Abstract
Epidemiology supports a causal link between air pollutant exposure and childhood asthma, but the mechanisms are unknown. We have previously reported that ozone exposure can alter the anatomic distribution of CD25+ lymphocytes in airways of allergen-sensitized infant rhesus monkeys. Here, we hypothesized that ozone may also affect eosinophil trafficking to allergen-sensitized infant airways. To test this hypothesis, we measured blood, lavage, and airway mucosa eosinophils in 3-month old monkeys following cyclical ozone and house dust mite (HDM) aerosol exposures. We also determined if eotaxin family members (CCL11, CCL24, CCL26) are associated with eosinophil location in response to exposures. In lavage, eosinophil numbers increased in animals exposed to ozone and/or HDM. Ozone + HDM animals showed significantly increased CCL24 and CCL26 protein in lavage, but the concentration of CCL11, CCL24, and CCL26 was independent of eosinophil number for all exposure groups. In airway mucosa, eosinophils increased with exposure to HDM alone; comparatively, ozone and ozone + HDM resulted in reduced eosinophils. CCL26 mRNA and immunofluorescence staining increased in airway mucosa of HDM alone animals and correlated with eosinophil volume. In ozone + HDM animal groups, CCL24 mRNA and immunofluorescence increased along with CCR3 mRNA, but did not correlate with airway mucosa eosinophils. Cumulatively, our data indicate that ozone exposure results in a profile of airway eosinophil migration that is distinct from HDM mediated pathways. CCL24 was found to be induced only by combined ozone and HDM exposure, however expression was not associated with the presence of eosinophils within the airway mucosa.
Keywords: ozone, allergen, infant, lung, eosinophil, chemokine
INTRODUCTION
Ozone is a major contributor to lung morbidity, particularly when exposure takes place within the first six to eight years after birth, during which human lung growth continues to progress. Numerous studies have reported on the detrimental effects of ambient ozone on lung function in young children, demonstrating both acute and long-term consequences of living in communities with high concentrations (Spektor et al., 1988; Kinney et al., 1989; Higgins et al., 1990; Spektor et al., 1991; Frischer et al., 1999; Burnett et al., 2001). In addition, ozone exposure can exacerbate pre-existing lung disease in children, such as allergic asthma (Castillejos et al., 1992; Romieu et al., 1996; Romieu et al., 1997; Mortimer et al., 2002). Although it is often considered to be an additive inflammatory stimulus, several studies provide evidence to support a more direct role for ozone in the etiology of airways disease. Schoolchildren have an approximate three fold increased risk of developing asthma if they play outdoor sports (versus no sports) in Southern California communities with high ambient ozone levels (McConnell et al., 2002). Infants of asthmatic mothers are at increased risk of developing respiratory symptoms when living in areas where ozone is near or just below federal standard levels, suggesting that certain sensitive populations are vulnerable to even low-level concentrations of airborne pollutants (Triche et al., 2006). More recently, children with genetic variants of arginase and tumor necrosis factor genes were found to have a reduced risk of asthma or symptoms associated with asthma; however these associations were dependent upon living in either a low ozone or high ozone environment (Lee et al., 2009; Salam et al., 2009).
In adults, ozone-induced airways inflammation is characterized predominantly by a neutrophilic influx, a finding that is replicated in several animal models (Campos et al., 1992; Hyde et al., 1992; Krishna et al., 1998; Holz et al., 1999; Jorres et al., 2000). The inflammatory effects of ozone within conducting airways of children are less understood, largely due to ethical concerns regarding the use of invasive sampling techniques. In a small population of healthy schoolchildren, it has been reported that neutrophil numbers in nasal passages significantly correlate with environmental ozone levels (Frischer et al., 1993). In the same study, circulating and nasal eosinophil numbers were not significantly elevated in response to ozone, yet concentration of eosinophil cationic protein was increased in nasal lavage. A contribution of the eosinophil in ozone-mediated airways inflammation is further implicated by Kopp and colleagues, where a dose dependent increase in ambient ozone exposure correlated with an increase in nasal eosinophil cationic protein concentration in a cohort of 144 schoolchildren (Kopp et al., 1999). Independent of atopy, concentration of urinary eosinophil protein X also significantly correlates with ozone exposure in schoolchildren (Frischer et al., 2001). The consistent observation of eosinophil activation markers in conjunction with ozone exposure in young children suggests a prominent role for the eosinophil as an inflammatory leukocyte in early life.
Within the lung, a number of chemokines have been identified as potential mediators of eosinophil trafficking into the airways. CCL5/RANTES, CCL7/MCP-3, CCL13/MCP-4, and CCL3/MIP-1alpha are all elevated in human asthmatics and associated with airways eosinophilia or eosinophil effector functions (Powell et al., 1996; Stellato et al., 1997; Harrison et al., 1999). Each member of this group of chemokines can induce intracellular signaling by binding to more than one chemokine receptor (CCR1, -2, 3, and -5), suggesting a diversity of functions in addition to eosinophil chemotaxis (Lilly and Daugherty, 2001). In contrast, the three members of the eotaxin family of chemokines (CCL11/eotaxin, CCL24/eotaxin-2, CCL26/eotaxin-3) are unique because they exclusively signal via the receptor, CCR3. The association of CCL11 and CCL24 mRNA positive cells with activated eosinophils in bronchial biopsies from asthmatic subjects suggests a role in persistence of eosinophilia in the airway wall (Ying et al., 1999). Expression of CCL26 is restricted to a 24–48 hour window following allergen challenge, and may be more important for new recruitment of eosinophils into the airways (Berkman et al., 2001; Ravensberg et al., 2005).
All three eotaxin family member peptides are synthesized by airway epithelial cells and can be differentially expressed by immunomodulatory cytokines, suggesting a complex regulatory mechanism. (Ying et al., 1997; Ying et al., 1999; Komiya et al., 2003; Heiman et al., 2005; van Wetering et al., 2007). Because a number of different cell types express CCR3 in addition to eosinophils, it is likely that eotaxins have additional important biological functions beyond promoting the activation and migration of eosinophils. CCL11, but not CCL24 or CCL26, is expressed during normal human fetal lung development, primarily by airway epithelium and may be important for proliferation via autocrine signaling through epithelial cell associated CCR3 (Haley et al., 2008). Human fibroblasts express CCR3 and can be induced to proliferate and synthesize collagen via CCL11 or CCL24 treatment (Puxeddu et al., 2006; Kohan et al., 2010). Eotaxins and CCR3 have also been recently been reported to play a key role in angiogenic processes associated with age-related macular degeneration, which is an eosinophil and mast cell-independent process (Takeda et al., 2009).
To date, we have a very limited understanding of the adjuvant mechanisms for air pollutants and how exposure results in a persistent asthma phenotype. Using the rhesus monkey as an animal model for infant lung development, we have reported that episodic ozone exposure during the postnatal growth period can alter the frequency and anatomic distribution of CD25+ lymphocytes in allergen-sensitized airways (Miller et al., 2009). In this current study, we hypothesized that the eosinophilic inflammatory response to allergic sensitization can be modulated in similar fashion by postnatal ozone exposure. To test our hypothesis, we measured circulating, airway lumen and airway mucosa eosinophils in 3-month old infant rhesus monkeys following combined ozone and house dust mite (HDM) exposure. We also determined if expression and localization of eosinophilic chemokines were affected by exposure, focusing on the three members of the eotaxin family.
