Abstract
Background and Purpose
The reduced incidence, morbidity and mortality of stroke among humans with strong social support have been well-documented; however, the mechanisms underlying this socially mediated phenomenon remain unknown, but may involve oxytocin (OT), a hormone that modulates some aspects of social behavior in humans and other animals.
Methods
In the present study, adult male mice were socially isolated (housed individually) or socially paired (housed with an ovariectomized female); social pairing increased hypothalamic OT gene expression. To determine whether a causal relationship exists between increased OT and improved stroke outcome, mice were treated with exogenous OT or OT receptor antagonist (OTA) beginning one week prior to induction of experimental stroke via middle cerebral artery occlusion (MCAO).
Results
Relative to social isolation, social housing attenuated infarct size, neuroinflammation, and oxidative stress following experimental stroke; the neuroprotective effect of social housing was eliminated by OTA treatment. In contrast, administration of OT to socially isolated mice reproduced the neuroprotection conferred by social housing. We further report evidence for a direct suppressive action of OT on cultured microglia, which is a key instigator in the development of neuroinflammation after cerebral ischemia.
Conclusions
These findings support the hypothesis that OT mediates the neuroprotective effect of social interaction on stroke outcome.
Keywords: Oxytocin, cerebral infarct, social interaction, neuroinflammation, oxidative stress
Social factors have a profound influence on disease outcome1. The diverse negative health effects of social isolation and lack of social support have been reported for rheumatoid arthritis2, renal disease3, and cancer4. The influence of social interactions on disease outcome is particularly well-described in the context of vascular disease, including cerebrovascular and cardiovascular disorders5; an effect that has been replicated in animal models of global6 and focal cerebral ischemia7, 8. Social interaction reduces neuronal damage caused by cerebral ischemia among both male and female mice, regardless of the sex and number of other mice in the cage 6–8. Furthermore, physical contact is an important component of the neuroprotective effect of social interaction 9. One likely mediator of psychosocial influences on disease outcomes is oxytocin (OT), a nonapeptide produced in the paraventricular and supraoptic nuclei of the hypothalamus, and released during physical contact. OT is both induced by and facilitates social behaviors10; exogenous administration of OT potentiates social behaviors11 and central blockade of OT signaling disrupts social memory, parental behaviors, and pair-bonding12. Indeed, exogenous OT protects against several of the physiological and behavioral consequences of social isolation, including autonomic dysfunction and depression13, stress-induced HPA axis activation and anxiety-like behavior14.
In addition to social buffering, recent data indicate that OT also may have anti-inflammatory and anti-oxidant properties. Exogenous OT administration alleviates tissue damage in a variety of animal models of injury15–18. Further, co-administration of an oxytocin receptor antagonist (OTA) blocks the cardioprotective effects of OT during cardiac ischemia in rats16. The protective actions of OT in these models may be associated with decreased levels of circulating pro-inflammatory cytokines15, 19 and decreased neutrophil infiltration to the site of injury15, 17, 19.
Taken together, the role of endogenous OT as a mediator of social behaviors and the protective role of OT in the pathophysiology of several diseases makes it a compelling candidate as the mediator of social influences on disease outcome. To our knowledge, no studies have directly assessed the role of OT in mediating social influences on any of the major causes of human morbidity and mortality. The present study was designed to investigate the effects of pharmacological manipulation of OT in mediating the influences of social interaction on stroke outcome. Specifically, we examined the effects of OT treatment and social housing conditions on histological, neuroendocrine, inflammatory and antioxidant measures following cerebral ischemia.
Methods and Materials
All procedures were approved by the Ohio State University and were conducted in accordance with NIH guidelines for the care and use of animals and under protocols approved by the institutional animal care and use committee. The experiments were conducted in accordance with recommendations from the Stroke Therapy Academic Industry Roundtable (STAIR20; specific details provided in supplemental material)
In brief, adult male C57/BL6 mice (23–30g; Charles River, Wilmington, Mass) were randomly assigned to an experimental groups and housed either individually (socially isolated) or with an ovariectomized female (pair housed) for a period of 1 week prior to surgery and throughout the reperfusion period. Alzet minipumps (Model 1002, Durect, Cupertino, CA) were connected via tubing to an ICV cannula (2.75mm projection, Plastics One, Roanoke VA) implanted into left lateral ventricle for constant infusion of OT, OTA or the vehicle (aCSF). Mice underwent MCAO or SHAM surgery; brains and sera were harvested 24 or 72 hours after reperfusion. Tissue samples were assessed for damage and glial activation using standard histological and gene expression assays; the individual collecting the data and performing the assays was not informed of group assignment. Oxidative stress and antioxidant enzyme activity were assessed in serum using commercial GPx (Calbiochem, San Diego, CA) and GSH/GSSG (Oxford Biomedical Research, Oxford, MI) kits.
