Abstract
We have previously reported that transplantation (Tx) of prevascularized donor islets as composite Islet-Kidneys (IK) reverses diabetic hyperglycemia in miniature swine. In order to test the potential clinical applicability of this strategy, we have extended it to a fully allogeneic non-human primate model. IKs were prepared in baboons by isolating islets from 50–70% partial pancreatectomies and injecting them under the autologous renal capsule, allowing vascularization before allogeneic Tx. Baboons with diabetes induced by stereptozotocin or total pancreatectomy, received composite I-Ks (n=3) or free islets under the renal capsule or intraportally (n=3), across fully allogeneic barriers with an immunosuppressive regimen consisting of ATG followed by MMF and tacrolimus. FBS of two of IK recipients decreased immediately after Tx and no insulin therapy was required throughout the experimental period (225 and 301 days). In contrast, all recipients of allogeneic free islets showed unstable FBS levels and required insulin within 2 months. We conclude that in addition to providing life-supporting renal function, fully allogeneic IKs from single primate donors can achieve glucose regulation without insulin therapy, while free islets do not. These results support the feasibility of composite allogeneic IK Tx as a potential cure for end-stage diabetic nephropathy.
Keywords: non-human primate, islet transplantation, kidney allograft, vascularization
Introduction
Islet cell transplants can potentially provide a cure for diabetes and have considerable appeal because of their relative safety in comparison to whole pancreas transplantation. Allogeneic islet transplants have successfully achieved normoglycemia in diabetic rodent models (1,2). Attempts to extend these results to primates, however, have met with limited success, and the results of clinical whole organ pancreas transplantation in correcting the hyperglycemia of diabetes have been superior to those of islet transplantation (3–5). In 2000, the “Edmonton Protocol” demonstrated significant increases in survival and function of islet transplants at one year in 4 of 12 patients; however, by 5 years, in a much larger series, only 10% of patients remained insulin independent (6,7). In addition to poorer function and graft survival, islet transplantation protocols require large numbers of islet equivalents (IE)/kg which often necessitates the use of 2 and sometimes 3 pancreata for a single recipient. More recently, the Minnesota group presented data showing an improvement in islet survival following T-cell depletion. In addition, they showed an improved rate of insulin independence (Hering B et al. XXIII International Congress of TTS), but graft outcome remained approximately 50% which is inferior to the results of other life-supporting organ transplantation such as kidneys or livers.
Experimental and clinical observations support the generalization that vascularized organs have prolonged graft function over non-vascularized grafts. We have reasoned that this survival advantage might be extended to islets by transplanting them as part of a composite vascularized graft. On this basis, we have developed the techniques required for constructing composite “islet-kidneys” (IK) by transplantation of autologous islets under the kidney capsule of the prospective donor 2–3 months prior to transplantation of the composite organ into pancreatectomized miniature swine (8,9). Allogeneic IK transplantation in MGH miniature swine, under conditions which allowed survival of the kidney graft, corrected blood sugar levels immediately in totally pancreatectomized recipient swine (9).
Although miniature swine have proven to be an excellent large-animal pre-clinical model with respect to many parameters of transplantation biology (10,11), studies in nonhuman primate models are necessary to demonstrate clinical applicability. Therefore, the aim of this study is to determine whether: 1) functional IKs can be prepared in baboons as was previously demonstrated in MGH miniature swine; 2) the autologous baboon IK is functional and capable of controlling blood sugar in the donor animal prior to allogeneic IK transplantation; and 3) the IK strategy is capable of correcting surgically or chemically-induced diabetes across an allogeneic barrier in baboons
Materials and Methods
(A) Animals
Male and female Hamadryas baboons were purchased from Mannheimer Foundation, Homestead, FL. MHC mismatching was assured by CML responses for MHC class-I responses and MLR for MHC class-II disparity.
(B) Surgeries and immunologic and histologic examination
(1) Living donor partial pancreatectomy for isolation of islets for isle-kidney (IK) preparation
Details are described in Supplemental Materials & Methods (online).
(2) Islet Preparation
Pancreata were infused with Liberase HI and subjected to digestion. The digested tissue was filtered through a mesh screen, applied to a discontinuous gradient and centrifuged for the purification. Islets were collected, washed and transplanted.
(3) Preparation of IK
IK preparation was performed in a similar manner to that used for MGH miniature swine (Yamada IK procedure (8)). Details are described in Supplemental Materials & Methods.
(4) Induction of diabetic hyperglycemia using streptozotocin
Recipient animals were given bolus doses of intravenous stereptozotocin (STZ) at 2000 mg/m^2 body surface area (almost equivalent to 130mg/kg) which is a substantially higher dose than that used for cynomologous monkeys (12).
(5) Induction of diabetic hyperglycemia using a total pancreatectomy or residual pancreatomy
When 2 doses of STZ failed to induce insulin dependent diabetes (IDDM), we removed the pancreas using a pancreaticoduodenectomy. The distal pancreas, to the level of the SMV was dissected free from its posterior attachments. Details are described in Supplemental Materials & Methods.
To assess function of autologous IKs, a residual pancreatectmy was performed in a procedure similar to the total pancreatectomy described above, however the distal pancreatectomy was not required, because it had already been removed.
(6) Transplantation of IKs across allogeneic barrier
Composite IKs were transplanted in a manner similar to living-related pediatric renal transplantation (9,13). Details are described in Supplemental Materials & Methods.
(7) Direct allogeneic transplantation in recipients
Donor islets were injected underneath the renal capsule as described above. Two recipients underwent allogeneic islet transplantation into the liver. This was done by injecting prepared islets using a 22-G angio catheter, into the ileocolic vein (8,9).
(8) Immulnoogic assays
MLR and CML were performed in the same procedure as that we have reported previously (14).
(9) Iv glucose tolerance test (ivGTT)
ivGTT was performed by administering an intravenous infusion of 0.5g/kg of glucose and checking serial blood sugar levels.
(10) Histologic examination
Sections were examined by a pathologist after hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Elastica-Masson Goldner (EMG) staining. Immunohistochemistry using rabbit anti-human CD3, mouse anti-human CD68, and guinea pig anti-insulin (Dako, Carpinteria, CA) were performed by the standard avidin-biotin-peroxidase complex technique.