MATERIALS AND METHODS
Animals and exposure
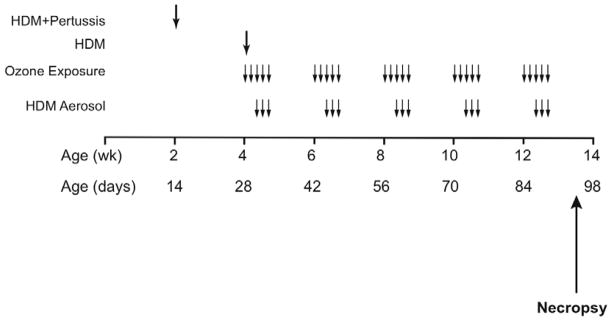
At 1–2 days of age, newborn male rhesus macaque (Macaca mulatta) monkeys were housed under filtered air conditions in 4.2 m3 capacity exposure chambers at the California Regional Primate Research Center, UC Davis. Care and housing of animals before, during and after treatment complied with the provisions of the Institute of Laboratory Animal Resources and conformed to practices of the American Association for Accreditation of Laboratory Animal Care. All animals at 2 weeks of age were sensitized to 10 μg HDM (Dermatophagoides farinae, Greer Laboratories, Lenoir, NC) in 10 mg aluminum hydroxide by subcutaneous injection. To mimic childhood vaccination, HDM sensitized animals also received heat-killed Bordetella pertussis intramuscularly as an adjuvant. At 4 weeks of age, all animals were given an additional subcutaneous injection of 10 μg HDM in 10 mg aluminum hydroxide. Starting at 30 days of age, animals were exposed to 5 cycles of filtered air (n= 6), HDM allergen (n= 6), ozone (n= 6), or ozone + HDM (n= 6). Each cycle consisted of ozone exposure for 5 days, followed by 9 days of filtered air (0.5 ppm at 8h/day). Animal groups not exposed to ozone remained in filtered air throughout each cycle. HDM aerosol exposures were on day 3–5 (2 h/day) of either filtered air exposure or ozone exposure. On days of combined ozone and HDM aerosol, exposures were conduced in sequence. For each day of combined ozone and HDM, animals were first exposed to 8 hours of ozone, followed by a 2 hour delay to allow for animal feeding, then exposure to 2 hours of HDM aerosol. A summary of the exposure protocol is presented in Figure 1. HDM aerosols were generated with a lyophilized extract of Dermatophagoides farinae diluted in PBS and nebulized with a high-flow rate nebulizer as previously described (Schelegle et al., 2001). HDM total protein concentration in each chamber was approximately 300 ug/m3 for each exposure day. All animals were necropsied at approximately 90 days of age. Necropsies of HDM-exposed animals occurred between 4 to 5 days following the last allergen or ozone exposure.
Figure 1. Experimental timeline for ozone and allergen exposure during postnatal development.
Infant rhesus monkeys were sensitized to HDM via subcutaneous (SQ) injection with adjuvant at day 14 and day 28. Starting at 30 days of age, monkeys were exposed to 5 cycles of ozone and/or HDM aerosol. Each cycle consisted of ozone exposure for 5 days, followed by 9 days of filtered air (0.5 ppm at 8h/day). HDM aerosol was delivered during the last 3 days of the ozone exposure period. Lavage and tissue specimens were collected at approximately 90 days of age.
Airway lavage and leukocyte counts
At necropsy, the right caudal lobe from each animal was lavaged with 35 ml of endotoxin-free PBS. Lavage samples were cyto-centrifuged and stained with a modified Wright’s stain (Diff-Quik, Sigma-Aldrich, St. Louis, MO). Differential leukocyte counts were determined by evaluating 300–340 cells using light microscopy.
Immunofluorescence staining for major basic protein
At necropsy, the left caudal lobe from each animal was inflated with a 50% v/v mixture of O.C.T (Sakura Finetek, Torrance, CA) and PBS, then sliced perpendicular to the long axis of the main intrapulmonary conducting airway into even blocks at 7–8 mm thickness per block. Each block from the left caudal lobe was numbered in sequence before embedding for cryosectioning. Cryosections of 5 μm thickness collected from the trachea and alternating numbered blocks were used for immunostaining. To detect eosinophils, cryosections were incubated with mouse anti-human major basic protein (MBP) monoclonal antibody (clone BMK13, BIODESIGN International, Saco, ME) at 0.5 μg/ml, followed by donkey anti-mouse ALEXA 488 (Molecular Probes, Eugene, OR) at 1:2000 dilution. Purified mouse IgG was used as a negative control. Immunofluorescence images were visualized on an Olympus (Ballerup, Denmark) PROVIS fluorescence microscope at 60x magnification. At least 10 random fields were imaged around one airway per section using a Zeiss camera (Zeiss, Oberkochen, Germany). Each image contained a region of epithelium and interstitium internal to the cartilaginous ring (if present). Four cryosections were sampled per animal, representing the most proximal portion (trachea) through the most distal portion of the intrapulmonary conducting airway (respiratory bronchiole). The volume of MBP+ cells within either epithelium or interstitium relative to surface area of basement membrane was measured as previously described (Miller et al., 2003).
RT-PCR analysis
Immediately following necropsy, conducting airways were isolated from the right caudal lobe by fresh tissue microdissection as previously described (Miller et al., 2003). RNA was isolated in TRIzol® Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was generated using random hexamers and MultiScribe™ Reverse Transcriptase (Applied Biosystems, Foster City, CA). Relative mRNA quantitation was analyzed using the comparative Ct method (Relative Quantitation of Gene Expression: ABI PRISM 7700 Sequence Detection System: User Bulletin #2: Rev B, Applied Biosystems).
Oligonucleotide primers for CCL11, CCL24, and CCL26 were designed for the TaqMan assay using the Primer Express™ Software (Applied Biosystems) based on human sequences obtained from the National Center for Biotechnology Information (NCBI) website (http://www.ncbi/nlm.nih.gov/); sequence details used in this study were previously reported (Chou et al., 2003). cDNA samples were amplified with the GeneAmp®7900HT Sequence Detection System using SYBR® Green PCR Master Mix (Applied Biosystems) as the detection fluorochrome. Commercially available Taqman primer/probe sets for rhesus CCR3 and rhesus carboxypeptidase 3 (CPA3) mRNAs (Applied Biosystems) were also used in this study. To control for loading variability, samples were normalized with values for 18s ribosomal RNA (CCL11, CCL24, CCL26) or GAPDH (CCR3, CPA3) according to the manufacturer’s instructions (Applied Biosystems).
Immunofluorescence staining for CCL11, CCL24, CCL26 and CCR3
Frozen cryosections for chemokine and chemokine receptor protein immunostaining were prepared in the same manner as those for major basic protein. Cryosections of midlevel airways (block 3 from the left caudal lobe) from each experimental group were stained with the following primary antibodies purchased from R&D Systems, Inc., (Minneapolis, MN): biotinylated purified polyclonal goat anti-human CCL11, biotinylated purified polyclonal goat anti-human CCL24, and biotinylated purified polyclonal goat anti-human CCL26. Antibody binding was detected with streptavidin ALEXA 488 (Molecular Probes, Eugene, OR) at 1:2000 dilution or goat anti-mouse ALEXA 488 (Molecular Probes) at 1 ug/ml. Mouse anti-rhesus macaque CCR3 antibody (clone 5B9) was used as previously reported (Zhang et al., 2002). All primary antibody concentrations were used at 1 μg/ml in 1% BSA in PBS. Purified MOPC 21 mouse IgG1 (ATCC, Manassas, VA) and total goat IgG (Sigma-Aldrich®, St. Louis, Missouri) were used as a negative control antibodies. Immunofluorescent images for CCL11, CCL24, and CCL26 were visualized on an Olympus (Ballerup, Denmark) PROVIS fluorescence microscope at 60x magnification. Immunofluorescent images for CCR3 were visualized on an Olympus BX61 fluorescence microscope at 60x magnification.
CCL11, CCL24, and CCL26 ELISA
DuoSet ELISA Development Systems kits (R&D Systems, Inc., Minneapolis, MN) for the detection of human CCL11, CCL24, and CCL26 protein were used to detect the concentration of chemokine protein in lavage samples, according to the manufacturer’s instructions. Lavage samples and standard curves using recombinant human CCL11, CCL24, and CCL26 were quantified with the VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA) using the Softmax® Pro Software (version 4.7.1).
Statistical analysis
All data are reported as mean ± SEM (standard error of the mean) unless indicated. Graphing and statistical analysis for all data were conducted usingPrism® 5 and Instat statistical analysis software (GraphPad Software, San Diego, CA). Treatment groups and airway levels were compared using either one- or two- way ANOVA, where p<0.05 was considered significant. The Tukey-Kramer Multiple Comparison Test was used for as a post-test for one-way ANOVA. Pearson’s R was utilized for correlation analysis.