Whole brain homogenates were harvested from additional cohorts of paired and isolated mice that did not undergo MCAO. In order to determine cell type-specific patterns of OTR expression, isolated cells were incubated with antibodies to CD11b, NeuN, GFAP and OTR. Finally, to confirm an anti-inflammatory role of microglial OTRs, microglial cultures were prepared from brain tissue collected from socially isolated mice. Following incubation with LPS and OT (or aCSF), microglial activation was determined using MHC class II expression. Details regarding the experimental animals and procedures used as well as data analysis are provided in SI Materials and Methods.
Results
Social housing condition and OT influence infarct size
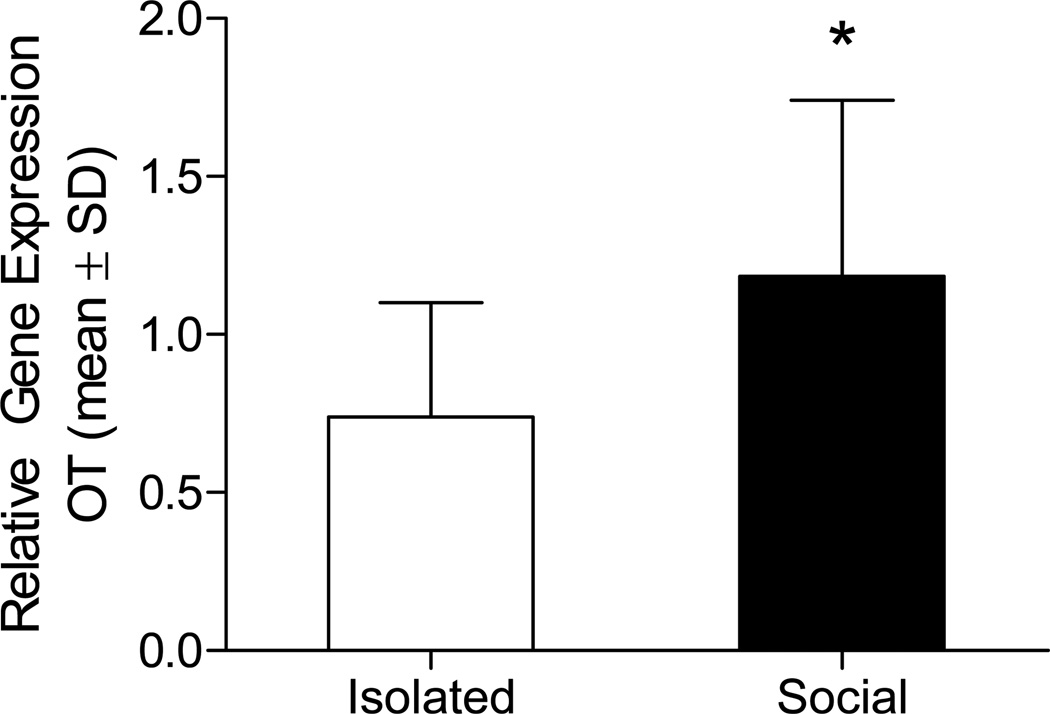
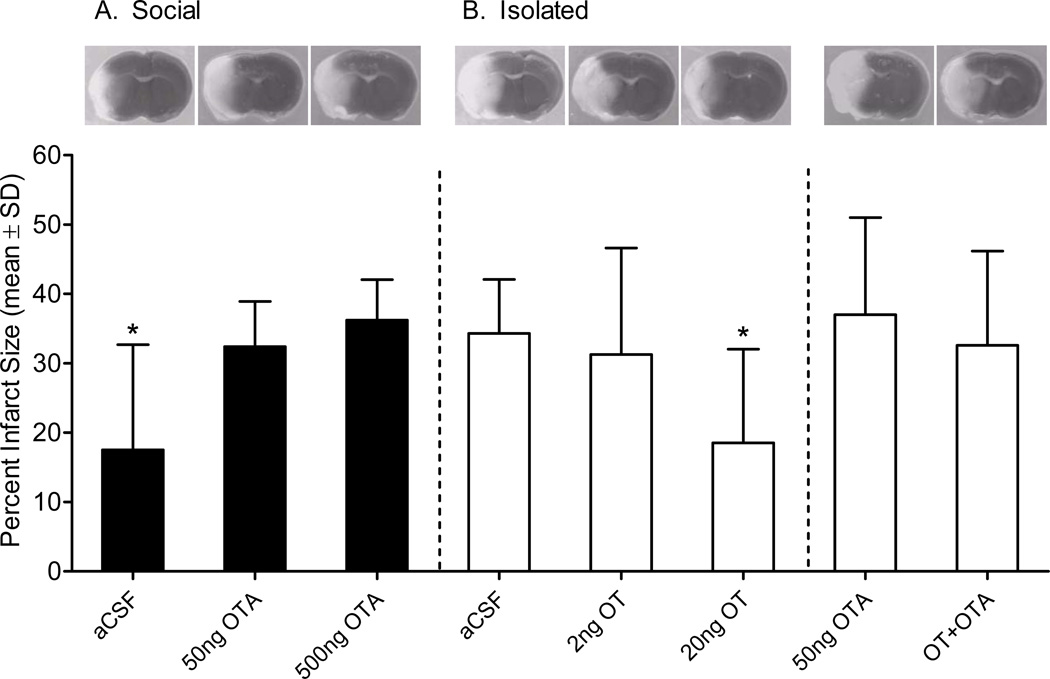
To examine a modulatory role of OT in this model, mice were socially housed or isolated and hypothalami were extracted 1 week later. RT-PCR confirmed an increase in OT mRNA in socially housed mice (t22 = 2.230, p = 0.036; Figure 1). To determine whether housing-induced increase in OT gene expression contributes to ischemic outcome, socially housed or isolated mice underwent middle cerebral artery occlusion (MCAO) or SHAM surgery, and were assessed for neuronal damage (infarct size) after 72 hours of reperfusion. Housing condition significantly influenced infarct size (Figure 2). Among vehicle treated (artificial cerebrospinal fluid; aCSF) groups, social housing decreased infarct size relative to social isolation (F1,14 = 7.598, P = 0.016). To determine whether the neuroprotective effect of social housing is mediated by endogenous OT, socially housed mice were treated with OTA. Daily central treatment of socially housed mice with either 50ng or 500ng of OTA increased infarct size (F1,20= 13.914, P = 0.001) relative to aCSF. Similarly, daily OT treatment of socially isolated mice dose-dependently reduced infarct size relative to aCSF-treated mice (F4,30 = 3.417, P = 0.020). The high dose (20ng/day) but not the low dose treatment (2ng/day) reduced infarct size relative to aCSF among socially isolated mice (P = 0.045). Moreover, co-infusion of OTA with the effective OT dose eliminated the neuroprotection conferred by OT treatment (P = 0.999 relative to aCSF), indicating a receptor mediated effect of OT treatment. Importantly, treatment of isolated mice with OTA alone did not significantly alter infarct volume relative to aCSF (P = 0.996), indicating that OTA is not neurotoxic.
Figure 1. Oxytocin mRNA gene expression in paired and isolated mice.
Oxytocin mRNA is elevated following 1 week of pair housing relative to social isolation (n = 12/group). * statistically different from socially isolated mice (P < 0.05).