Results
Successful preparation of islet-kidney (IK) in baboons
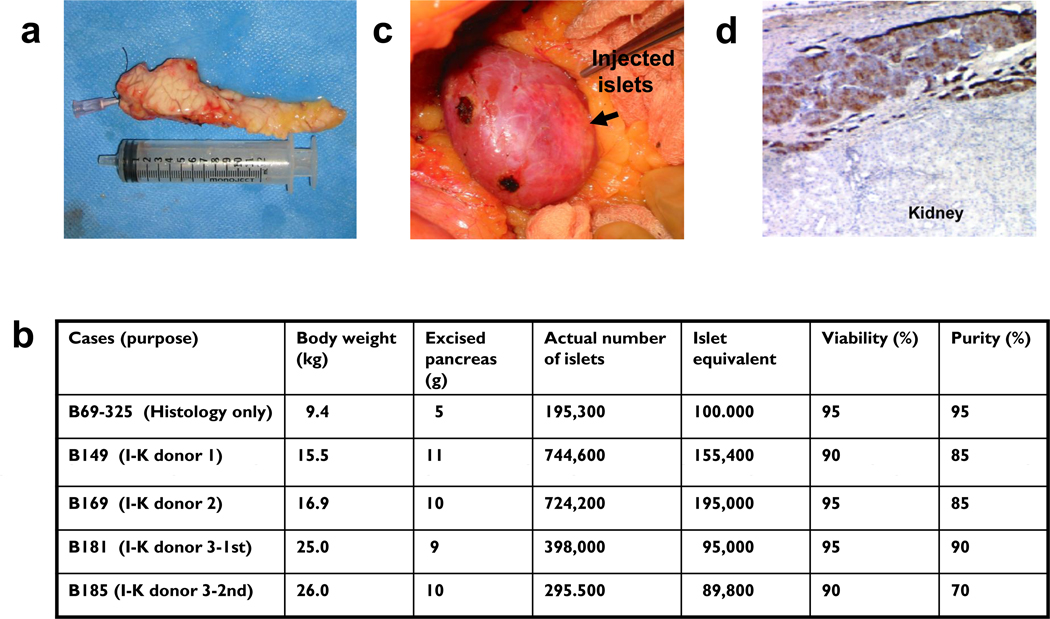
Five baboons underwent partial (50–70%) pancreatectomy (PPtx) of the pancreatic body and tail (Fig. 1a). We isolated 195.3×03 islets, 100×103IE by a 50% PPx (5 gram pancreas, B69-325), and 744.6×103 islets and 155.4×103IE (B149), 744.6×103 islets and 195.0×103IE (B169), 378.0×103 and 95.0×103IE (B181) and 295.5×103 and 89.8×103 IE (B185) by 70% PPxs respectively from 9 to 10 grams of each pancreas (Fig. a and b). The islets were then injected under the animal’s native renal capsule on the same day (Fig. 1c). One baboon, B39-325, was used for histologic analysis only. Histologic analysis revealed the presence of numerous insulin-producing β cells under the renal capsule, demonstrating that islets are capable of engrafting in this site in baboons at 4 months after IK preparation (Fig 1d). The other baboons (B149, B169, B181 and B185) served as allogeneic IK donors. No biopsies were performed on the baboons prepared for allogeneic donation because we did not want to decrease the number of islets in the IKs and because we were unsure whether local inflammation from the biopsy might lead to islet destruction or diminish islet function.
Fig 1.
a) Macroscopic finding of an excised pancreas for islet-kidney preparation. b) Islet-kidney preparation in three baboons: Body weight, weight of excised pancreas, actual number of islets, islet-equivalent, and viability and purity of isolated islets. One IK (B39-325) was used for histology only (panel d) and four I-Ks (B149, B169, B181 and B185) were used for allogeneic transplantation in three baboons. c) Injection of autologous islets under self renal capsule was through a flank incision (IK preparation). d) Insulin positive islet cells under the renal capsule 4 months after autologous islet injection (B39-325. ×100. Insulin staining).
Autologous composite islet-kidneys regulate blood sugar levels following a total pancreatectomy
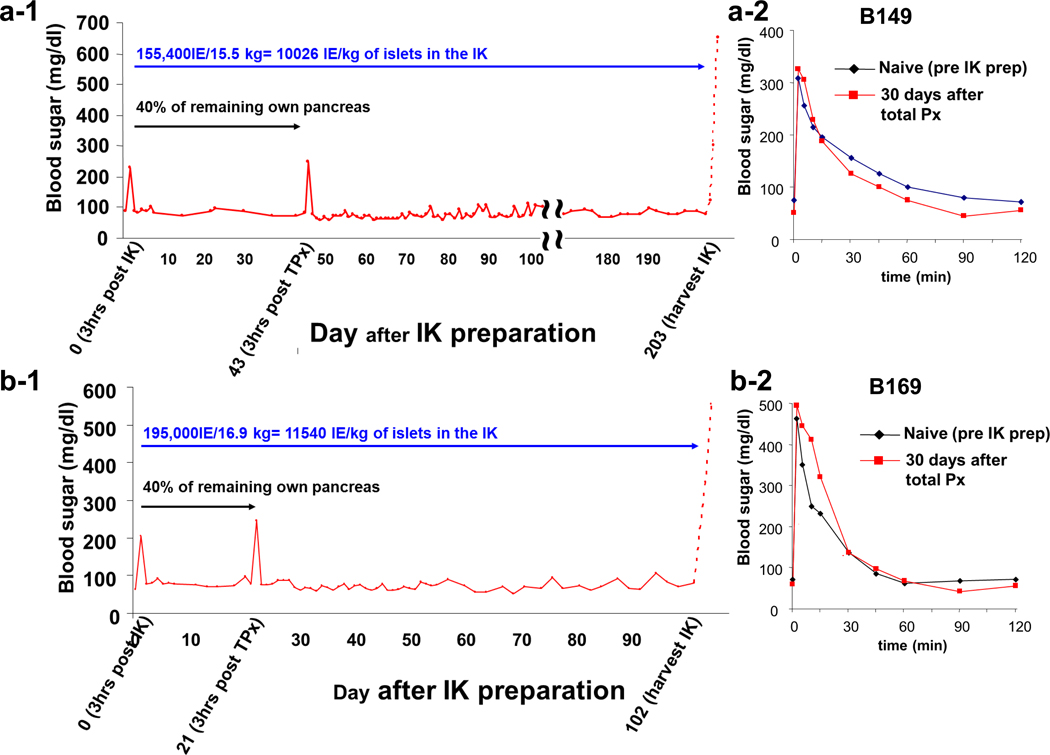
All animals, including the one used for histologic analysis, remained normoglycemic after implantation. To assess islet function, two of four potential IK donors (B149 and B169 in Fig 1b) were totally pancreatectomized surgically (i.e. residual pancreatectomy) at days 21 and 43, respectively, following IK preparation. Fasting blood sugar (FBS) levels remained normal, with the exception of transient perioperative hyperglycemia, which spontaneously resolved (Fig 2a-1, 2b-1). ivGTT were performed 30 days following total pancreatectomy. In both animals, ivGTT demonstrated normal regulation of blood glucose following pancreatectomy (Fig 2a-2, 2b-2). These results indicated successful engraftment and function of autologous islets after preparation of the IK. The remaining baboon was not subjected to total pancreatectomy for reasons unrelated to this study.
