Abstract
Background and Purpose
Brain iron overload plays a detrimental role in brain injury after intracerebral hemorrhage (ICH). A recent study found that minocycline acts as an iron chelator and reduces iron-induced neuronal death in vitro. The present study investigated if minocycline reduces iron overload after ICH and iron-induced brain injury in vivo.
Methods
This study was divided into four parts. (1) Rats with different sizes of ICH were euthanized 3 days later for serum total iron and brain edema determination. (2) Rats had an ICH treated with minocycline or vehicle. Serum iron, brain iron, and brain iron handling proteins were measured. (3) Rats had an intracaudate injection of either saline, iron, iron+minocycline or iron+macrophage/microglia inhibitory factor and were used for brain edema and neuronal death measurements. (4) Rats had an intracaudate injection of iron and were treated with minocycline. The brains were used for edema measurement.
Results
After ICH, serum total iron and brain non-heme iron increased and these changes were reduced by minocycline treatment. Minocycline also reduced ICH-induced upregulation of brain iron handling proteins and neuronal death. Intracaudate injection of iron caused brain edema, blood-brain barrier leakage and brain cell death, all of which were significantly reduced by co-injection with minocycline.
Conclusions
The current study found that minocycline reduces iron overload after ICH and iron-induced brain injury. It is also well known minocycline is an inhibitor of microglial activation. Minocycline may be very useful for ICH patients because both iron accumulation and microglia activation contribute to brain damage following ICH.
Keywords: brain edema, cerebral hemorrhage, iron, minocycline
Intracerebral hemorrhage (ICH) is a subtype of stroke with high morbidity and mortality1. Evidence suggests that iron is involved in ICH-induced brain injury2. After ICH, iron concentrations in surrounding brain can reach very high levels. Thus, our previous studies showed an increase in brain non-heme iron after ICH in rats, and this remains high for at least one month 3. Brain iron overload after ICH causes brain edema in the acute phase and brain atrophy later. We have now demonstrated that an iron chelator, deferoxamine, reduces ICH-induced brain edema, neuronal death, brain atrophy and neurological deficits in young rats 4–6, aged rats 7 and pigs 8. Clinical data also suggest a role of iron in ICH-induced brain injury. Recent studies found that high levels of serum ferritin, an iron storage protein, are independently associated with poor outcome and severe brain edema in ICH patients 9, 10.
Minocycline is a semi-synthetic second-generation derivative of tetracycline. It is a highly lipophilic compound and penetrates the brain-blood barrier (BBB) easily. It has a clear neurovascular protective effect in animal models of ICH and cerebral ischemia11–14 and it is in current clinical trial for ischemic stroke patients. Minocycline has been reported to provide neuroprotection by reducing the inflammatory response to injury, including inhibiting microglia, matrix metalloproteinase and poly(ADP-ribose) polymerase-1 (PARP-1) activation 15,16. For example, it inhibits macrophage/microglia activation after ICH in rats 17. Evidence indicates that there is an inflammatory component to ICH-induced brain injury 18. However, a recent study has shown that minocycline also attenuates iron neurotoxicity in cortical neuronal cultures by chelating iron 19.
Therefore, the present study investigated whether minocycline can attenuate iron overload and brain injury after ICH and whether minocycline reduces iron-induced brain injury in vivo.
Materials and Methods
Animal Preparation and Intracerebral Injection
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of 160 male Sprague-Dawley rats (weighed 275–300g, Charles River Laboratories) were used in this study. Septic precautions were utilized in all surgical procedures and body temperature was maintained at 37.5 °C. Rats were anesthetized with pentobarbital (45 mg/kg, i.p.) and the right femoral artery was catheterized for continuous blood pressure monitoring and blood sampling. Blood from the catheter was used to determine pH, PaO2, PaCO2, hematocrit and glucose. It was also the source for the intracerebral blood injection. The animals were positioned in a stereotactic frame (Kopf Instruments). Rats received an injection into the right basal ganglia and the coordinates were 0.2 mm anterior to bregma, 5.5 mm ventral, and 4.0 mm lateral to midline.