Figure 2. Social housing condition and oxytocin influence infarct size.
Social housing reduces infarct size relative to isolation (aCSF: social n = 8, isolated n =8); (A) however, daily treatment of socially housed mice with OTA (50ng n = 8 and 500ng n = 9) eliminates the neuroprotective effect of social housing on infarct size. (B) Daily treatment of socially isolated mice with 20ng (n = 11) OT (but not 2ng, n = 8) reduces infarct size. OTA infusion alone (n = 6) or with OT (n = 6) does not affect infarct size. Representative TTC photomicrographs are shown above each group. * Statistically different from the socially isolated aCSF-treated mice (P < 0.05).
Because stroke is itself a potent stressor, and social isolation exacerbates stress-induced glucocorticoid release21, circulating corticosterone concentrations were assessed in all drug and housing conditions. OT treatment of isolated mice did not reduce circulating corticosterone relative to aCSF, indicating that the neuroprotective effects of the high dose of OT is likely independent of circulating glucocorticoids. (SI Results and SI Table 1).
Social housing and OT influence neuroinflammation
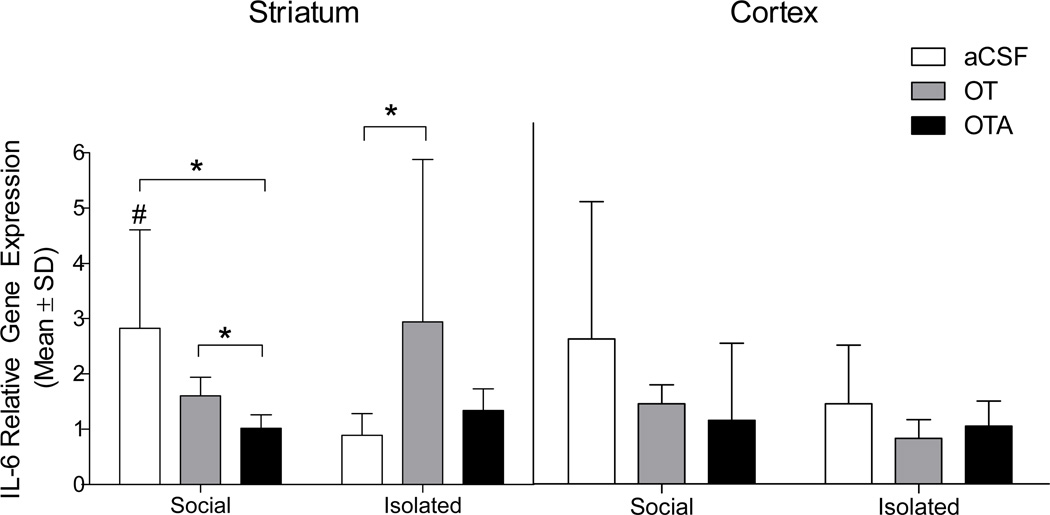
Focal cerebral ischemia triggers a marked neuroinflammatory response, particularly in the cortical and striatal regions of the ischemic hemisphere. Central interleukin-6 (IL-6) is neuroprotective in ischemia and we recently reported a role for IL-6 as a mediator of the neuroprotection conferred by social housing. Among aCSF-treated groups, pair housing increased striatal IL-6 mRNA (U = 6.0, P = 0.032) relative to social isolation, reaffirming the relationship between central IL-6 and neuroprotection after stroke22. In keeping with this pattern, IL-6 mRNA expression was reduced in socially housed mice treated with OTA (U = 3.0, P = 0.05). OT was administered to socially housed mice, however, while OT treatment resulted in increased expression of IL-6 mRNA relative to OTA treatment (U = 1.0, P = 0.014), we did not observe an additive effect of social housing and OT treatment. On the other hand, treatment of socially isolated mice with OT increased IL-6 mRNA expression relative to aCSF (U = 7.0, P = 0.05, Figure 3). Further, OTA administration to socially isolated mice did not alter IL-6 mRNA expression relative to aCSF treatment, indicating 1) that endogenous OT signaling is low in isolated mice and not further antagonized with the 50ng dose of OTA, and 2) central administration of this dose of OTA does not independently influence the neuroinflammatory response to cerebral ischemia. Additional PCR and histological gliotic data are included in supplemental materials and figures.