RESULTS
Eosinophils and eotaxins in the airway lumen
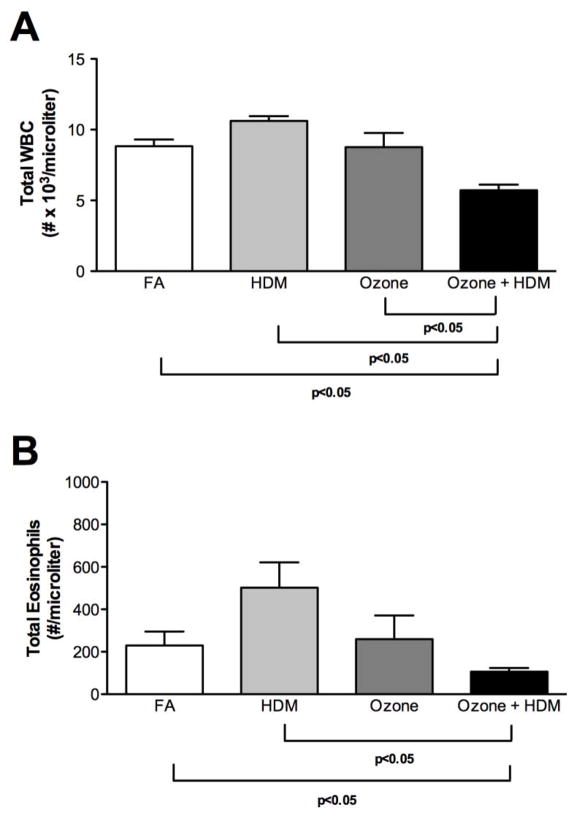
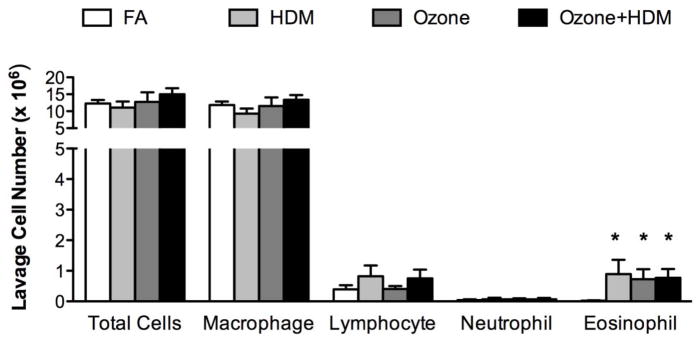
At 3 months of age, monkeys were evaluated at 4–5 days following the last ozone and/or allergen exposure cycle. As shown in Figure 2A, combined ozone and HDM exposure resulted in a significant reduction in peripheral blood WBC numbers, with no effect of ozone or HDM alone in comparison with filtered air control animals. Blood eosinophil numbers were also significantly reduced at 3 months of age in the combined ozone and HDM exposure animal group in comparison with filtered air or HDM alone groups (Fig. 2B). Lavage eosinophil numbers were significantly increased in HDM, ozone, and ozone + HDM exposure groups, relative to filtered air control animals (Fig. 3). There was no effect of exposure regimen on lavage macrophage, lymphocyte, and neutrophil numbers.
Figure 2. Effect of ozone and allergen exposure on peripheral blood WBC and eosinophil numbers at 3 months of age.
Peripheral blood samples were collected from infant monkeys at necropsy, 4–5 days following the last allergen and/or ozone exposure. Samples obtained from both 4 and 5 day necropsy timepoints were pooled. Each column represents the mean ± SE values for 6 animals, each group treated with filtered air (FA), house dust mite (HDM), ozone, or HDM + ozone as described in Materials and Methods. (A) WBC, (B) eosinophils.
Figure 3. Effect of ozone and allergen exposure on lavage cell numbers at 3 months of age.
Lavage samples were collected from infant monkeys at necropsy, 4–5 days following the last allergen and/or ozone exposure. Samples obtained from both 4 and 5 day necropsy timepoints were pooled. Columns represent the mean ± SE cell numbers for 6 animals, each group treated with filtered air (FA), house dust mite (HDM), ozone, or HDM + ozone as described in Materials and Methods. *p<0.05 as compared with filtered air control animals.
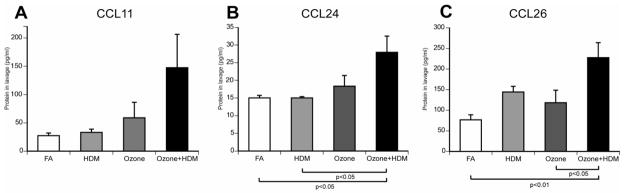
To determine if expression of eotaxins may contribute to recruitment of eosinophils into the airway lumen following ozone and/or HDM exposure, we measured CCL11, CCL24, and CCL26 protein concentration in lavage fluid (Fig. 4). There was a trend towards increased CCL11 protein concentration as a result of combined ozone and HDM exposure, relative to filtered air controls, HDM alone or ozone alone (p=0.0611)(Fig. 4A). CCL24 protein concentration in lavage was increased with combined ozone and HDM exposure, relative to filtered air controls or HDM alone (Fig. 4B). CCL26 protein concentration in lavage was also increased in response to combined ozone and HDM exposure, relative to filtered air controls and ozone alone (Fig. 4C). There was a trend towards increased CCL26 protein in lavage samples from the HDM alone animal group, but this did not reach statistical significance (p=0.0564). There were no significant correlations between lavage protein concentration of CCL11, CCL24 or CCL26 and lavage eosinophil number within exposure groups.
Figure 4. Effect of ozone and allergen exposure on lavage CCL11, CCL24, and CCL26 protein in infant monkeys.
Lavage samples were collected as for Figure 3. Each column represents the average concentration (pg/ml) ± SEM for CCL11 (A), CCL24 (B), and CCL26 (C) protein in lavage. n= 6 for each exposure group.
Eosinophils and eotaxins in the airway mucosa
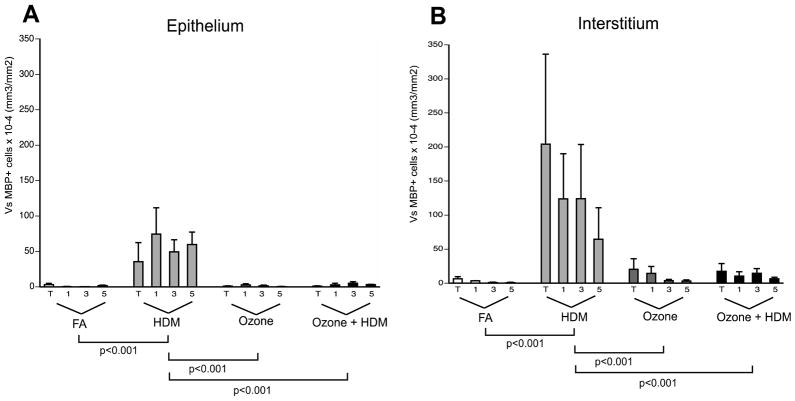
To determine if ozone and HDM exposure had a differential effect on eosinophil accumulation in the airway mucosa of infant monkeys, eosinophil volume was measured in four different conducting airway generations using stereological methods (Fig. 5). Overall, eosinophil volume was higher within the interstitium versus the epithelial compartment in all animal groups evaluated. Eosinophil distribution in airway epithelium correlated with the eosinophil distribution in airway interstitium (Pearson’s R=0.9986, p=0.0014). Eosinophil volume was most abundant in airway epithelium (Fig. 5A) and interstitium (Fig. 5B) in response to HDM alone, relative to filtered air controls, ozone alone, or ozone + HDM groups. There were no significant differences in epithelial and interstitial eosinophil volume between filtered air controls and ozone-exposed groups, although there was a trend towards increased interstitial eosinophil volume in combined ozone and HDM animals as compared to filtered air controls (p=0.06). There was no preferential accumulation of eosinophils in large versus small airways within exposure groups.