Fig 2.
Blood sugar levels after I-K preparation in B149 (a-1) and B169 (b-1). Both animals had stable BS levels even after removal of residual pancreas at Days 43 and 21 respectively. Glucose tolerance test demonstrated normal regulation of BS in both animals (a-2 and a-3)
The autologous composite IKs were removed and transplanted in allogeneic recipients on days 203 and 102 post-IK preparation. After IK nephrectomy, donor blood sugar levels immediately increased (Fig 2a-1 and 2b-1 dashed lines), indicating that autologous islets, at numbers achieved by partial pancreatectomy, injected under the native renal capsule, were capable of maintaining normal blood sugar levels after residual pancreatectomy. These animals were euthanized 2 days after IK donation, as they had no other life-supporting kidney available.
Long term function of life-supporting allogeneic composite islet-kidney grafts across a fully allogeneic barrier with chronic immunosuppression
(a) Induction of insulin dependent diabetes (IDDM) in recipients
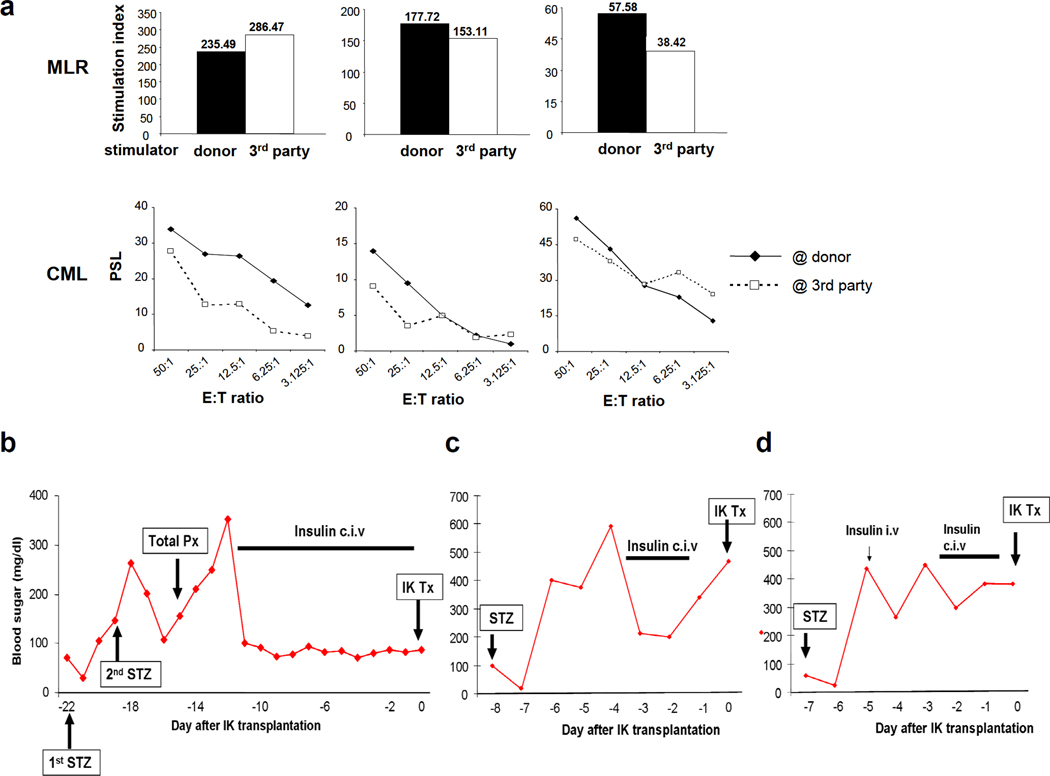
Three naïve recipients were determined to be class-I and class-II MHC mismatched to the IK donors, as assessed by CML and MLR assays (Fig 3a). All recipients were given bolus doses of intravenous stereptozotocin (STZ) at 2000 mg/m^2 body surface area.
Fig. 3.
MLR (upper panel) and CML (lower panel) showed that all recipients of IKs (B170, B184 and B195) were fully allogeneic to their recipient across both Class I and Class II barriers. Blood sugar levels after induction of IDDM (b and c). B170 required total pancreatectomy for the induction of IDDM (b) while a single dose of STZ at 2000 mg/m^2 body surface area induced IDDM in B184 (c) and B195 (d)
Recipient B170 demonstrated a transient decrease in BS, presumably due to insulin release from destroyed islets 6–12 hours following administration of STZ. However, despite the bolus of high-dose STZ, blood sugar (BS) did not increase markedly thereafter (Fig 3b). We then administered a 2nd dose of the same amount of STZ. BS increased to 300mg/dl the next day, but again decreased to less than 100 by 2 days after the 2nd dose. As the animal was still not diabetic, we performed a total pancreatectomy, with a bile duct reconstruction. High BS levels persisted thereafter, and treatment with a continuous infusion of regular insulin was required. Insulin therapy was continued for 10 days, during recuperation from total pancreatectomy and was then stopped at the time of IK transplantation.
Chemically-induced diabetes was successfully achieved following a single dose of STZ in the 2nd (B184) and the 3rd (B195) recipients (Fig 3c and d). After 3 days of elevated BS, we initiated a continuous infusion of regular insulin to prevent ketoacidosis before allogeneic IK transplantation. In order to re-confirm chemically-induced IDDM, insulin treatment was stopped 12 hours prior to IK transplantation, with a BS rise to 480mg/dL (B184) and 385mg/dL (B195).