Figure 3. Relative gene expression of interleukin-6 following MCAO.
Striatal IL-6 mRNA is elevated in socially housed (aCSF n = 6) and OT-treated mice (n = 7) relative to social isolation (aCSF n = 7). OT treatment increased IL-6 expression relative to OTA in socially housed mice, while OTA treatment did not influence IL-6 expression in isolated mice. * indicates a statistically significant difference between indicated groups (P < 0.05). Data are expressed as a ratio of ischemic to non-ischemic hemisphere. # significantly different from socially isolated aCSF-treated mice (P < 0.05).
Post-MCAO antioxidant capacity and oxidative stress
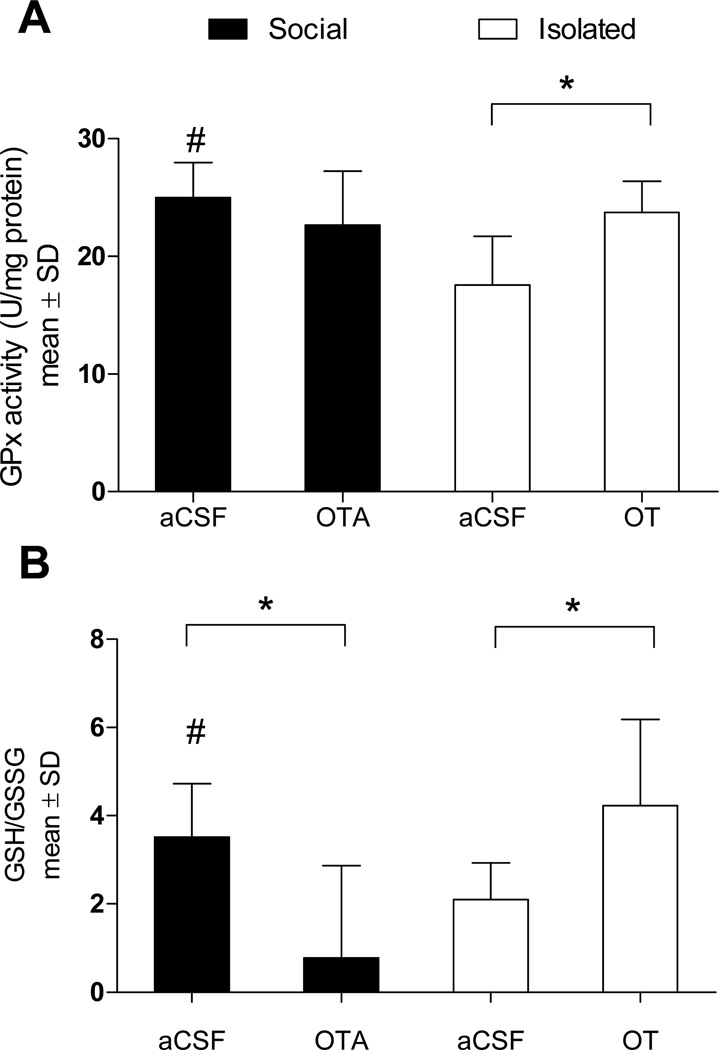
Several studies have established that OT has antioxidant properties; OT scavenges peroxinitrite, inhibits lipid peroxidation, and attenuates NADPH-dependent superoxide activity23–25. Brain antioxidant levels (glutathione peroxidase; GPx) were increased in socially housed (F1,13 = 7.816, P = 0.016) and OT-treated mice (F1,12 = 9.949, P = 0.009) relative to aCSF-treated socially isolated mice (Figure 4A). Oxidative stress was attenuated by social housing (F1,11 = 4.660, P = 0.05) as well as OT treatment (F1,11 = 5.066, P = 0.048) relative to aCSF-treated isolated mice. Among socially housed mice, OTA treatment significantly increased oxidative stress (F1,15 = 4.722, P = 0.045; Figure 4B). Thus, modulation of post-ischemic oxidative stress is a second mechanism through which OT may be mediating social neuroprotection.