Figure 5. Effect of ozone and allergen exposure on airway mucosa eosinophils in infant monkeys.
The volume of eosinophils within the airway wall was determined by immunostaining of cryosections obtained from the left caudal lobe of each animal, tissues were collected at necropsy (3 months of age). Eosinophils were identified as immunofluorescence positive for major basic protein. Each group was treated with filtered air (FA), house dust mite (HDM), ozone, or HDM + ozone as described in Materials and Methods. Values for each column represent the average volume of eosinophils relative to the surface area of basement membrane ± SEM. Blocks T, 1, 3, and 5 correspond to the trachea (T) and regions progressively sampled from the most proximal (1) to distal (5) airways of the lobe. For each airway generation, n= 6 animals for each of the experimental groups. Eosinophil volume was separately determined for the airway epithelial compartment (A) and interstitial compartment (B).
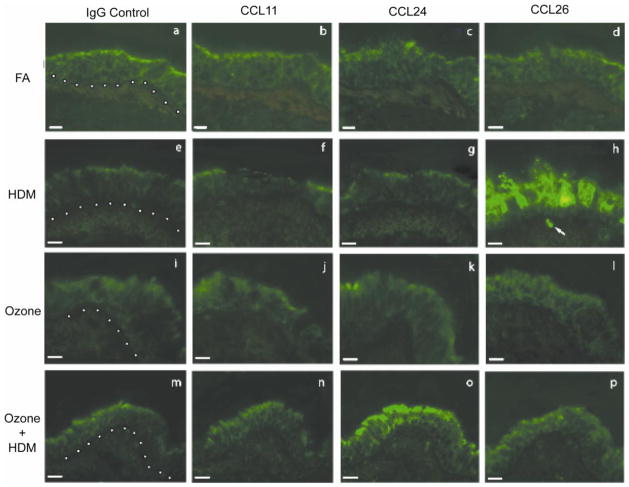
Because ozone and HDM exposure (alone and in combination) resulted in differential anatomic compartmentalization of eosinophils in the lung, we next investigated if airway mucosal expression of eotaxins associated with exposure regimen. Cryosections obtained from midlevel airways of each of the four animal exposure groups were evaluated for CCL11 CCL24, and CCL26 protein by immunofluorescence staining (Fig. 6). Airways from filtered air control animals showed little CCL11, CCL24, or CCL26-associated immunofluorescence staining (Fig. 6b–d). CCL11 immunofluorescence staining in ozone and/or HDM animal groups was comparable to filtered air control animals (Fig. 6b, f, j, n). CCL24 immunofluorescence staining was detectable only in the ozone + HDM animal group; positive signal was primarily localized to the airway epithelium (Fig. 6o). CCL26 was highly expressed in airway epithelium in response to HDM alone (Fig. 6h), with comparatively reduced immunofluorescence staining in combination with ozone exposure (Fig. 6p). In addition to epithelial cells, we observed CCL26 immunofluorescence in association with leukocytes within the interstitium of airway mucosa from animals exposed to HDM alone (arrow in Fig. 6h).
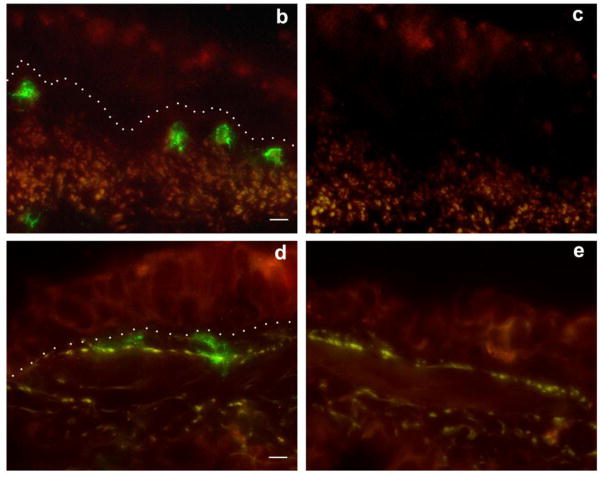
Figure 6. Effect of ozone and allergen exposure on CCL11, CCL24 and CCL26 protein expression in infant monkey airways.
Immunofluorescence staining for CCL11, CCL24 and CCL26 was conducted on adjacent cryosections obtained from a midlevel intrapulmonary airway of the left caudal lobe from each animal at 3 months of age. One representative infant monkey from each FA (a–d), HDM (e–h), ozone (i–l), and ozone + HDM (m–p) exposure group is shown. Purified goat IgG was used as a negative control (a, e, i, m). Each image panel contains a portion of the airway lumen (top), airway epithelium (center), and airway interstitium (bottom). The dotted line represents the basement membrane zone in panels a, e, i and m. The arrow in (h) points to a CCL26+ cell within the airway interstitium (scale bar= 10 μm).
Eotaxins and CCR3 gene expression in the airway mucosa
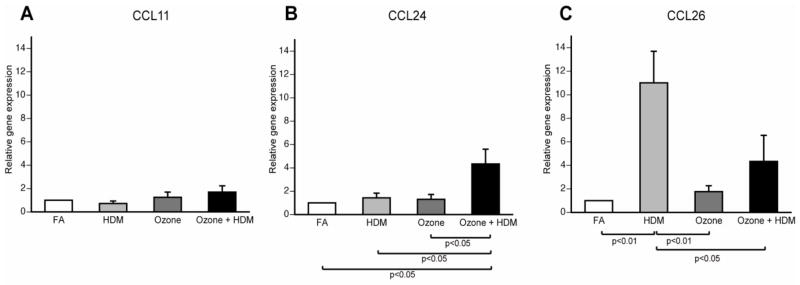
We next determined if CCL11, CCL24, and CCL26 mRNA expression corresponded with airway mucosa immunofluorescence staining for these chemokines. Infant monkey conducting airways were isolated and separated from parenchyma at necropsy, then evaluated for CCL11, CCL24, and CCL26 mRNA expression. As shown in Figure 7A, there were no differences in CCL11 mRNA expression for ozone and/or HDM animals. Ozone or HDM alone also had no effect on CCL24 mRNA expression within airways as compared with filtered air controls (Fig. 7b). In contrast, there was an approximate 5-fold increase in airway CCL24 mRNA expression in response to combined ozone and HDM exposure, relative to filtered air, HDM alone, or ozone alone. CCL26 mRNA expression was approximately 10-fold higher in response to HDM exposure versus filtered air controls (Fig. 7c). Exposure to ozone alone did not alter CCL26 mRNA expression compared to filtered air controls. Ozone and HDM combined exposure showed an approximate 3-fold decrease in CCL26 mRNA expression in airway tissue as compared to HDM alone.
Figure 7. Effect of ozone and allergen exposure on CCL11, CCL24, and CCL26 mRNA expression in infant monkey airways.
CCL11, CCL24 and CCL26 mRNA expression was determined by real-time RT-PCR analysis of microdissected conducting airways isolated from the right caudal lobe of each animal at 3 months of age. Each column represents the change in gene expression for CCL11 (A), CCL24 (B), and CCL26 (C) relative to filtered air control infant monkeys. Columns represent the mean ± SE for 6 animals, each group treated with filtered air (FA), house dust mite (HDM), ozone, or ozone + HDM as described in Materials and Methods.