Experiment Groups
This study was divided into 4 parts. In the first part, rats (n=6 for each group) had an intracaudate injection of 10μL or 100μL autologous whole blood. Rats were euthanized at day 3 for serum total iron and brain water content determination. Normal or sham operation rats (n=4) were used as controls. In the second part, rats had an intracerebral injection of 100μl autologous whole blood, and the rats were treated with minocycline (45 mg/kg, i.p. at 2 and 12 hours after ICH, followed by 22.5mg/kg twice a day up to 7 days) or vehicle. This dose of minocycline can reduce ICH-induced brain edema14. Rats were euthanized 1, 3 and 7 days later for serum total iron determination, immunohistochemistry and Western blot assay (n=9 for each group). In addition, rats (n=6 for each group) were euthanized 3 days later for brain non-heme iron determination. In the third part, rats (n=15 each group) had intracaudate injection of 50μl of saline, FeCl2 (0.5mmol/L), FeCl2 (0.5mmol/L) + minocycline (MC; 0.5mmol/L; Sigma) or FeCl2 (0.5mmol/L) + macrophage/microglia inhibitory factor (MIF; 0.5mmol/L; American Peptide Co, Inc.). Rats were euthanized at 24 hours and the brains were used for brain edema, BBB disruption and brain cell death measurements. In the fourth part, rats had intracaudate injection of 50μl of FeCl2 (0.5mmol/L), and the rats were treated with minocycline (45 mg/kg, i.p. immediately and 12 hours after iron injection) or vehicle (n=5 each group). Rats were euthanized at 24 hours for brain edema measurement.
Serum Total Iron Determination
Venous blood samples were drawn for total serum iron measurement before euthanasia. The blood samples were centrifuged after clotting, the serum separated and total iron levels measured by a QuantiChrom Iron Assay Kit (Bioasssay Systems).
Immunohistochemistry
Immunohistochemistry was performed as previously described.3 Primary antibodies were polyclonal rabbit anti-human ferritin IgG (DACO;1:500 dilution), monoclonal mouse anti-rat NeuN IgG (Millipore; 1:500). Normal rabbit IgG or mouse IgG was used as negative controls.
Western Blot Analysis
Western blot analysis was performed as described earlier.3 The primary antibodies were polyclonal goat anti-rat ferritin-L-chain (1:1000 dilution; Abnova), polyclonal rabbit anti-rat ferritin-H-chain(1:2000 dilution; Cell Signaling), polyclonal rabbit anti-human transferrin (1:2000 dilution; Dako), monoclonal mouse anti-human transferrin receptor (1:2000 dilution; Invitrogen), polyclonal sheep anti-rat ceruloplasmin(1:2000 dilution; Abcam) or polyclonal goat anti-mouse albumin antibody (1:20000 dilution; BETHYL Laboratories Inc.). The secondary antibodies were goat anti-rabbit IgG, goat anti-mouse IgG, rabbit anti-goat IgG (1:4000 dilution; Bio-Rad) and rabbit ant-sheep IgG (1:4000 dilution; Millipore).
Non-heme Brain Tissue Iron Determination
Rats were euthanized 3 days after ICH and the brains were perfused with PBS. A coronal slice (4 mm thick) around the injection needle tract was cut, divided into ipsilateral and contralateral sides, and weighed. Non-heme brain tissue iron was determined according to the method described previously.3
Brain Water and Ion Contents
Animals were reanesthetized, the brain was removed, and a coronal tissue slice (4mm thick) around the injection needle tract was cut. Five tissue samples from each brain were obtained: the ipsilateral and contralateral cortex, the ipsilateral and contralateral basal ganglia, and the cerebellum. Brain samples were then dried at 100°C for 24 hours to obtain the dry weight and water content calculated as: (wet weight−dry weight)/wet weight. The dehydrated samples were digested in 1 mL of 1 mol/L nitric acid for 1 week. Sodium and potassium contents of this solution were measured by flame photometry. Sodium and potassium ion contents were expressed in milliequivalents per kilogram of dehydrated brain tissue (mEq/kg dry weight).