Figure 4. Antioxidant enzyme activity and oxidative stress levels.
(A) GPx activity is increased in OT-treated (n = 6) and socially housed (n = 6) mice relative to social isolation (n = 7). (B) Likewise, relative to social isolation, oxidative stress is reduced following OT treatment or social housing, but is significantly elevated in socially housed mice following OTA treatment (n = 7). * Statistically significant difference between indicated groups (P < 0.05). # indicates a statistically significant difference from socially isolated aCSF-treated mice (P < 0.05).
Evidence for glial and neuronal oxytocin receptor expression
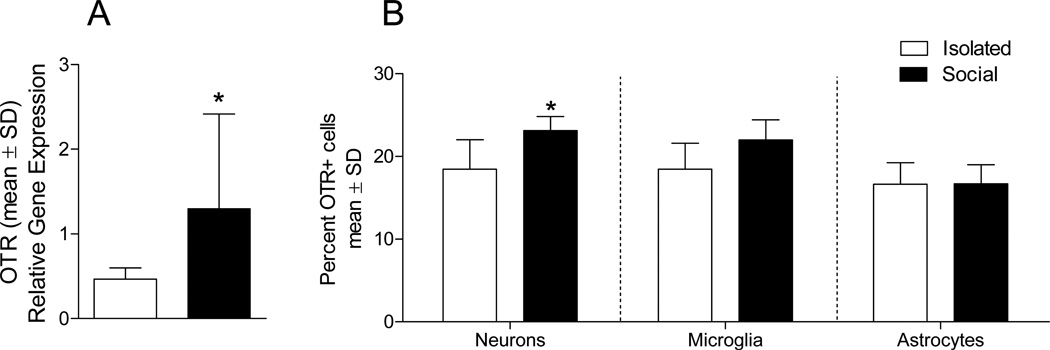
The ability of OT and social interaction to modulate the pathophysiological response to cerebral ischemia likely indicates 1) endogenous changes in OTR expression as a result of social experiences and 2) the presence of OTR on cell populations that mediate neuroinflammation. OTR mRNA and protein expression was assessed on neuronal and glial cell populations of socially housed and isolated mice using RT-PCR and flow cytometry, respectively. As previously reported, both neuronal (NeuN-positive) and astroglial (GFAP-positive) cells express OTR26. Social housing increased neuronal OTR mRNA (t19 = 2.047, P = 0.05) (Figure 5A) and protein (t9 = 2.200, P = 0.05) (Figure 5B) expression relative to isolation. Additionally, approximately 16% of CD11b-positive cells express OTR, regardless of housing condition. (Figure 5B).
Figure 5. Oxytocin receptor mRNA and gene expression.
(A) Expression of OTR mRNA in enriched neurons is increased in socially housed (n = 12) relative to isolated (n = 9) mice. (B) Percent OTR expression in socially housed and isolated mice is shown on neuronal (NeuNpositive), astroglial (GFAP-positive) and microglial (CD11b-positive) cell populations assessed using flow cytometry, n = 5–6 per group. * indicates a statistically significant social condition difference, (P > 0.05).
OT modulates microglial reactivity
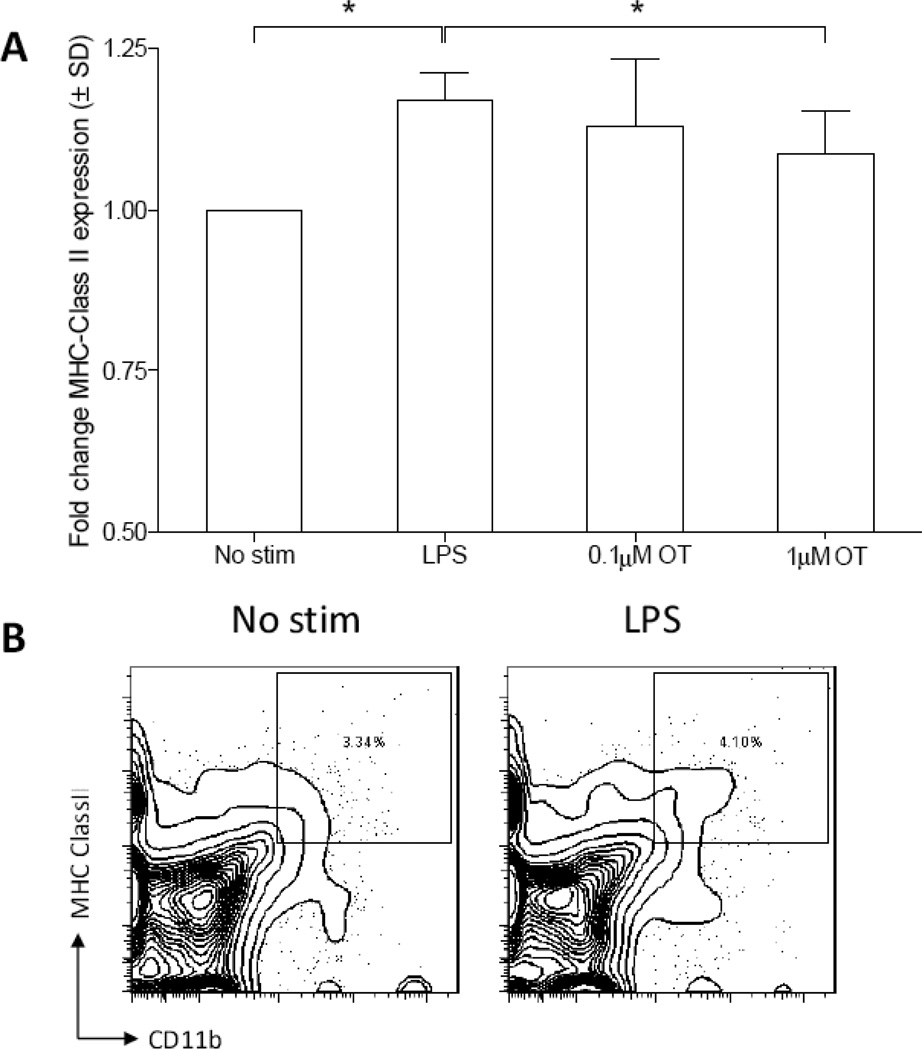
In order to determine whether microglial OTR play a regulatory role during microglial activation, enriched microglia were incubated with or without OT (0.1 or 1 µM), then stimulated with 1µg/mL of lipopolysaccharide (LPS). Activated microglia act as antigen-presenting cells and up-regulate major histocompatibility complex class II (MHC Class II) expression, thus MHC Class II expression was used as a measure of microglial reactivity. As expected, primary microglial cultures stimulated with LPS increased expression of MHC Class II relative to the non-stimulated control (Figure 6; t12 = 9.715,P = 0.0001). OT dose-dependently attenuated LPS-stimulated MHC Class II expression relative to LPS alone (t12 = 2.578, P = 0.024). Taken together, these in vitro data indicate the potential for a direct role of OT in inhibiting microglial reactivity.