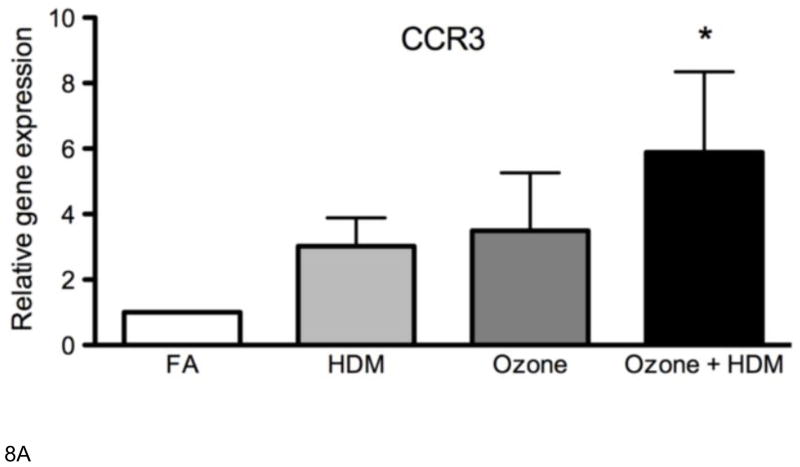
In parallel with lavage eotaxin concentration, we compared mucosal eotaxin mRNA levels with mucosal eosinophil volume. CCL26 mRNA expression significantly correlated with eosinophil volume in airway epithelium and interstitium for all animal exposure groups (Table 1). There was no association of CCL11 mRNA with eosinophil volume in airway mucosa, regardless of animal exposure group. Although CCL24 mRNA levels also did not correspond to eosinophil volume, it did correlate with CCL24 protein concentration in lavage (Table II). Because we observed significant CCL24 protein and mRNA exclusively in the combined ozone and HDM exposure group but did not find a corresponding association with eosinophil accumulation, we evaluated airway tissues for CCR3 mRNA expression to determine if other lung cells may be responding to this eotaxin family member. As shown in Figure 8a, CCR3 mRNA was significantly increased in combined ozone and HDM exposure as compared to filtered air controls. There was a modest, but not statistically significant increase in CCR3 mRNA for HDM alone and ozone alone groups. Immunofluorescence staining for CCR3 in midlevel airways of HDM alone animals showed positive signal associated with bilobed leukocytes (Fig. 8b). In comparison, CCR3 immunofluorescence positive cells in ozone + HDM airways are larger in size and have a morphologically distinct appearance (8d). Airway tissues were also evaluated for the chymase positive mast cell marker, CPA3; mRNA expression was detected at low levels and there was no significant effect of exposure (data not shown).
Table I.
Correlation Between CCL11, CCL24, and CCL26 mRNA Expression with Volume of Airway Mucosa Eosinophils.
| Pearson’s R | p value | ||
|---|---|---|---|
| CCL11 mRNA vs. | eos(epithelium) | −0.6976 | 0.3024 |
| eos(interstitium) | − 0.6757 | 0.3243 | |
| CCL24 mRNA vs. | eos(epithelium) | − 0.2222 | 0.7778 |
| eos(interstitium) | − 0.2051 | 0.7949 | |
| CCL26 mRNA vs. | eos(epithelium) | 0.9588 | 0.0412* |
| eos(interstitium) | 0.9646 | 0.0354* | |
Table II.
Correlation Between Airway Mucosa mRNA with Lavage Protein for CCL11, CCL24, and CCL26.
| Pearson’s R | p value | |
|---|---|---|
| CCL11 mRNA in tissue vs. CCL11 protein in lavage | 0.9234 | 0.0766 |
| CCL24 mRNA in tissue vs. CCL24 protein in lavage | 0.9667 | 0.0333* |
| CCL26 mRNA in tissue vs. CCL26 protein in lavage | 0.3365 | 0.6635 |
Figure 8. Effect of ozone and allergen exposure on CCR3 mRNA and protein expression in infant monkey airways.
(A) CCR3 mRNA expression was determined by real-time RT-PCR analysis of microdissected conducting airways isolated from the right caudal lobe of each animal at 3 months of age. Each column represents the change in gene expression for CCR3 relative to filtered air control infant monkeys. Columns represent the mean ± SE for 6 animals, each group treated with filtered air (FA), house dust mite (HDM), ozone, or ozone + HDM as described in Materials and Methods. (B–E) Immunofluorescence staining for CCR3 was conducted on adjacent cryosections as described for Figure 6. One representative infant monkey from HDM (b–c) and ozone + HDM (d–e) exposure group is shown. Mouse IgG1 clone MOPC 21 was used as a negative control (c, e). The dotted line represents the basement membrane zone in panels b and d. (scale bar= 10 μm).
DISCUSSION
Although much has been described regarding the immunological impact of allergen exposures in the lung, little is known about the biological mechanisms for air pollutant effects on developing pulmonary and immune cells during early childhood. To directly assess how these environmental challenges can affect the lung during infancy, we conducted this study in the rhesus macaque monkey as a non-human primate model of childhood development. We have previously reported that cyclic ozone exposure alters the frequency and anatomic distribution of CD25+ lymphocytes in allergen-sensitized infant monkeys (Miller et al., 2009). The purpose of this present study was to determine if eosinophils, which are an important parameter of the asthma phenotype, also exhibit differential recruitment patterns to the lung in response to ozone and allergen exposures. Using a cyclic regimen to mimic the episodic nature of air pollutant exposure, we found that inhalation of ozone, HDM, or a combination of ozone + HDM starting at 30 days of age resulted in recruitment of airway lumen eosinophils in infant monkeys (Fig. 3). In contrast, significant accumulation of eosinophils in airway mucosa was observed only with HDM exposure; ozone alone or in combination with HDM significantly reduced eosinophils in airway mucosa (Fig. 5). Concurrent evaluation of peripheral blood showed that combined exposure decreased total WBC and eosinophils (Fig. 2). By directly comparing eosinophil abundance in airway lavage and tissue with expression of eotaxin family members, we have been able to demonstrate that ozone does not uniformly function as an additive or inhibitory factor in conjunction with the inflammatory effects of allergen. In addition, by distinguishing the expression of eotaxins at specific sites within the lung, we have also found expression of CCL24 is uniquely induced by combined ozone and allergen exposure. Finally, the observed reduction in circulating WBC and eosinophils with combined exposure demonstrates that the effect of ozone is not limited to sites of ozone deposition in the lung; systemic responses were also elicited.
We have shown that HDM treatment of 2-month old rhesus monkeys (aerosol only) and 6-month old rhesus monkeys (systemically sensitized and aerosol) result in airway mucosa eosinophilia (Miller et al., 2003; Schelegle et al., 2003). Findings from this current study with HDM alone (systemically sensitized and aerosol) in 3-month old rhesus monkeys are consistent with our published data on tissue eosinophils, thereby establishing that eosinophil recruitment to the airway wall is a predictable outcome of aeroallergen exposure in the very young. In 2-month old monkeys, HDM aerosol exposure resulted in the induction of CCL26 mRNA and protein expression in airway mucosa, primarily by epithelial cells and airway nerves (Chou et al., 2005). Here, we found similar results in 3-month old monkeys with HDM alone, with a trend towards increased lavage CCL26 protein. These data support a prominent role for CCL26, but not CCL11 or CCL24, as a mediator of eosinophil trafficking to the airway wall in response to HDM exposure. In a study with human adult asthmatics, CCL11 and CCL24 mRNA expression in bronchial biopsies was shown to be constitutively elevated in comparison with control subjects, whereas CCL26 mRNA was enhanced at 24 hours after allergen challenge (Berkman et al., 2001). This suggests that CCL26, but not CCL11 or CCL24, may be responsible for new eosinophil recruitment into the airway wall following allergen challenge. In a separate study with mild adult asthmatics, CCL24 and CCL26, but not CCL11 was found to be responsible for ongoing airway eosinophilia 48 hours after allergen challenge (Ravensberg et al., 2005). These data from human subjects reinforce the notion that CCL26 plays a key role in recruitment of airway eosinophils following an allergen challenge. The lack of correlation between CCL11 and CCL24 mRNA expression with tissue eosinophilia is in contrast to rodent studies, where only double and not single knock out mice for CCL11 and CCL24 significantly decreased peribronchial eosinophilia following ovalbumin challenge (Pope et al., 2005b). In light of a lack of a defined function for CCL26 in rodents (Pope et al., 2005a), these disparities may be due to species and age related differences with regards to expression of chemokines within the lung.