(b) Clinical course following allogeneic islet-kidney transplantation in fully MHC mismatched IDDM recipients
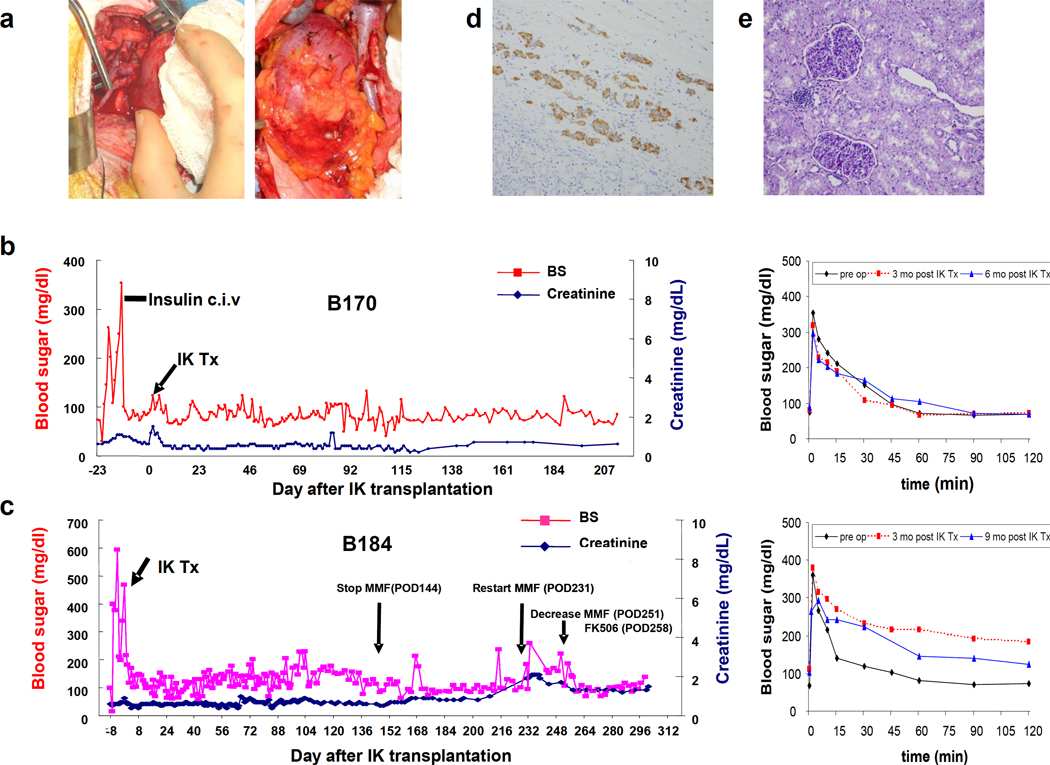
Unlike our published swine model, in which the protocol for prolonging kidney survival involved the induction of renal allograft tolerance (9), chronic immunosuppression was used to prevent rejection of the kidney grafts in the present study. The immunosuppressive regimen chosen was similar to that used in the clinic, including: 1) rabbit ATG at 20mg/kg on day -3 for partial T-cell depletion prior IK transplant, 2) low-dose FK506 starting on day −4 to maintain blood levels between 20 and 25ng/ml during the first 2 weeks, and 3) MMF at 100mg/kg administered starting on day −2 to maintain blood levels between 3 and 5ng/mL. On the day of IK transplantation, both native kidneys were removed and the IK grafts were transplanted in a manner similar to clinical pediatric transplantation (Fig 4a). Adipose tissue in Gerota’s capsule was intentionally preserved to prevent damage of the vascularized islets. Recipient B170 received an IK graft from the donor in B149. Recipient B184 received an IK graft from the donor in B169.
Fig 4.
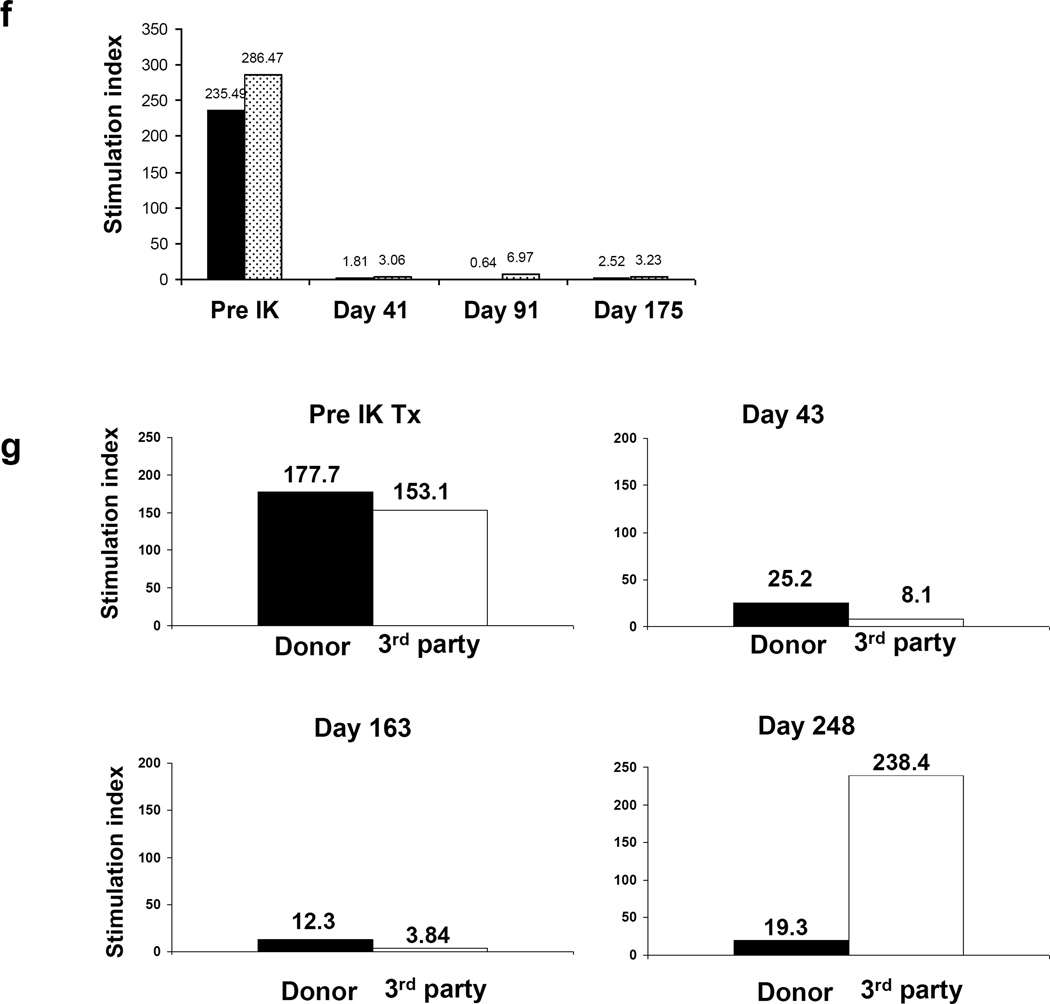
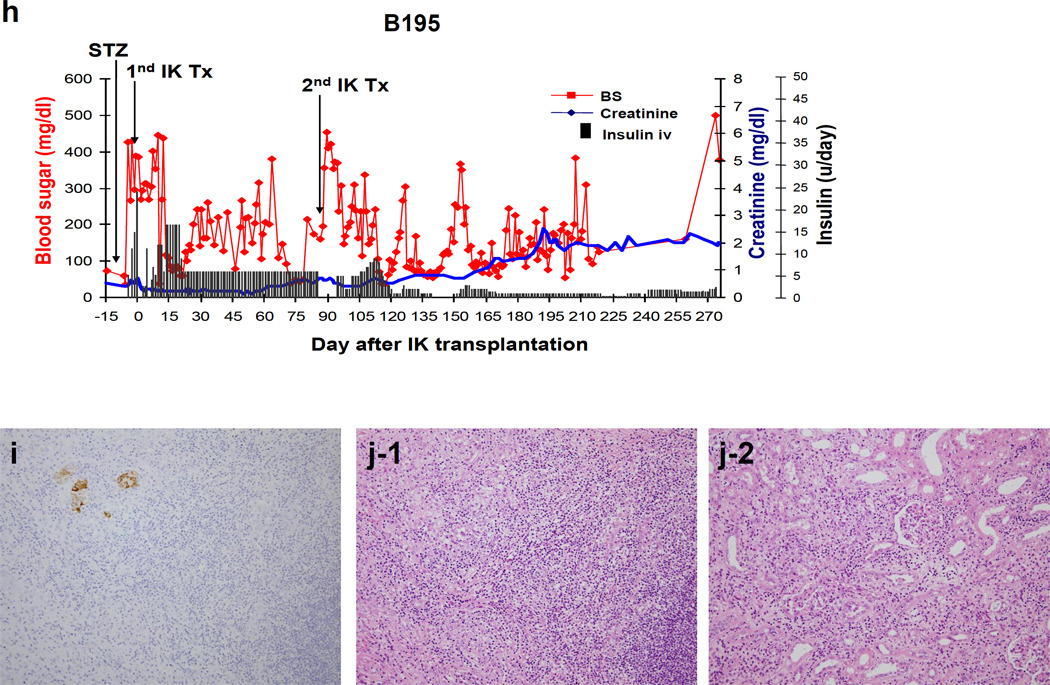
a) Representative macroscopic pictures of transplanted IKs across allogeneic barriers. IKs turned pink immediately after completion of renal vein and arterial anastomoses. b-left and c-left) Blood sugar levels after allogeneic I-K transplants in two recipients (see details in main text). No insulin treatment was required throughout the experimental period. b-right and c-right) 0.5g/kg ivGTT after IK transplants. Blood sugar levels were well-regulated at both 3 and 6 months post I-K transplantation in B170. B184 had slightly high blood sugar levels at 3 months (red line), but better regulation was seen at day 283 (blue line). d and e) An excised IK showed numerous insulin positive cells (d. ×200. Insulin staining) and only a minimal cell infiltrate in an IK graft (e. ×200. PAS). f and g) MLR responses after IK transplants in B170 (f) and B184 (g). h) Blood sugar levels after allogeneic IK transplants in B195. Two IKs were transplanted separately. Insulin treatment markedly decreased after the 2nd IK transplantation, however, the animal lost BS regulation with increasing creatinine (h)(see details in main text). i) Insulin staining shows that a small number of islets remained (i-1. ×200). J) Both islet area (j-1) and the kidney graft (j-2) of the IK had T cell infiltrates (×200. H&E), indicating insufficient immunosuppression leading to IK graft rejection. i (insulin staining) and j (H&E staining) are in the same area using sequential cuts (ie. back to back sections).