Figure 6. Oxytocin suppresses microglial reactivity in vitro.
(A) Microglial MHC class II expression is up-regulated following a 24 hour LPS challenge (1µg/mL). Pre-incubation with the high dose (1 µM) but not the low dose (0.1 µM) OT attenuates LPS-stimulated MHC class II expression, however this effect is blocked by co-incubation with OTA (1 µM), n = 7 per treatment condition. (B) MHC Class II expression was measured on total CD11b+ cells as gated above. Graph reflects fold change of LPS alone or LPS+OT stimulation. * indicates a statistically significant difference between indicated groups, (P > 0.05).
Discussion
The influence of social interaction on disease outcome suggests an endogenous signaling pathway links the psychosocial environment to disease pathophysiology. The data presented here suggest that OT is a mediator of social interaction-induced neuroprotection. The present findings indicate that relative to social isolation, either social interaction or chronic central administration of OT leads to a reduction in ischemic damage, as well as measures of neuroinflammation and oxidative stress. A role for OT is supported by the findings that 1) chronic OTR antagonism during social housing blocks the protection conferred by social interaction, 2) OT and OTR expression are altered in healthy animals following 1 week of social interaction, and 3) OT exerts a direct anti-inflammatory effect on cultured microglia.
Data in the current study indicate an increase in brain IL-6 mRNA expression in socially housed and OT-treated mice. Further, serum concentrations of IL-6 in the same cohort of mice were reduced in OT-treated mice (SI Results and SI Table 1). This is consistent with an anti-inflammatory IL-6 profile, as central IL-6 is neuroprotective in ischemia22; however, contrary to its central actions, peripheral IL-6 promotes acute phase protein induction and is thus indicative of a pro-inflammatory state27. While these data may reflect a reduction in neuronal injury in the socially housed or OT-treated mice, we have recently reported that a single treatment with an IL-6 neutralizing antibody prior to ischemia both increases infarct size and eliminates the social buffering effect8. There also is a direct reciprocal relationship between OT and cytokine signaling both in vivo and in vitro15, 17, 19, 24, 28. The OTR gene contains response elements for inflammatory mediators including nuclear factor-IL-6, as well as nuclear factor-κB, acute phase response factor, and binding sites for activator protein-128, 29. Up-regulation of proinflammatory cytokines such as IL-6 or interleukin-1β modulates both OT and OTR gene transcription28, 30. Additionally, OT attenuates LPS-stimulated IL-6 secretion from cultured peripheral macrophages and endothelial cells24.