Fluoro-Jade C Staining
Brain sections were kept in 0.06% potassium permanganate (KMnO4) for 15 minutes and rinsed in distilled water, sections were stained by gently shaking for 30 minutes in working solution of Fluoro-Jade C composed of 10 ml 0.01% Fluoro-jade C in distilled water and 90 ml 0.1% acetic acid, then rinsed in distilled water for three times. After dried with a blower, slides quickly dipped into xylol and covered after mounted by DPX (Electron Microscopy Sciences, Inc.). 8
DNA Damage Measurements
The DNA polymerase I-mediated biotin-dATP nick-translation (PANT) assay and the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) technique were performed on brain sections to detect DNA single- and double-strand breaks according to the method we used in our previous studies.20
Cell Counting
Cell counting was performed on brain coronal sections. Three high-power images (x40 magnification) were taken around the hematoma or iron injection site using a digital camera. Fluoro-Jade C, NeuN, PANT and TUNEL positive cells were counted on these 3 areas from each rat brain section.
Statistical Analysis
All the data in this study are presented as mean ± SD. Data were analyzed by Student t test and one-way analysis of variance (ANOVA). A level of P<0.05 was considered statistically significant.
Results
Physiological variables
All physiological variables were measured immediately before the injection. Mean arterial blood pressure, blood pH, PaO2, PaCO2, and blood glucose level were within normal ranges (mean arterial blood pressure, 80–120 mmHg; PO2, 80–120 mmHg; PCO2, 35–45 mmHg; hematocrit, 38–43 %; blood glucose, 80–120 mg/dl).
Minocycline reduces increased total iron levels in serum after ICH
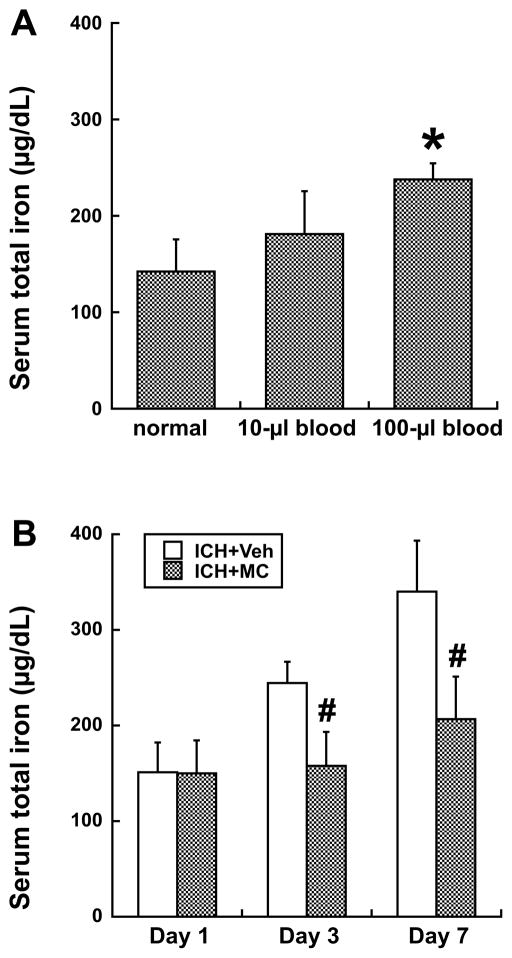
In the normal rats, serum iron concentration was 143±32 μg/dL. To test the effects of hematoma size on serum iron levels, rats had an intracaudate injection of 10- or 100-μL autologous blood. Three says after ICH, serum total iron increased. The bigger clot resulted in higher serum iron levels (238 ± 17 vs. 182 ± 44 μg/dL in the 10-μL blood group, p<0.05, Fig 1A). They also caused more severe perihematomal brain edema (79.7 ± 0.6 vs. 78.4 ± 0.3% in the 10-μL blood group, p<0.01) at day 3. Control water content was about 78%.
Figure 1.