Figure 4b and 4c show FBS, creatinine levels and ivGTT tests following IK transplantation in B170 and B184 recipients. Baboon B170, which underwent total pancreatectomy, showed slightly elevated (120–130mg/dL) FBS along with a transient rise in creatinine for 3 days following transplantation, likely due to mild ischemic damage of the IK during transplantation. However, both renal and islet function then returned to normal levels and remained relatively stable thereafter (Fig 4b-left). ivGTT were performed at 3 and 6 months following IK transplantation and blood sugar levels were regulated in a similar pattern to that seen before induction of IDDM (Fig 4b-right). No insulin therapy was required following IK transplantation, indicating that the composite IK graft cured both surgically-induced diabetes and renal failure. We sacrificed the baboon, with normal graft function, on day 255 for complete histology.
Baboon B184, in which chemically-induced IDDM was achieved successfully with a single dose of STZ, also maintained stable creatinine levels after IK transplantation. FBS levels were also relatively stable, although at a higher level than in baboon B170 and again, no insulin therapy was required to maintain these levels (Fig 4c). Due to catheter-related issues at day 144, we discontinued continuous intravenous MMF administration (Fig 4c, arrow). An increase in creatinine level to 1.9 on day 231 led to elective re-insertion of a central venous line for hydration and resumption of MMF at 70mg/kg, which was followed by a decrease in creatinine to 1.6 within ten days. Although tolerance induction was not the goal of these experiments, baboon B184 demonstrated donor-specific hyporesponsiveness in MLR (see below). Immunosuppression was tapered starting on day 251, with MMF decreasing from 70 to 40mg/kg/day and FK506 decreasing from 0.15 to 0.06 mg/kg/day by day 258. During this tapering period, both creatinine and BS levels were stable, indicating that the tapering was successful. ivGTT were performed at 3 and 9 months following IK transplantation. Blood sugar levels were slightly elevated at 3 months (red line), but better regulation was observed with decreasing immunosuppression at day 283 (blue line) as described above (Fig 4c-right). However, the baboon developed respiratory failure from pneumonia, undoubtedly associated with long-term central IV catheters and was euthanized on POD 301. Creatinine at time of death was 1.3 and BS was 138. Histology of the excised IK graft showed numerous insulin staining positive cells under renal capsule (Fig 4d) and only a minimal cell infiltrate without tubulitis or glomerulitis, was seen in the renal graft (Fig 4e).