In contrast with HDM, eosinophil accumulation in 3 month-old animals that received ozone alone or in combination with HDM was limited to the airway lumen. This suggests that chemotactic factors would be predominantly secreted into the airway lumen with ozone exposure. Lavage eosinophil numbers were not significantly greater in ozone groups, which indicates that the overall quantity of eosinophils recruited to the lung by ozone (lumen and mucosa) was likely to be less than that for HDM alone. In this study, we focused on evaluation of conducting airways for the presence of eosinophils. As yet, we do not know the anatomic (airway) site for eosinophil migration from the peripheral blood into the airway lumen, particularly in response to ozone exposure. Eosinophils may have been recruited from the distal alveolar airways, however we did not observe substantial numbers of eosinophils within lung parenchyma specimens collected at 4–5 days post allergen challenge (data not shown). Interestingly, ozone exposure does not reduce HDM-induced lung eosinophilia at 6 months of age in the infant monkey; eosinophilia was observed in both airway lumen and mucosa (Schelegle et al., 2003). It is possible that attenuation of airway tissue eosinophils by ozone exposure in younger animals is the result of enhanced emigration from the epithelial compartment, however we would expect that this would result in an additive effect with HDM in lavage eosinophil numbers. The attenuating effects of ozone may also be attributed to increased granulocyte apoptosis via repression of anti-apoptotic proteins transcription (Fievez et al., 2001); but we would also concurrently expect a decline in eosinophil number in lavage. Determination of the mechanisms involved in site-specific distribution of eosinophils within the airways may be critical to understanding the initiation of inflammatory airway diseases. Rodents sensitized to allergen exhibit increased emigration of eosinophils from the airway tissue into the lumen; however, in rodents with pre-existing viral infections, eosinophils tend to remain in the airway wall after allergen challenge (Sorkness et al., 2007). In humans, regional variations in eosinophil distribution within the airways are exclusive to asthmatics, but not cystic fibrosis patients (Haley et al., 1998).
Distinct eosinophil trafficking patterns at different stages of postnatal development may be attributed to specific expression of cytokines and chemokines that are unique to the age of the animal (Johnston et al., 2006). In 3-month old monkeys, CCL11 in airways was not significantly affected by HDM or ozone alone, but there was a trend towards increased lavage protein with combined ozone and HDM exposure. Because all chemokine expression parameters were evaluated 4–5 days after ozone or allergen exposure in our studies, it is possible that CCL11 mRNA expression in lung tissue and CCL11 protein in lavage fluid returned to baseline levels at the time of analysis. Kinetic studies in mice show that CCL11 mRNA levels decrease within 12 hours of ovalbumin challenge, whereas CCL11 protein levels in lavage decrease over 48 hours (Pope et al., 2005b). The limited CCL11 expression at the time point evaluated in this current study is further supported by other laboratories which establish a role for eotaxin in the recruitment of eosinophils to asthmatic airways within the first 24 hours of the late phase allergic response (Brown et al., 1998). We observed that CCL26 mRNA and protein in airway tissue from HDM-exposed monkeys was reduced by ozone, but found significant concentrations of CCL26 protein in lavage from combined HDM and ozone animal groups. This may explain the discordant finding of abundant eosinophils in lavage and few eosinophils in tissue with ozone + HDM, but the cellular source for CCL26 in lavage is unknown. The finding of CCL24 mRNA and protein expression in response to combined ozone and HDM exposure is unique; to the best of our knowledge this is the first report of a chemokine that is exclusively expressed in the context of combined exposures. It is also noteworthy that significant (or near significant) levels of CCL11, CCL24 and CCL26 protein in lavage were found only in the combined ozone and HDM animal group, yet there were no differences in lavage eosinophil numbers between animal groups. Although we did not evaluate eosinophils for parameters of activation in infant monkeys, CCL11, CCL24, and CCL26 have been reported to enhance degranulation and superoxide anion generation in this cell population (Badewa et al., 2002).
Airway CCL24 mRNA and protein expression was significantly enhanced only in response to combined ozone and HDM exposure, and did not correlate with the presence of tissue eosinophils, CCL11, and CCL26. As yet we do not know what the physiologic role of CCL24 is within infant airways, but functions in addition to eosinophil chemotaxis may be considered. van Wetering and colleagues have reported that in vitro induction of CCL24 and CCL26 via Th2 cytokines is dependent upon the state of airway epithelial cell differentiation (van Wetering et al., 2007). Specifically, CCL26 was preferentially released by mucociliary differentiated cultures, whereas CCL24 was preferentially released by squamous differentiated cultures. This study lends support to the idea that the differentiated state of the epithelium, either via environmental challenge, developmental delay, or a combination of both, may dictate the type of inflammatory response mediated by the lung. All eotaxin family members signal via CCR3, have autocrine capabilities, and can alter the expression of other eotaxins in airway epithelial cells (Saito et al., 2000; Abonyo et al., 2005). Because CCL24 was only increased in response to combined ozone and HDM exposure, it is tempting to speculate that CCL24 may inhibit HDM-induced CCL26. However, mRNA levels and lavage protein concentration for CCL24 were considerably less than that for CCL26. Binding affinity for human CCL24 for both macaque and human CCR3 is 2–3 fold higher than that for human CCL26, therefore we cannot discount the possibility of CCL24 and CCL26 interacting at the receptor level (Zhang et al., 2002). Significantly increased levels of CCR3 mRNA expression in airway mucosa from ozone + HDM animals suggests that other cell types besides eosinophils may respond to CCL24 in the mucosa. CCR3 is highly expressed on chymase positive mast cells (Romagnani et al., 1999), but we found little expression of the chymase positive mast cell marker, CPA3 (Irani et al., 1991; Romagnani et al., 1999); this finding is consistent with previously published data indicating that ozone and allergen exposed infant rhesus monkeys have very few mast cells within intrapulmonary airways (Van Winkle et al., 2010). Immunofluorescence staining in infant airways suggests that the phenotype of CCR3+ cells induced by combined ozone + HDM is similar to MHC class II+ CCR3+ dendritic cells that we have previously observed in conjunction with airway nerve fiber bundles (Figure 8d)(Chou et al., 2005). Future studies will include immunophenotyping to fully characterize the CCR3+ cell type(s) within infant airways exposed to ozone and HDM.
In conclusion, our results suggest that the postnatal period of development is a dynamic period with regards to ability of the lung to respond to environment challenges. The inflammatory mechanisms of ozone that mediate eosinophil responses during infancy are distinct from those of HDM. Data from combined exposures do not support a uniformly additive or inhibitory effect of ozone with regards to eosinophils and eotaxins. Rather, the interaction of ozone effects on the allergen-sensitized lung results in a distinct profile of eosinophil trafficking in both peripheral blood and lung.
Highlights.
Ozone can modulate the localization of eosinophils in infant allergic airways.
Expression of eotaxins within the lung is affected by ozone and allergen exposure.
CCL24 induction by ozone and allergen exposure is not linked to eosinophilia.
Acknowledgments
We thank Sarah Davis, Brian Tarkington, Lei Putney, and Justin Fontaine for expert technical assistance during the course of this study. Susie Nishio contributed to manuscript preparation. We would also like to recognize the valuable scientific feedback provided by staff scientists and collaborators within the CNPRC Respiratory Diseases Unit. This work was supported by National Institutes of Health grants ES-00628 (D.M. Hyde), ES-11617 (E.M. Postlethwait), HL-81286 (L.A. Miller), AI065567 (L.A. Miller), HL-07013 (R. Wu), ES-007059 (R. Rice), RR-00169 (B.M. Klein) and EPA STAR Grant 832947 (L.A. Miller).