Further support for a potent anti-inflammatory component of OT-mediated neuroprotection comes from the discovery that OT attenuates gliosis in vivo and microglial activation in vitro. In the current study, social housing and OT treatment attenuated CD11b and GFAP expression in vivo, while OTA treatment increased CD11b expression similar to levels observed in socially isolated mice (SI Figure 1). CD11b-positive microglia contribute substantially to ischemic injury, treatment that inhibits microgliosis reduces infarct size and attenuates pro-inflammatory cytokine production31. The identification of OTR expression on CD11b-positive cells in the brain provides a mechanistic link that may further explain attenuation of neuroinflammation by OT. To determine whether OT could be acting directly on microglia via the OTR receptor, microglia were enriched and incubated in the presence of OT; Indeed, OT attenuated LPS-induced MHC Class II expression in cultured microglia. These data re consistent with a recent publication demonstrating that OT reduces LPS-stimulated IL-6 and superoxide production in cultured macrophages and endothelial cells24. Given the role of microglia in the production of pro-inflammatory cytokines and reactive oxygen species following cerebral ischemia, these data indicate that the anti-oxidant and anti-inflammatory effects of OT may be driven by OTR signaling directly on resident microglia and invading macrophages.
Oxidative stress, marked by production of free radicals, antioxidant depletion and lipid peroxidation occurs rapidly after the onset of ischemia and is a detrimental consequence of the return of blood flow to the affected brain area during reperfusion32. In the current study, both social housing and exogenous OT treatment increased antioxidant activity (GPx) and attenuated oxidative stress. It remains to be determined whether the reduction in oxidative stress in the current study is a function of reduced neuronal damage in OT-treated and socially housed mice, or whether it is a contributing factor to the developing ischemic injury. However, OT has been shown to act as a free radical scavenger and to reduce lipid peroxidation23, 24. Further, the anti-oxidant capacity of OT has also been reported in models of renal and hepatic ischemia/reperfusion injury15, 19. Importantly, the reduction of oxidative damage in these studies was accompanied by an attenuation of pro-inflammatory cytokines15, 19. Thus, it is likely that the neuroprotective and anti-inflammatory effects of OT in cerebral ischemia are in part mediated by its antioxidant properties.
Taken together, these data indicate a regulatory role for OT as the mechanism by which social interaction influences ischemia pathophysiology. Of note, OT is part of a tightly regulated neuro-endocrine system that is both affected by and can exert influence on cerebral ischemia outcome. For example, OT regulates (and is regulated by) hypothalamo-pituitary-adrenal (HPA) axis activity33. Indeed, OT is released in response to physical and psychological stressors, and evokes a potent anxiolytic effect 34. Given the highly stressful nature of cerebral ischemia 21, HPA axis hormones (i.e. CORT, adrenal corticotropin hormone and corticotropin releasing factor) cannot be discounted as playing a potentially critical role in modulating the effects of OT on measures of ischemia outcome. Further, hypothalamic release of OT is typically concurrent with release of the structurally related neuropeptide vasopressin (AVP)35. AVP is a potent vasodilator and regulator of brain osmolarity, and thus plays a pivotal role in cerebral ischemia outcome36. Importantly, OTRs have a low affinity for AVP37 indicating this neuropeptide as another potential contributing factor to social neuroprotection during cerebral ischemia.
Overall, the results from this study support the growing body of evidence that social interaction and support improve pathological and functional measures of stroke outcome. On nearly all measures assessed in the current study, the neuroprotection conferred by social housing can be mimicked in socially isolated mice via chronic central infusion of OT. These data further extend recent research on the neuroinflammatory and antioxidant properties of OT and emphasize that OT is uniquely suited to integrate psychosocial stimuli with pathophysiological responses to tissue injury. Taken together, these data support a role for OT as a mediating factor of social modulation of health outcomes.
Supplementary Material
Acknowledgments
We thank Megan Cochran for technical support, Zach Weil for critiquing this manuscript, and Dr. Maurice Manning for his generous contribution of the selective oxytocin antagonist used in these experiments.
Sources of Funding
This work was supported by grants from the American Heart Association (EIA to A.C.D. and predoctoral fellowship to K.K.), NINDS Core Grant P30 NS045758 (to A.C.D.), NINDS RO1NS40267–05 (to A.C.D.), and NHLBI RO1HL080249–01 (to A.C.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicting financial interests.