Immunologic responses following transplantation were assessed by MLR. Recipient B170 showed general hyporesponsiveness, likely due to continuous immunosuppression (Fig 4e). In contrast, recipient B184, in which immunosuppression was tapered more aggressively, displayed donor-specific hyporesponsiveness at day 248 (Fig 4g). Notably, the baboon was generally hyporesponsive in MLR assays tested at days 43 and 163 during period of continuous immunosuppression with MMF and FK506. However, by day 248, after immunosuppression was decreased markedly, the baboon showed a restored third party allogeneic MLR response, but anti-donor responses remained low.
In contrast to the previous baboons, in the third IK recipient (B195) the islet isolation per gram of excised pancreas was less efficient (Fig. 1c) than in previous donors and required insulin treatment following IK transplantation (Fig 4h). Six units of insulin per day were necessary to maintain FBS levels below 250mg/dL. We transplanted a 2nd IK graft from a different donor to increase the total number of islets at day 86. The insulin requirement decreased to 1–2 units following the 2nd transplant and the animal was occasionally insulin-free, suggesting that augmentation of the islet number improved blood sugar regulation (Fig. 4 red line). However, 50 days after the 2nd IK transplant (145 days after the first transplant) FBS levels fluctuated again and there was also an increase in creatinine levels (Fig 4h blue line); treatment with two to three units of insulin was instituted thereafter (Fig. 4h black bar). The animal was sacrificed at day 274. Histology showed a small number of islets remaining in the IK (Fig. 4 i) but cellular infiltrates were seen in both the islet tissue and the kidney of the IK graft (Fig 4j-1, j-2), indicating cellular rejection of the IK with chronic immunosuppression.
Allogeneic islets in suspension were rejected following transplantation
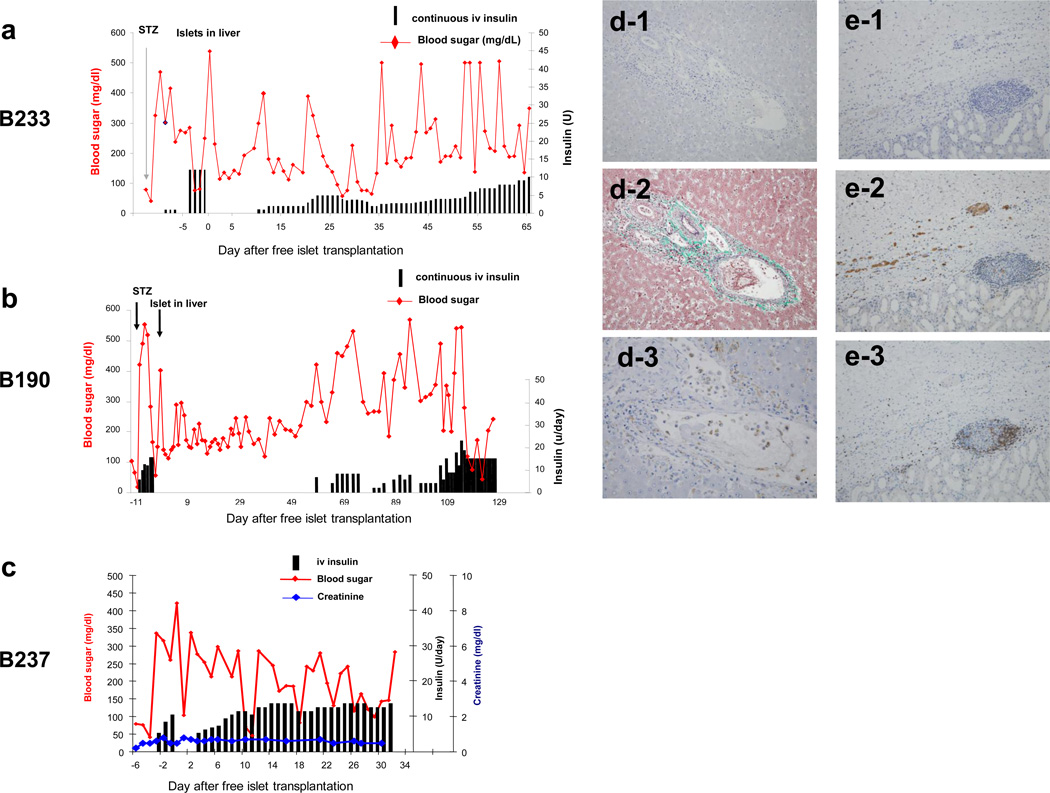
In order to determine whether the prevascularization of islets as a part of a donor kidney, rather than the immunosuppression, was responsible for the success of transplantation, allogeneic islet suspensions were administered to control baboons. These islets were injected either under the recipient’s native renal capsule or intrahepatically via the SMV in three baboons. All grafts had greater than 480.0×103 islets with at least 85% viability and 90% purity at the time of transplantation. The first baboon B233 received islets at 19,200IE/kg (85% viability, 90% purity and 481.8×103 isolated islets). FBS levels remained under 200mg/dL without insulin therapy for the first 9 postoperative days (Fig. 5a) but then rose above 300mg/dL, and the animal was started on insulin. The insulin requirement continued to increase until the baboon was euthanized. The next baboon B190 received a large number of islets (32,000IE/kg, 95% viability, 95% purity and 549.1×103 isolated islets) intra-hepatically. This larger number of transplanted islets appeared to prolong the insulin-free period compared to recipient B233 (Fig 5b). However, FBS levels increased gradually thereafter and rose above 250 mg/dl by day 50, leading to initiation of insulin therapy on day 60. More than 15 units of insulin per day were required starting from day 110 and the baboon was euthanized at day 129 (Fig 5b).