Footnotes
CONFLICTS OF INTEREST STATEMENT
The authors of this manuscript declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abonyo BO, Alexander MS, Heiman AS. Autoregulation of CCL26 synthesis and secretion in A549 cells: a possible mechanism by which alveolar epithelial cells modulate airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L478–488. doi: 10.1152/ajplung.00032.2005. [DOI] [PubMed] [Google Scholar]
- Badewa AP, Hudson CE, Heiman AS. Regulatory effects of eotaxin, eotaxin-2, and eotaxin-3 on eosinophil degranulation and superoxide anion generation. Exp Biol Med (Maywood) 2002;227:645–651. doi: 10.1177/153537020222700814. [DOI] [PubMed] [Google Scholar]
- Berkman N, Ohnona S, Chung FK, Breuer R. Eotaxin-3 but not eotaxin gene expression is upregulated in asthmatics 24 hours after allergen challenge. Am J Respir Cell Mol Biol. 2001;24:682–687. doi: 10.1165/ajrcmb.24.6.4301. [DOI] [PubMed] [Google Scholar]
- Brown JR, Kleimberg J, Marini M, Sun G, Bellini A, Mattoli S. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol. 1998;114:137–146. doi: 10.1046/j.1365-2249.1998.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett RT, Smith-Doiron M, Stieb D, Raizenne ME, Brook JR, Dales RE, Leech JA, Cakmak S, Krewski D. Association between ozone and hospitalization for acute respiratory diseases in children less than 2 years of age. Am J Epidemiol. 2001;153:444–452. doi: 10.1093/aje/153.5.444. [DOI] [PubMed] [Google Scholar]
- Campos MG, Segura P, Vargas MH, Vanda B, Ponce-Monter H, Selman M, Montano LM. O3-induced airway hyperresponsiveness to noncholinergic system and other stimuli. J Appl Physiol. 1992;73:354–361. doi: 10.1152/jappl.1992.73.1.354. [DOI] [PubMed] [Google Scholar]
- Castillejos M, Gold DR, Dockery D, Tosteson T, Baum T, Speizer FE. Effects of ambient ozone on respiratory function and symptoms in Mexico City schoolchildren. Am Rev Respir Dis. 1992;145:276–282. doi: 10.1164/ajrccm/145.2_Pt_1.276. [DOI] [PubMed] [Google Scholar]
- Chou DL, Daugherty BL, McKenna EK, Hsu W, Tyler NK, Plopper CG, Hyde DM, Schelegle ES, Gershwin LJ. Chronic aeroallergen during infancy enhances eotaxin-3 expression in airway epithelium and nerves. Am J Resp Cell Mol Biol. 2005;33:1–8. doi: 10.1165/rcmb.2004-0236RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievez L, Kirschvink N, Dogne S, Jaspar F, Merville MP, Bours V, Lekeux P, Bureau F. Impaired accumulation of granulocytes in the lung during ozone adaptation. Free Radic Biol Med. 2001;31:633–641. doi: 10.1016/s0891-5849(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Frischer T, Kuehr J, Pullwitt A, Meinert R, Forster J, Studnicka M, Koren H. Ambient ozone causes upper airways inflammation in children. Am Rev Respir Dis. 1993;148:961–964. doi: 10.1164/ajrccm/148.4_Pt_1.961. [DOI] [PubMed] [Google Scholar]
- Frischer T, Studnicka M, Gartner C, Tauber E, Horak F, Veiter A, Spengler J, Kuhr J, Urbanek R. Lung function growth and ambient ozone: a three-year population study in school children. Am J Respir Crit Care Med. 1999;160:390–396. doi: 10.1164/ajrccm.160.2.9809075. [DOI] [PubMed] [Google Scholar]
- Frischer T, Studnicka M, Halmerbauer G, Horak FJ, Gartner C, Tauber E, Koller DY. Ambient ozone exposure is associated with eosinophil activation in healthy children. Clincial and Experimental Allergy. 2001;31:1213–1219. doi: 10.1046/j.1365-2222.2001.01155.x. [DOI] [PubMed] [Google Scholar]
- Haley KJ, Sunday ME, Porrata Y, Kelley C, Twomey A, Shahsafaei A, Galper B, Sonna LA, Lilly CM. Ontogeny of the eotaxins in human lung. American journal of physiology Lung cellular and molecular physiology. 2008;294:L214–224. doi: 10.1152/ajplung.00086.2007. [DOI] [PubMed] [Google Scholar]
- Haley KJ, Sunday ME, Wiggs BR, Kozakewich HP, Reilly JJ, Mentzer SJ, Sugarbaker DJ, Doerschuk CM, Drazen JM. Inflammatory cell distribution within and along asthmatic airways. Am J Respri Crit Care Med. 1998;158:565–572. doi: 10.1164/ajrccm.158.2.9705036. [DOI] [PubMed] [Google Scholar]
- Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med. 1999;159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- Heiman AS, Abonyo BO, Darling-Reed SF, Alexander MS. Cytokine-stimulated human lung alveolar epithelial cells release eotaxin-2 (CCL24) and eotaxin-3 (CCL26) J Interferon Cytokine Res. 2005;25:82–91. doi: 10.1089/jir.2005.25.82. [DOI] [PubMed] [Google Scholar]
- Higgins IT, D’Arcy JB, Gibbons DI, Avol EL, Gross KB. Effect of exposures to ambient ozone on ventilatory lung function in children. Am Rev Respir Dis. 1990;141:1136–1146. doi: 10.1164/ajrccm/141.5_Pt_1.1136. [DOI] [PubMed] [Google Scholar]
- Holz O, Jorres RA, Timm P, Mucke M, Richter K, Koschyk S, Magnussen H. Ozone-induced airway inflammatory changes differ between individuals and are reproducible. Am J Respir Crit Care Med. 1999;159:776–784. doi: 10.1164/ajrccm.159.3.9806098. [DOI] [PubMed] [Google Scholar]
- Hyde DM, Hubbard WC, Wong V, Wu R, Pinkerton K, Plopper CG. Ozone-induced acute tracheobronchial epithelial injury: relationship to granulocyte emigration in the lung. Am J Respir Cell Mol Biol. 1992;6:481–497. doi: 10.1165/ajrcmb/6.5.481. [DOI] [PubMed] [Google Scholar]
- Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase. Selective localization to MCTC cells. Journal of immunology. 1991;147:247–253. [PubMed] [Google Scholar]
- Johnston CJ, Holm BA, Gelein R, Finkelstein JN. Postnatal Lung Development: Immediate-Early Gene Responses Post Ozone and LPS Exposure. Inhalation Toxicol. 2006;18:875–883. doi: 10.1080/08958370600822466. [DOI] [PubMed] [Google Scholar]
- Jorres RA, Holz O, Zachgo W, Timm P, Koschyk S, Muller B, Grimminger F, Seeger W, Kelly FJ, Dunster C, Frischer T, Lubec G, Waschewski M, Niendorf A, Magnussen H. The Effect of Repeated Ozone Exposures on Inflammatory Markers in Bronchoalveolar Lavage Fluid and Mucosal Biopsies. Am J Respir Crit Care Med. 2000;161:1855–1861. doi: 10.1164/ajrccm.161.6.9908102. [DOI] [PubMed] [Google Scholar]
- Kinney PL, Ware JH, Spengler JD, Dockery DW, Speizer FE, Ferris BG., Jr Short-term pulmonary function change in association with ozone levels. Am Rev Respir Dis. 1989;139:56–61. doi: 10.1164/ajrccm/139.1.56. [DOI] [PubMed] [Google Scholar]
- Kohan M, Puxeddu I, Reich R, Levi-Schaffer F, Berkman N. Eotaxin-2/CCL24 and eotaxin-3/CCL26 exert differential profibrogenic effects on human lung fibroblasts. Annals of allergy, asthma & immunology: official publication of the American College of Allergy. Asthma, & Immunology. 2010;104:66–72. doi: 10.1016/j.anai.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Komiya A, Nagase H, Yamada H, Sekiya T, Yamaguchi M, Sano Y, Hanai N, Furuya A, Ohta K, Matsushima K, Yoshie O, Yamamoto K, Hirai K. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225:91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kopp MV, Ulmer C, Ihorst G, Seydewitz HH, Frischer T, Forster J, Kuehr J. Upper airway inflammation in children exposed to ambient ozone and potential signs of adaptation. Eur Respir J. 1999;14:854–861. doi: 10.1034/j.1399-3003.1999.14d22.x. [DOI] [PubMed] [Google Scholar]
