulsky MI. Increased oxidative stress in atherosclerosis-predisposed regions of the mouse aorta. Life Sci. 2010;87:100–10. doi: 10.1016/j.lfs.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 18.Keizer PL, Burgering BM, Dansen TB. Forkhead Box O as a Sensor, Mediator, and Regulator of Redox Signaling. Antioxid Redox Signal. 2010 Sep 20; doi: 10.1089/ars.2010.3403. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 20.Klingenberg R, Hansson GK. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. Eur Heart J. 2009;30:2838–44. doi: 10.1093/eurheartj/ehp477. [DOI] [PubMed] [Google Scholar]
- 21.Lee KS, Iijima-Ando K, Iijima K, Lee WJ, Lee JH, Yu K, Lee DS. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J Biol Chem. 2009;284:29454–61. doi: 10.1074/jbc.M109.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 23.Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160:2145–55. doi: 10.1016/S0002-9440(10)61163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–20. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, Liu Z-P. Foxo4 regulates TNFα-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Molecular and Cellular Biology. 2007;27:2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu ZP, Wang Z, Yanagisawa H, Olson EN. Phenotypic Modulation of Smooth Muscle Cells through Interaction of Foxo4 and Myocardin. Developmental Cell. 2005;9:161–270. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Mori N, Shirakawa F, Shimizu H, Murakami S, Oda S, Yamamoto K, Eto S. Transcriptional regulation of the human interleukin-6 gene promoter in human T-cell leukemia virus type I-infected T-cell lines: evidence for the involvement of NF-kappa B. Blood. 1994;84:2904–11. [PubMed] [Google Scholar]
- 28.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–40. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 29.Nishina PM, Verstuyft J, Paigen B. Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J Lipid Res. 1990;31:859–869. [PubMed] [Google Scholar]
- 30.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009a;119:136–45. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puig O, Tjian R. Nutrient availability and growth: regulation of insulin signaling by dFOXO/FOXO1. Cell Cycle. 2006;5:503–5. doi: 10.4161/cc.5.5.2501. [DOI] [PubMed] [Google Scholar]
- 33.Raines EW, Ferri N. Thematic review series: The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J Lipid Res. 2005;46:1081–92. doi: 10.1194/jlr.R500004-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Ronnebaum SM, Patterson C. The FoxO family in cardiac function and dysfunction. Annu Rev Physiol. 2010;72:81–94. doi: 10.1146/annurev-physiol-021909-135931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 36.Sadowitz B, Maier KG, Gahtan V. Basic science review: Statin therapy--Part I: The pleiotropic effects of statins in cardiovascular disease. Vasc Endovascular Surg. 2010;44:241–51. doi: 10.1177/1538574410362922. [DOI] [PubMed] [Google Scholar]
- 37.Senokuchi T, Liang CP, Seimon TA, Han S, Matsumoto M, Banks AS, Paik JH, DePinho RA, Accili D, Tabas I, Tall AR. Forkhead transcription factors (FoxOs) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. Diabetes. 2008;57:2967–76. doi: 10.2337/db08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin HS, Lee HJ, Nishida M, Lee MS, Tamura R, Yamashita S, Matsuzawa Y, Lee IK, Koh GY. Betacellulin and amphiregulin induce upregulation of cyclin D1 and DNA synthesis activity through differential signaling pathways in vascular smooth muscle cells. Circ Res. 2003;93:302–10. doi: 10.1161/01.RES.0000086803.64109.9E. [DOI] [PubMed] [Google Scholar]
- 39.Souza HP, Cardounel AJ, Zweier JL. Mechanisms of free radical production in the vascular wall. Coron Artery Dis. 2003;14:101–7. doi: 10.1097/00019501-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–50. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 42.Van der Heiden K, Cuhlmann S, Luong le A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 43.van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal. 2010 Aug 1; doi: 10.1089/ars.2010.3419. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–68. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W, Cao Q, Peng Y, Zhang Q-J, Castrillon D, DePinho R, Liu ZP. FoxO4 Inhibits NF-κB and Protects Mice Against Colonic Injury and Inflammation. Gastroenterology. 2009;137:1403–1. doi: 10.1053/j.gastro.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.