Fig 5.
a and b) Blood sugar levels (red lines) and insulin requirements (bars) after allogeneic islet transplantation into host livers. c) Blood sugar levels (red line), insulin requirements (bars) and serum creatinine levels (blue line) after allogeneic islets under host renal capsule. d) Insulin staining (d-1. ×200), Elastica-Masson Goldner (d-2. ×200) and CD68 staining (d-3. ×600) of allogeneic islets in the recipient's liver. e) Insulin staining (e-1), CD68 staining (e-2) and CD3 staining (e-3) of allogeneic islets under recipient's renal capsule (×200), demonstrating that the allogeneic islets were rejected in the recipient’s liver (d) and under the renal capsule (e) by a cellular mechanism.
The third baboon (B237) in this group received allogeneic islets under the recipient’s renal capsule at 17,833IE/kg (85% viability and 95% purity of 586.5×103 islets). FBS levels remained elevated and insulin treatment was required from day 3. It was not possible to effectively control the FBS even with greater than 12 units of daily insulin; treatment over 30 days and the animal died at day 33 (Fig 5c red line and bars). Host renal function, as assessed by serum creatinine, was stable following transplantation (Fig. 5 blue line).
Histology of allogeneic islets in excised recipient livers (B190, B233) showed no insulin positive cells. Fibrin (EMG staining Fig 5 d-2) and macrophage infiltrate (CD68 positive cells) were seen. Histology of allogeneic islets under recipient’s renal capsules (B237) also showed no insulin positive cells. CD68 positive macrophages (Fig 5 e-2) and CD3 positive cells (Fig 5 e-3) were seen under renal grafts, suggesting some islets have engrafted but were rejected by cellular mechanisms.
These results confirmed that vascularization of islets prior to transplantation, as part of an IK graft, was essential for long-term function.
Discussion
The incidence of diabetes and its sequellae make it an important public health concern and uncontrolled diabetes mellitus may lead to significant patient morbidity and mortality (15) (16). Many diabetics with end-stage renal disease (ESRD) are forced to begin hemodialysis and almost 50% of diabetic patients die within three years of initiating hemodialysis, making this combination particularly morbid (17). Combined kidney-pancreas transplantation has been shown to correct both labile blood sugar and renal failure in these patients and to decrease the risk of complication associated with diabetes, such as microvascular disease (18). However, pancreas transplantation is a technically challenging procedure associated with a number of post-operative complications (18).
Composite IK transplantation avoids the surgical complications associated with pancreas transplantation, while maintaining the benefits of kidney transplantation and euglycemia. IK transplantation could be particularly relevant for the population of juvenile diabetic patients that receive living-related kidney-transplants for ESRD. Using a tolerance-induction protocol, we have previously reported in MGH miniature swine that IKs were capable of restoring normoglycemia to pancreatectomized recipients immediately, in addition to maintaining renal function long-term (9). When donor free islets were injected under the donor renal capsule and then immediately re-vascularrized in pacncreatectomized recipients with the same tolerance-inducing regimen as in MGH miniature swine, allogeneic islets were rejected by day 31 with evidence of cellular rejection in both islets and kidneys (9). Since induction of tolerance in living-related, kidney recipients has recently been accomplished (19), the IK strategy could have important clinical applicability to this pediatric population. The present study demonstrates that IK are also capable of correcting diabetes in primates, indicating that combining this approach with tolerance induction could correct both diabetes and renal failure, without the need for chronic immunosuppession, in juvenile diabetics suffering from end-stage diabetic nephropathy.
Our study shows that prevascularization of the islets as part of an IK is essential to success, since suspensions of comparable numbers of allogeneic islets transplanted intrahepatically or as a non-vascularized graft underneath the renal capsule were not successful in reversing diabetes. Furthermore, in contrast to the clinical setting, where multiple pancreata are required for each transplant, euglycemia in this model was established with islets harvested from a single organ. One possible reason for this difference is the fact that non-vascularized tissue grafts must establish a new vascular supply in order to survive. During the process of revascularization, tissue grafts are more susceptible to failure from nonspecific causes, such as inadequate oxygen or nutrient supply. In addition, while vascularized grafts are relatively tolerogenic, skin and tissue grafts are immunogenic (9,20–22), probably because they sensitize the host by releasing cells or antigens into the lymphatic system.
In this study, we showed that allogeneic islets which were transplanted intrahepatically had a lower insulin requirement in the early post-Tx period as compared to allogeneic non-vascularized islets transplanted beneath the recipient’s renal capsule. This is potentially related to the revascularization of transplanted islets. Because the liver is far more vascular than the renal capsule, in the allogeneic setting nonvascularized tissues are more likely to survive, at least temporarily, in the liver versus the renal capsule. However, unlike the space beneath the renal capsule, the liver contains large numbers of Kupffer cells capable of phagocytosing allogeneic islets (23,24). Thus, in the allogeneic setting, non-vascularized pancreatic islets are lost due to (i) anoxia when transplanted under the renal capsule, or (ii) phagocytosis in the liver, that are both enhanced by the allogeneic cellular responses. We suggest that the location for islet transplantation be reconsidered and propose that the renal subcapsular site as a more suitable location for islet injection, especially if performed prior to allogeneic transplantation. Minimization of hyperglycemia and absence of the inhibitory effects of FK506 during the revascularization process of autologous islet-kidneys, may contribute to enhance islet function following allogeneic transplantation of vascularized IK grafts, hypotheses that are being tested in miniature swine (Vallabhajosyula et al., unpublished).
Two major concerns may arise when considering the clinical application of this strategy. One is glucose intolerance in the donor after IK preparation, and the other is surgical risk associated with IK preparation. In this study, baboon recipients of IK preparation had no evidence of exocrine pancreatic insufficiency, such as food intolerance, changes in stool consistency or unexpected changes in body weight. This safety issue was also observed in our MGH miniature swine IK model, which had longer follow-up. Importantly for clinical applicability, even after 70% pancreatectomy, donor animals have remained normoglycemic and retained normal pancreatic exocrine function for over one year (8). Clinically, the University of Minnesota has published extensively on the use of living-related pancreas transplantation (25). Despite removing a large percentage of the organ, less than 3% of the donors in this series developed diabetes. Those who did develop DM were sufficiently controlled with oral hypoglycemics and donor survival rate in that study was 100% (25). If donors are screened carefully, this procedure is sufficiently safe to be clinically acceptable as a source of pancreas procurement (Sutherland D.E., personal communication). To minimize surgical intervention, we envision preparation of vascularized IK grafts using a laparoscopic approach, several months prior to kidney transplantation. In addition, we found that in the baboon which received two IK grafts and had impaired BS regulation, the islets were isolated less efficiently as compared to the other recipients (Fig. 1c). These donors had undergone a prior laparotomy which caused adhesion of pancreas and adversely affected the harvest of the organ. Therefore thorough screening of donors, including surgical histology as well as islet function may be essential to maximize benefit of the IK strategy as well as to ensure the safety of the donors. We are currently attempting to develop non-invasive assays, including an islet oxygen conception assay, to assess islet function in non-human primates.