- Krishna MT, Madden J, Teran LM, Biscione GL, Lau LCK, Withers NJ, Sandstrom T, Mudway I, Kelly FJ, Walls A, Frew AJ, Holgate ST. Effects of 0.2 ppm ozone on biomarkers of inflammation in bronchoalveolar lavage fluid and bronchial mucosa of healthy subjects. Eur Respir J. 1998;11:1294–1300. doi: 10.1183/09031936.98.11061294. [DOI] [PubMed] [Google Scholar]
- Lee YL, McConnell R, Berhane K, Gilliland FD. Ambient ozone modifies the effect of tumor necrosis factor G-308A on bronchitic symptoms among children with asthma. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.02014.x. [DOI] [PubMed] [Google Scholar]
- Lilly CM, Daugherty BL. A novel LPS-inducible CCR3 activator: why so many CCR3 ligands? Am J Respir Cell Mol Biol. 2001;25:673–675. doi: 10.1165/ajrcmb.25.6.f222. [DOI] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- Miller LA, Gerriets JE, Tyler NK, Abel K, Schelegle ES, Plopper CG, Hyde DM. Ozone and allergen exposure during postnatal development alters the frequency and airway distribution of CD25+ cells in infant rhesus monkeys. Toxicol Appl Pharmacol. 2009;236:39–48. doi: 10.1016/j.taap.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Plopper CG, Hyde DM, Gerriets JE, Pieczarka E, Tyler N, Gershwin LJ, Schelegle ES, Van Winkle LS. Immune and Airway Effects of House Dust Mite Aeroallergen Exposures During Postnatal Development of the Infant Rhesus Monkey. Clin Exp Allergy. 2003;33:1686–1694. doi: 10.1111/j.1365-2222.2003.01812.x. [DOI] [PubMed] [Google Scholar]
- Mortimer KM, Neas LM, Dockery DW, Redline S, Tager IB. The effect of air pollution on inner-city children with asthma. Eur Respir J. 2002;19:699–705. doi: 10.1183/09031936.02.00247102. [DOI] [PubMed] [Google Scholar]
- Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a Cooperative Mechanism Involving Interleukin-13 and Eotaxin-2 in Experimental Allergic Lung Inflammation. J Biol Chem. 2005a;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005b;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- Powell N, Humbert M, Durham SR, Assoufi B, Kay AB, Corrigan CJ. Increased expression of mRNA encoding RANTES and MCP-3 in the bronchial mucosa in atopic asthma. Eur Respir J. 1996;9:2454–2460. doi: 10.1183/09031936.96.09122454. [DOI] [PubMed] [Google Scholar]
- Puxeddu I, Bader R, Piliponsky AM, Reich R, Levi-Schaffer F, Berkman N. The CC chemokine eotaxin/CCL11 has a selective profibrogenic effect on human lung fibroblasts. The Journal of allergy and clinical immunology. 2006;117:103–110. doi: 10.1016/j.jaci.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Ravensberg AJ, Ricciardolo FL, van Schadewijk A, Rabe KF, Sterk PJ, Hiemstra PS, Mauad T. Eotaxin-2 and eotaxin-3 expression is associated with persistent eosinophilic bronchial inflammation in patients with asthma after allergen challenge. J Allergy Clin Immunol. 2005;115:779–785. doi: 10.1016/j.jaci.2004.11.045. [DOI] [PubMed] [Google Scholar]
- Romagnani P, De Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, Romagnani S, Marone G. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. The American journal of pathology. 1999;155:1195–1204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Meneses F, Ruiz S, Huerta J, Sienra JJ, White M, Etzel R, Hernandez M. Effects of intermittent ozone exposure on peak expiratory flow and respiratory symptoms among asthmatic children in Mexico City. Arch Environ Health. 1997;52:368–376. doi: 10.1080/00039899709602213. [DOI] [PubMed] [Google Scholar]
- Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, White MC, Etzel RA. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. Am J Respir Crit Care Med. 1996;154:300–307. doi: 10.1164/ajrccm.154.2.8756798. [DOI] [PubMed] [Google Scholar]
- Saito H, Shimizu H, Akiyama K. Autocrine regulation of eotaxin in normal human bronchial epithelial cells. Int Arch Allergy Immunol. 2000;122(Suppl 1):50–53. doi: 10.1159/000053633. [DOI] [PubMed] [Google Scholar]
- Salam MT, Islam T, Gauderman WJ, Gilliland FD. Roles of arginase variants, atopy, and ozone in childhood asthma. J Allergy Clin Immunol. 2009;123:596–602. 602 e591–598. doi: 10.1016/j.jaci.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, Wong VJ, Joad JP, Pinkerton KB, Wu R, Evans MJ, Hyde DM, Plopper CG. Allergic Asthma Induced in Rhesus Monkeys by House Dust Mite (Dermatophadoides farinae) Am J Pathol. 2001;158:333–341. doi: 10.1016/S0002-9440(10)63973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, Baker GL, Pantle LM, Joad JP, Pinkerton KE, Wu R, Evans MJ, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol. 2003;191:74–85. doi: 10.1016/s0041-008x(03)00218-7. [DOI] [PubMed] [Google Scholar]
- Sorkness RL, Herricks KM, Szakaly RJ, Lemanske RF, Jr, Rosenthal LA. Altered allergen-induced eosinophil trafficking and physiological dysfunction in airways with preexisting virus-induced injury. Am J Physiol Lung Cell Mol Physiol. 2007;292:L85–91. doi: 10.1152/ajplung.00234.2006. [DOI] [PubMed] [Google Scholar]
- Spektor DM, Lippmann M, Lioy PJ, Thurston GD, Citak K, James DJ, Bock N, Speizer FE, Hayes C. Effects of ambient ozone on respiratory function in active, normal children. Am Rev Respir Dis. 1988;137:313–320. doi: 10.1164/ajrccm/137.2.313. [DOI] [PubMed] [Google Scholar]
- Spektor DM, Thurston GD, Mao J, He D, Hayes C, Lippmann M. Effects of single- and multiday ozone exposures on respiratory function in active normal children. Environ Res. 1991;55:107–122. doi: 10.1016/s0013-9351(05)80167-7. [DOI] [PubMed] [Google Scholar]
- Stellato C, Collins P, Ponath PD, Soler D, Newman W, La Rosa G, Li H, White J, Schwiebert LM, Bickel C, Liu M, Bochner BS, Williams T, Schleimer RP. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J Clin Invest. 1997;99:926–936. doi: 10.1172/JCI119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, Ambati J. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triche EW, Gent JF, Holford TR, Belanger K, Bracken MB, Beckett WS, Naeher L, McSharry JE, Leaderer BP. Low-level ozone exposure and respiratory symptoms in infants. Environ Health Perspect. 2006;114:911–916. doi: 10.1289/ehp.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wetering S, Zuyderduyn S, Ninaber DK, van Sterkenburg MA, Rabe KF, Hiemstra PS. Epithelial differentiation is a determinant in the production of eotaxin-2 and -3 by bronchial epithelial cells in response to IL-4 and IL-13. Mol Immunol. 2007;44:803–811. doi: 10.1016/j.molimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Van Winkle LS, Baker GL, Chan JK, Schelegle ES, Plopper CG. Airway mast cells in a rhesus model of childhood allergic airways disease. Toxicological sciences: an official journal of the Society of Toxicology. 2010;116:313–322. doi: 10.1093/toxsci/kfq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, Mackay CR, Daugherty BL, Springer MS, Durham SR, Williams TJ, Kay AB. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–3516. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- Zhang L, Soares MP, Guan Y, Matheravidathu S, Wnek R, Johnson KE, Meisher A, Iliff SA, Mudgett JS, Springer MS, Daugherty BL. Functional expression and characterization of macaque C-C chemokine receptor 3 (CCR3) and generation of potent antagonistic anti-macaque CCR3 monoclonal antibodies. J Biol Chem. 2002;277:33799–33810. doi: 10.1074/jbc.M205488200. [DOI] [PubMed] [Google Scholar]