A) Serum total iron concentration at day 3 in normal rats or those that had a 10μL or 100μL blood injection into right basal ganglia. Values are mean±SD; n=4~6, *p<0.05, compared with the other groups. B) Serum total iron concentration at days 1, 3 and 7 in rats had ICH (100μL blood) with or without minocycline (MC) treatment. Values are mean±SD; n=6, #p<0.01, compared with ICH+vehicle group.
A time course showed that total serum iron levels after 100-μL ICH were low at day 1, increased significantly at day 3, and stayed at high levels at day 7 (Fig 1B). Sham operation did not increase serum iron levels significantly at days 1 and 7(e.g., day 7: 169.4±5.9 μg/dL). Minocycline reduced serum total iron levels at both day 3 (158±36 vs. 245±22 μg/dL in the vehicle-treated group, p<0.01) and day 7 (206±45 vs. 341±53 μg/dL in the vehicle-treated group, p<0.01).
Minocycline reduces brain iron overload and neuronal death after ICH
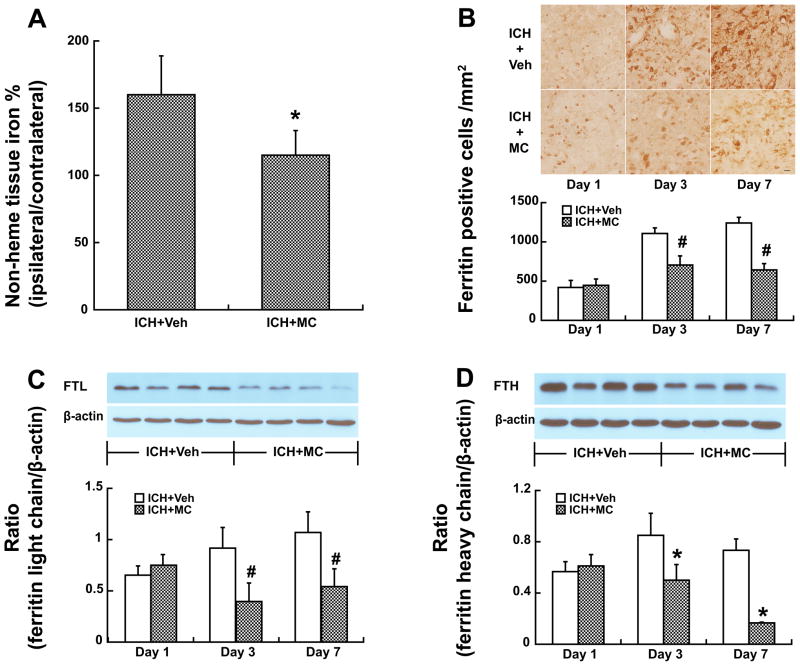
Lysis of erythrocytes resulted in a buildup in non-heme iron in brain tissue. Minocycline reduced brain non-heme iron accumulation 3 days after ICH (ipsilateral/contralateral: 115.0 ± 18.2 vs. 160.2 ± 28.7% in the vehicle-treated group, p<0.05, Fig 2A).
Figure 2.
A) Brain non-heme iron levels, as ratio of ipsilateral to contralateral hemisphere, three days after ICH. Values are mean±SD; n=6, *p<0.05, compared with ICH+vehicle group. B) Ferritin immunoreactivity in the ipsilateral basal ganglia at days 1, 3 and 7 after ICH. Values are mean ± SD; n=5, #P<0.01, compared with ICH+vehicle group, Scale bar=20μm. C, D) Ferritin-L-chain (C) and ferritin-H-chain (D) protein levels in the ipsilateral basal ganglia at days 1, 3 and 7 after ICH. Values are mean ± SD; n=4, #p<0.01, *p<0.05, compared with ICH+vehicle group.
Ferritin, an iron storage protein, was upregulated after ICH. Ferritin positive cells were less in minocycline-treated animals (Fig 2B, e.g. day 7: 643 ± 80 vs. 1238 ± 75 cells/mm2 in the vehicle-treated group, p<0.01). Western blot analysis showed that both ferritin-L-chain (FTL) and ferritin-H-chain (FTH) protein levels were lower in minocycline-treated group at both day 3 and day 7 (Fig 2C & 2D).