One recipient of IK had less regulation of BS compared to other two recipients of IKs, with evidence of T cell infiltrates in both the renal grafts and islet tissue. In contrast, the other recipients maintained islet function as long as the kidney was not rejecting, suggesting that the immune response to the islets in IK grafts are kidney-dependent. In order to avoid rejection of kidneys as well as enhancing the procedure’s clinical applicability, we are currently working on approaches to combine our IK strategy with tolerance induction. Preliminary results have demonstrated that tolerance induction is possible using IK grafts in rhesus monkeys (Pathiraja V and Yamada K et al, unpublished data). Although this procedure necessitates additional donor surgery, by combining this technology with laparoscopic construction of the IK, we hope to develop a safe, clinically applicable protocol for tolerance induction of living-related vascularized islet kidneys.
Supplementary Material
Acknowledgements
We thank Dr. Curt Cetrulo, Dr. Isabel Hanekamp and Dr. Joseph Scalea for their helpful review of this manuscript; and Rebecca Wark for editorial assistance. All experiments were performed in accordance with the NIH Guidelines for Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Funding Source: This work was supported by NIH NIDDK U01AI074634-01.
Abbreviations
- ATG
Anti-Thymocyte Globulin
- BS
Blood sugar
- CML
Cell Mediated Lympholysis
- FBS
Fasting Blood Sugar
- H&E
Hematoxylin and Eosin
- IDDM
Insulin Dependent Diabetes
- IE
Islet Equivalent
- IK
Islet-Kidney
- ivGTT
intravenous Glucose Tolerance Test
- MGH
Massachusetts General Hospital
- MLR
Mixed Lymphocyte Reaction
- PPtx
Partial pancreatectomy
- STZ
Streptozotocin
- Tx
Transplantation
Footnotes
Conflicts of Interest
None of authors have any of conflict interest to declare with regard to the content of this article.
References
- 1.Nicolls MR, Coulombe M, Yang H, Bolwerk A, Gill RG. Anti-LFA-1 therapy induces long-term islet allograft acceptance in the absence of IFN-gamma or IL-4. J Immunol. 2000;164:3627–3634. doi: 10.4049/jimmunol.164.7.3627. [DOI] [PubMed] [Google Scholar]
- 2.Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune diabetes through allogeneic tolerance and reversal of autoimmunity. Diabetes. 2004;53:376–383. doi: 10.2337/diabetes.53.2.376. [DOI] [PubMed] [Google Scholar]
- 3.Cretin N, Buhler L, Fournier B, et al. Human islet allotransplantation: world experience and current status. Dig Surg. 1998;15:656–662. doi: 10.1159/000018672. [DOI] [PubMed] [Google Scholar]
- 4.Paty BW, Lanz K, Kendall DM, Sutherland DE, Robertson RP. Restored hypoglycemic counterregulation is stable in successful pancreas transplant recipients for up to 19 years after transplantation. Transplantation. 2001;72:1103–1107. doi: 10.1097/00007890-200109270-00021. [DOI] [PubMed] [Google Scholar]
- 5.Brunicardi FC, Shackleton CR. Whole-organ versus islet pancreatic transplantation. Curr Opin Gen Surg. 1994:179–185. [PubMed] [Google Scholar]
- 6.Shapiro AM, Lakey JRT, Ryan EA, et al. Islet Transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000 doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 7.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 8.Kumagai N, O'Neil JJ, Barth RN, et al. Vascularized islet-cell transplantation in miniature swine. I. Preparation of vascularized islet kidneys. Transplantation. 2002;74:1223–1230. doi: 10.1097/00007890-200211150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai N, LaMattina JC, Kamano C, et al. Vascularized islet cell transplantation in miniature Swine: islet-kidney allografts correct the diabetic hyperglycemia induced by total pancreatectomy. Diabetes. 2002;51:3220–3228. doi: 10.2337/diabetes.51.11.3220. [DOI] [PubMed] [Google Scholar]
- 10.Sachs DH. MHC Homozygous Miniature Swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as Models in Biomedical Research. Ames, Iowa: Iowa State University Press; 1992. pp. 3–15. [Google Scholar]
- 11.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Shapira MY, Hai AA, Tsirigotis P, Resnick IB, Or R, Slavin S. Hematopoietic stem cell therapy for malignant diseases. Ann Med. 2007;39:465–473. doi: 10.1080/07853890701472323. [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α-1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 14.Griesemer AD, Hirakata A, Shimizu A, et al. Results of Gal-Knockout Porcine Thymokidney Xenografts. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol. 2000;164:3079–3086. doi: 10.4049/jimmunol.164.6.3079. [DOI] [PubMed] [Google Scholar]
- 21.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 22.Mezrich JD, Haller GW, Arn JS, Houser SL, Madsen JC, Sachs DH. Histocompatible miniature swine: an inbred large-animal model. Transplantation. 2003;75:904–907. doi: 10.1097/01.TP.0000054839.43852.BF. [DOI] [PubMed] [Google Scholar]
- 23.Clayton HA, Davies JE, Sutton CD, Bell PR, Dennison AR. A coculture model of intrahepatic islet transplantation: activation of Kupffer cells by islets and acinar tissue. Cell Transplant. 2001;10:101–108. [PubMed] [Google Scholar]
- 24.Bottino R, Fernandez LA, Ricordi C, et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316–323. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 25.Humar A, Gruessner RW, Sutherland DE. Living related donor pancreas and pancreas-kidney transplantation. Br Med Bull. 1997;53:879–891. doi: 10.1093/oxfordjournals.bmb.a011656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.