References
- 1.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 2.Strating MMH, Suurmeijer TPBM, Van Schuur WH. Disability, social support, and distress in rheumatoid arthritis: Results from a thirteen year prospective study. Arthritis Care & Research. 2006;55:736–744. doi: 10.1002/art.22231. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SD, Sharma T, Acquaviva K, Peterson RA, Patel SS, Kimmel PL. Social support and chronic kidney disease: An update. Advances in chronic kidney disease. 2007;14:335–344. doi: 10.1053/j.ackd.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel D, Sephton SE. Psychoneuroimmune and endocrine pathways in cancer: Effects of stress and support. Seminars in clinical neuropsychiatry. 2001;6:252–265. doi: 10.1053/scnp.2001.26995. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda A, Iso H, Kawachi I, Yamagishi K, Inoue M, Tsugane S. Social support and stroke and coronary heart disease: The jphc study cohorts ii. Stroke. 2008;39:768. doi: 10.1161/STROKEAHA.107.496695. [DOI] [PubMed] [Google Scholar]
- 6.Weil Z, Norman G, Barker J, Su A, Nelson R, DeVries A. Social isolation potentiates cell death and inflammatory responses after global ischemia. Molecular psychiatry. 2008;13:913–915. doi: 10.1038/mp.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craft TKS, Glasper ER, McCullough L, Zhang N, Sugo N, Otsuka T, et al. Social interaction improves experimental stroke outcome. Stroke. 2005;36 doi: 10.1161/01.STR.0000177538.17687.54. 2006. [DOI] [PubMed] [Google Scholar]
- 8.Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proceedings of the National Academy of Sciences. 2009;106:5895. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karelina K, Norman GJ, Zhang N, DeVries AC. Social contact influences histological and behavioral outcomes following cerebral ischemia. Exp Neurol. 2009;220:276–282. doi: 10.1016/j.expneurol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Uvnas-Moberg K. Physiological and endocrine effects of social contact. The integrative neurobiology of affiliation. 1997;807:146–163. doi: 10.1111/j.1749-6632.1997.tb51917.x. [DOI] [PubMed] [Google Scholar]
- 11.Carter CS. Developmental consequences of oxytocin. Physiology & behavior. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 12.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in neuroendocrinology. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grippo AJ, Trahanas DM, Zimmerman I, Robert R, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windle R, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 15.Düsünceli F, Iseri SÖ, Ercan F, Gedik N, Yegen C, Yegen BÇ. Oxytocin alleviates hepatic ischemia-reperfusion injury in rats. Peptides. 2008;29:1216–1222. doi: 10.1016/j.peptides.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Houshmand F, Faghihi M, Zahediasl S. Biphasic protective effect of oxytocin on cardiac ischemia/reperfusion injury in anaesthetized rats. Peptides. 2009;30:2301–2308. doi: 10.1016/j.peptides.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Iseri SÖ, Sener G, Sag lam B, Gedik N, Ercan F, Yeg en BÇ. Oxytocin protects against sepsis-induced multiple organ damage: Role of neutrophils. Journal of Surgical Research. 2005;126:73–81. doi: 10.1016/j.jss.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Ondrejcakova M, Ravingerova T, Bakos J, Pancza D, Jezova D. Oxytocin exerts protective effects on in vitro myocardial injury induced by ischemia and reperfusion. Canadian journal of physiology and pharmacology. 2009;87:137–142. doi: 10.1139/Y08-108. [DOI] [PubMed] [Google Scholar]
- 19.Tugtepe H, Sener G, Biyikli NK, Yuksel M, Cetinel S, Gedik N, et al. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regulatory peptides. 2007;140:101–108. doi: 10.1016/j.regpep.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK. 2006 curt p. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. Journal of Cerebral Blood Flow & Metabolism. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Moosmann B, Behl C. Secretory peptide hormones are biochemical antioxidants: Structure-activity relationship. Molecular pharmacology. 2002;61:260. doi: 10.1124/mol.61.2.260. [DOI] [PubMed] [Google Scholar]
- 24.Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, et al. Oxytocin attenuates nadph-dependent superoxide activity and il-6 secretion in macrophages and vascular cells. American Journal of Physiology-Endocrinology And Metabolism. 2008;295:E1495. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides. 2005;26:483–491. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Wang YF, Hatton GI. Mechanisms underlying oxytocin-induced excitation of supraoptic neurons: Prostaglandin mediation of actin polymerization. Journal of neurophysiology. 2006;95:3933. doi: 10.1152/jn.01267.2005. [DOI] [PubMed] [Google Scholar]
- 27.Smith CJ, Emsley HCA, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC neurology. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid B, Wong S, Mitchell B. Transcriptional regulation of oxytocin receptor by interleukin-1 {beta} and interleukin-6. Endocrinology. 2001;142:1380. doi: 10.1210/endo.142.4.8107. [DOI] [PubMed] [Google Scholar]
- 29.Murasawa S, Matsubara H, Kijima K, Maruyama K, Mori Y, Inada M. Structure of the rat v1a vasopressin receptor gene and characterization of its promoter region and complete cdna sequence of the 3 -end. Journal of Biological Chemistry. 1995;270:20042. doi: 10.1074/jbc.270.34.20042. [DOI] [PubMed] [Google Scholar]
- 30.Rauk PN, Friebe-Hoffmann U. Interleukin 1 down regulates the oxytocin receptor in cultured uterine smooth muscle cells. American Journal of Reproductive Immunology. 2000;43:85–91. doi: 10.1111/j.8755-8920.2000.430204.x. [DOI] [PubMed] [Google Scholar]
- 31.Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13496. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner DS, Sheng H, Batini -Haberle I. Oxidants, antioxidants and the ischemic brain. Journal of experimental biology. 2004;207:3221. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 33.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mrna expression in specific forebrain regions associated with modulation of hypothalamo–pituitary–adrenal activity. The Journal of neuroscience. 2004;24:2974. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann ID. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo-pituitary-adrenal axis. Progress in Brain Research. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 35.Neumann I, Ludwig M, Engelmann M, Pittman Q, Landgraf R. Simultaneous microdialysis in blood and brain: Oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- 36.Kozniewska E, Romaniuk K. Vasopressin in vascular regulation and water homeostasis in the brain. J Physiol Pharmacol. 2008;59:109–116. [PubMed] [Google Scholar]
- 37.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81:629. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.