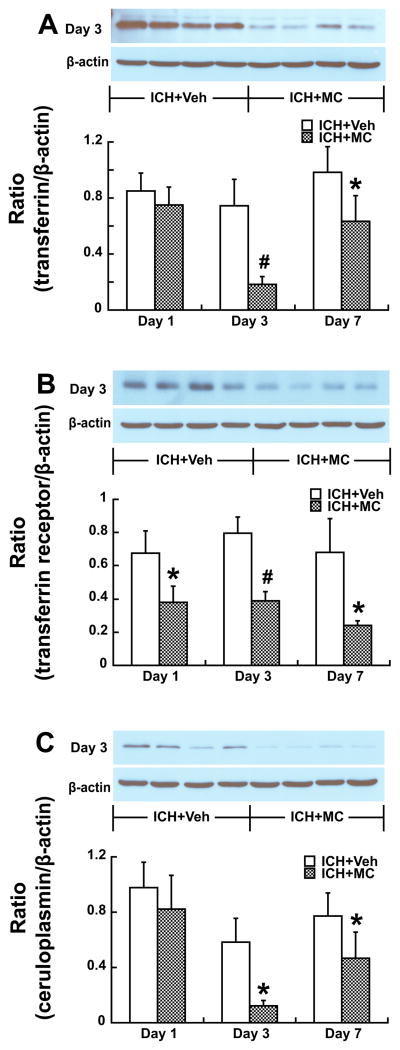
Transferrin (Tf), transferrin receptor (TfR) and ceruloplasmin (CP) are involved in iron metabolism and our previous studies have showed an increase of those iron-handling proteins in the brain after ICH. Minocycline reduced Tf, TfR and CP levels significantly (Fig 3).
Figure 3.
A) Transferrin (Tf), B) transferrin receptor (TfR) and C) ceruloplasmin (CP) protein levels in the ipsilateral basal ganglia at days 1, 3 and 7 after ICH treated with or without minocycline (MC). Values are mean ± SD; n=4, #p<0.01, *p<0.05, compared with ICH+vehicle group).
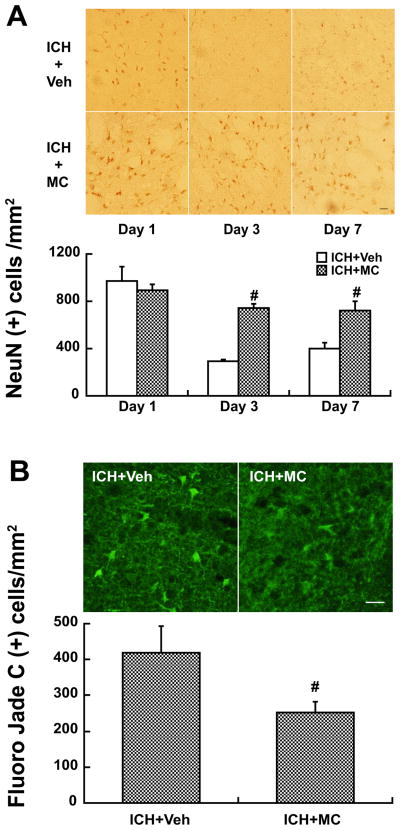
NeuN staining and Fluoro-Jade C staining were used to assess live and dead neurons, respectively. The number of NeuN-positive neurons in the ipsilateral basal ganglia was significantly higher in the minocycline-treated group (e.g. day 3: 743 ± 33 vs. 295 ± 16 cells/mm2 in the vehicle-treated group, p<0.01, Fig 4A). Fluoro-Jade C positive cells were less in the minocycline-treated group at day 1 (254 ± 29 vs. 419 ± 75 cells/mm2 in vehicle group, p<0.01, Fig 4B). Our previous study showed that minocycline also reduces perihematomal brain edema14.
Figure 4.
Cells positive for NeuN (A) at days 1, 3 and 7 and Fluoro-Jade C (B) at day 1 in the ipsilateral basal ganglia after ICH in rats treated with vehicle or minocycline (MC). Values are mean ± SD; n=5, #p<0.01, compared with ICH+vehicle group. Scale bar = 20μm.
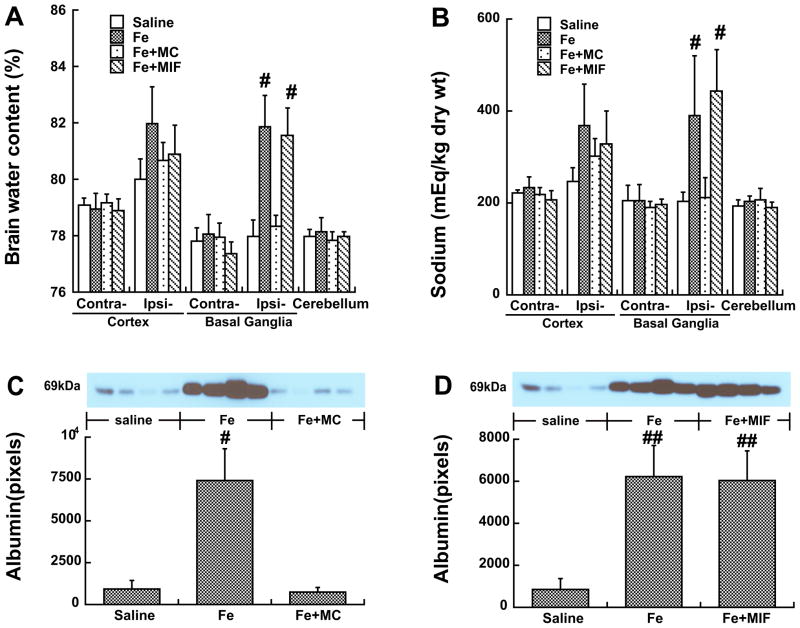
Co-injection of minocycline, but not MIF, attenuates iron-induced brain edema, BBB disruption and brain cell death
Intracerebral injection of iron caused brain edema. Co-injection of iron with minocycline reduced iron-induced brain edema in the ipsilateral basal ganglia at day 1 (78.3±0.4 vs. 81.9±1.1% in the iron group, p<0.01, Fig 5A). This was associated with a decrease of brain sodium content (212±44 vs. 391±129 mEq/kg dry wt in the iron group, p<0.01, Fig 5B) and a less loss of potassium content (451±49 vs. 353±66 mEq/kg dry wt in the iron group) in the ipsilateral basal ganglia. The co-injection of iron with MIF, however, did not reduce iron-induced brain edema (Fig 5A).
Figure 5.
Brain water (A) and sodium (B) contents 24 hours after the injection of saline, FeCl2, FeCl2+MC or FeCl2+MIF into the right basal ganglia. Values are mean ± SD; n=6, #p<0.01 vs. saline or Fe+MC group. Protein levels of albumin (C, D) in the ipsilateral basal ganglia 24 hours after the injection. Values are means ± SD; n=4, #p<0.01vs. other groups, ##p<0.01 vs. saline.
Brain albumin, a marker of BBB disruption, was measured by Western blot analysis. Albumin in the ipsilateral basal ganglia was markedly increased one day after iron injection. Minocycline, but not MIF coinjection, reduced iron-induced BBB leakage (p<0.01, Fig 5C & 5D).
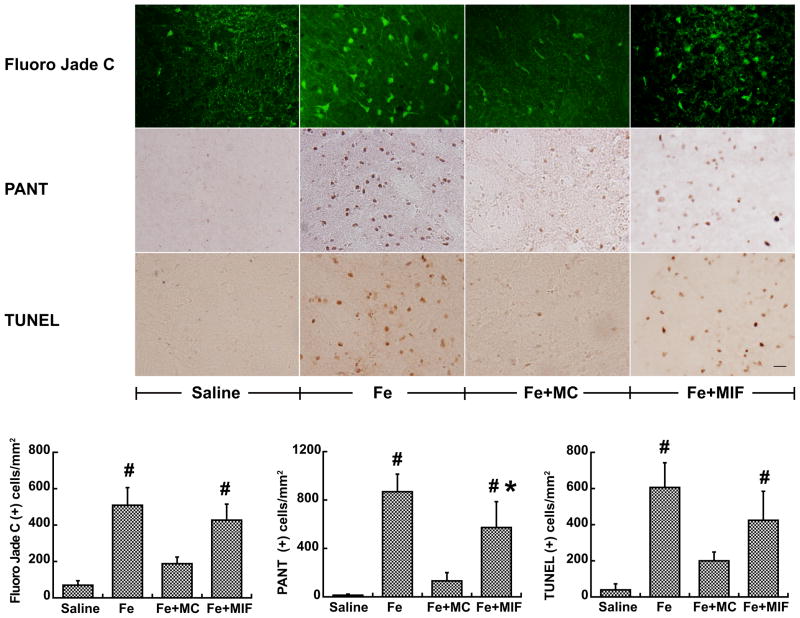
Intracerebral injection of iron also caused neuronal death and DNA damage. Fluoro-Jade C, PANT and TUNEL positive cells in the ipsilateral basal ganglia were markedly decreased in the iron and minocycline co-injection group at day 1 (e.g. Fluoro-Jade C: 189±34 vs. 508±98 cells/mm2 in the iron alone group, p<0.01, Fig 6). MIF also reduced iron-induced single chain DNA damage (576±216 vs. 867±146 cells/mm2 in FeCl2 group, p<0.05, Fig 6). However, MIF did not reduce the number of Fluoro-Jade C and TUNEL positive cells (Fig 6).
Figure 6.
The number of Fluoro-Jade C (A), PANT (B) and TUNEL (C) positive cells in the ipsilateral basal ganglia at 24h after the injection of saline, FeCl2, FeCl2+MC and FeCl2+MIF. Values are means ± SD; n=5, #p<0.01, compared with saline or Fe+MC group; *p<0.05, vs. Fe group. Scale bar = 20μm.
However, systemic minocycline treatment starting at the time of iron injection did not reduce iron-induced brain edema (82.8±0.3 vs. 82.7±0.7% in the vehicle-treated group, p>0.05).
Discussion
The major findings of current study are: 1) serum total iron levels were increased after ICH and this was reduced by systemic use of minocycline; 2) minocycline reduced brain iron overload after ICH; 3) minocycline treatment reduces ICH-induced neuronal death; and 4) minocycline attenuates iron-induced brain edema formation and BBB disruption, an effect not found with a microglia inhibitor, MIF.
It is well known that brain iron overload occurs after experimental ICH and causes perihematomal brain edema, neuronal death, brain atrophy and neurological deficits. Clinically blood levels of ferritin, an iron storage protein, are increased in ICH patients and associated with brain edema development and functional outcome. In this study, we found that serum total iron is increased after ICH and minocycline can reduce this increase. The causes of higher serum iron levels after ICH are unknown and could be related to: 1) iron released from the hematoma; 2) complement system activation, as occurs after ICH, which might cause systemic hemolysis; 3) iron redistribution from tissues following ICH. Future studies should determine whether serum iron levels are correlated with ICH-induced brain injury and whether serum iron is a new biomarker of ICH-injury brain injury.
Minocycline acts as an iron chelator and reduces ICH-induced brain iron overload. Both brain non-heme iron and brain iron handling protein levels are decreased following minocycline treatment. Evidence shows that minocycline is an iron chelator.21 For example, absorption of minocycline is significantly decreased by administration with iron supplements 22 and skin hyperpigmentation, an adverse effect of long-term minocycline therapy, may be related to insoluble minocycline–iron chelation products.23
Recent evidence has also shown that minocycline can attenuate iron neurotoxicity in cortical neuronal cultures19. Treatment of cultured cortical neurons with 10-μM ferrous sulfate for 24 h caused significant neuronal death and increases in malondialdehyde. Minocycline prevents this injury, with near-complete protection at the concentration of 30 μM2. To test whether minocycline can reduce iron-induced brain injury in vivo, rats received an intracerebral injection of iron with or without minocycline. In the proof of concept study, 50 μl of iron (0.5mmol/L) was injected because the concentration of iron in rat red blood cells is approximately 10 mM. Minocycline is an inhibitor of microglial activation, therefore, MIF24, 25 was used as a control. We found that minocycline, but not MIF, attenuates iron-induced brain edema and BBB disruption. We have previously found that MIF, with the dose and route of administration used here, is capable of inhibiting ICH-induced microglial activation25.
Iron is not the only cause of brain injury after ICH. There is considerable evidence for there being an inflammatory component 18 including that linked to microglia activation 14, 24, 26. Minocycline is a potent inhibitor of microglia activation and has been reported to provide neuroprotection by inhibiting microglia 27. It is a highly lipophilic compound that penetrates the brain-blood barrier easily 28. Minocycline has been found neuroprotective in both hemorrhagic and ischemic animal models 11–15, 25, 29. In the current study, although MIF did not reduce iron-induced brain edema, both minocycline and MIF reduced single strand DNA damage caused by iron suggesting a role of microglia in iron-induced neuronal death.
In summary, minocycline reduces iron overload after ICH and iron-induced brain injury. These effects, along with microglia and other actions, suggest that minocycline may be a new treatment for ICH patients.
Acknowledgments
Funding:
This study was supported by grants NS-039866, NS-052510 and NS-057539 from the National Institutes of Health (NIH) and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
Footnotes
Disclosures: None.
References
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Keep R, Hua Y, Schallert T, Hoff J, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 5.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: The role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 6.Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: Effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–2863. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- 7.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: Therapeutic time window and optimal duration. Stroke. 2010;41:375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez de la Ossa N, Sobrino T, Silva Y, Blanco M, Millan M, Gomis M, Agulla J, Araya P, Reverte S, Serena J, Davalos A. Iron-related brain damage in patients with intracerebral hemorrhage. Stroke. 2010;41:810–813. doi: 10.1161/STROKEAHA.109.570168. [DOI] [PubMed] [Google Scholar]
- 10.Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke. 2008;39:1165–1170. doi: 10.1161/STROKEAHA.107.501213. [DOI] [PubMed] [Google Scholar]
- 11.Machado LS, Sazonova IY, Kozak A, Wiley DC, El-Remessy AB, Ergul A, Hess DC, Waller JL, Fagan SC. Minocycline and tissue-type plasminogen activator for stroke: Assessment of interaction potential. Stroke. 2009;40:3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: Improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol. 2007;207:227–237. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Yang S, Xi G, Fu G, Keep RF, Hua Y. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res. 2009;31:183–188. doi: 10.1179/174313209X385680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(adp-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Annals of Neurology. 2003;53:731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 19.Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun. 2009;386:322–326. doi: 10.1016/j.bbrc.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Research. 2002;953:45–52. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- 21.Grenier D, Huot MP, Mayrand D. Iron-chelating activity of tetracyclines and its impact on the susceptibility of actinobacillus actinomycetemcomitans to these antibiotics. Antimicrob Agents Chemother. 2000;44:763–766. doi: 10.1128/aac.44.3.763-766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leyden JJ. Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk, and iron. J Am Acad Dermatol. 1985;12:308–312. doi: 10.1016/s0190-9622(85)80041-4. [DOI] [PubMed] [Google Scholar]
- 23.Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: An update. Acta Dermatovenerol Croat. 2009;17:123–126. [PubMed] [Google Scholar]
- 24.Wang J, Tsirka SE. Tuftsin fragment 1–3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 2005;36:613–618. doi: 10.1161/01.STR.0000155729.12931.8f. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Yang S, Xi G, Song S, Fu G, Keep RF, Hua Y. Microglial activation and brain injury after intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:59–65. doi: 10.1007/978-3-211-09469-3_13. [DOI] [PubMed] [Google Scholar]
- 26.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: Effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 27.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. Journal of Neuroscience. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein NC, Cunha BA. Tetracyclines. Medical Clinics of North America. 1995;79:789–801. doi: 10.1016/s0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]