Abstract
Objective
Myeloperoxidase (MPO) is a leukocyte-derived enzyme that appears to be directly involved in atherosclerosis development. We evaluated the association of circulating MPO with coronary and aortic atherosclerosis in a large, multiethnic population.
Methods and Results
Plasma levels of MPO were measured in 3294 subjects participating in the Dallas Heart Study, a probability-based population sample. Coronary artery calcification (CAC) was measured by EBCT, and abdominal aorta plaque prevalence (AP) and burden (APB), as well as abdominal aorta wall thickness (AWT) were determined by MRI. Associations between MPO and atherosclerosis phenotypes were assessed in multivariable analyses adjusting for traditional atherosclerosis risk factors. MPO levels in the 4th compared with 1st quartile independently associated with prevalent AP (OR 1.41, 95% CI 1.08–1.84), APB (beta coefficient 0.23, p=0.02), and AWT (beta coefficient 0.04, p=0.03), but not with prevalent CAC (OR 0.84, 95% CI 0.61–1.17). MPO remained associated with aortic atherosclerosis phenotypes but not coronary calcification after adjustment for other inflammatory biomarkers. A significant interaction was observed between race/ethnicity, MPO and AP (pinteraction=0.038), such that MPO levels in the 4th vs 1st quartile associated with prevalent AP in African Americans, (OR 1.81, 95% CI 1.23–2.65) but not in White or Hispanic participants (OR 0.99, 95% CI 0.68–1.44).
Conclusion
Higher levels of MPO associated with aortic but not coronary atherosclerosis, with significant associations limited to African American participants. These findings suggest that MPO might be a novel risk factor contributing to racial disparities in peripheral vascular disease.
Keywords: Myeloperoxidase, atherosclerosis, peripheral vascular disease, African American
Introduction
Inflammation plays a critical role in the initiation and progression of atherosclerosis. Myeloperoxidase (MPO), a bactericidal enzyme present in neutrophils, monocytes and macrophages, also has pro-inflammatory functions that may contribute to vascular injury. In vitro, MPO contributes to peroxidation of low density lipoprotein (LDL) and high density lipoprotein (HDL) as well as consumption of nitric oxide which may contribute to endothelial damage.1, 2 In vivo experimental evidence further supports a potential role of MPO in atherosclerosis development in both animal models3, 4 and in human studies.5, 6 MPO has also been investigated as a potential biomarker of coronary atherosclerosis and of the corresponding risk for adverse clinical outcomes. Several studies have reported associations between higher levels of MPO and a greater extent of coronary artery disease,7–9 and increased risk of adverse cardiac events.10
Fewer data are available regarding an association between MPO and peripheral vascular disease (PVD), which affects about 8 million Americans. Although risk factors for PVD overlap with those of CAD, smoking is particularly important as a PVD risk factor; moreover, PVD also disproportionately affects African Americans.11 Although prior studies have identified several inflammatory biomarkers associated with atherosclerosis in the aorta and peripheral vascular beds,12–14 little is known about the association of MPO and PVD, and whether MPO may contribute to racial disparities in PVD outcomes. In the present study, we performed a comprehensive evaluation of the association between MPO and atherosclerosis in multiple different vascular beds in a large, multi-ethnic population.
Methods
Study Population
The Dallas Heart Study (DHS) is a probability-based population sample of 6101 Dallas County residents.15 African Americans and women were oversampled intentionally to achieve a final cohort of approximately 50% African Americans and 50% women. Following an initial in-home visit for collection of survey data, body mass index and measurement of blood pressure, participants between the ages of 30 and 65 were invited to participate in a second visit where they provided in-home fasting blood and urine specimens. Those completing visit two were invited to and to a third visit at UT Southwestern Medical Center, where imaging studies including cardiac magnetic resonance imaging and electron beam computed tomography were performed. Demographics, blood pressure and body composition were similar between subjects completing visits 1 and 2, and laboratory data were similar between those completing visits 2 and 3.15 The present study includes 3294 DHS subjects from visit 2 who underwent measurement of MPO.
Definition of Variables
Hypertension was defined as an average systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medication. Hypercholesterolemia was defined as a fasting low density lipoprotein ≥160 mg/dL, a total cholesterol ≥240 mg/dL, or use of a statin medication. Low high density lipoprotein was defined as HDL-C <40 mg/dL in men and <50mg/dL in women. Diabetes was defined as a fasting blood glucose level of ≥126 mg/dL, or a non-fasting blood glucose level of ≥200 mg/dL, or self-reported diabetes with use of any glucose lowering medication. Body mass index was calculated based on measured body weight and height. Race was self-reported.
Atherosclerosis Assessment
Personnel who performed atherosclerosis measurements were blinded to all participant data. Coronary artery calcification (CAC) was determined as the average score on two consecutive electron beam computed tomography (EBCT) scans. Agreement between the two scans, as assessed by the intraclass correlation coefficient, was 0.96. Prevalent CAC was defined as >10 Agastaton units, which was a data-derived threshold selected to maximize the signal to noise ratio, as previously described.16 The abdominal aorta was assessed using a 1.5 Tesla whole-body MRI system (Intera, Philips Medical Systems), using a free-breathing, ECG-gated, T2-weighted turbo spin-echo (black-blood) sequence, with 6 transverse slices of the infrarenal abdominal aorta obtained as described previously.17 Aortic plaque (AP) was defined as a hyper-intense signal volume that protruded ≥1 mm from the endoluminal surface of the aortic wall, and was manually contoured in each image.18 Total vascular area (TVA) and total plaque area (TPA) were calculated as the summation of vessel area and plaque area for all 6 slices. Aortic plaque burden (APB) was then calculated by the formula: 100 × (TPA/TVA). Aortic wall thickness (AWT) was calculated by dividing the total vessel wall area by the aortic circumference in each slice, as previously described.19 Mean AWT was then determined by the summation of AWT for each slice divided by number of total slices (n=6). In 70 subjects, an interobserver variability analysis demonstrated an intraclass correlation coefficient between two observers of 0.94 and a mean interobserver difference of 4.2 + 6.6%.20
Measurement of MPO and Other Biomarkers
Venous blood was collected in standard blood collection tubes containing citrate EDTA and samples were maintained at 4°C for ≤4 hours and then centrifuged (1430g for 15 minutes) at 4°C. Plasma was then removed, aliquoted, and frozen at −80°C until assays were performed. MPO was measured from thawed frozen plasma at Alere San Diego, Inc (San Diego, CA) Inc using a sandwich assay on a Luminex 200 reader (Austin, TX) and modified paramagnetic Luminex beads from Radix Biosolutions (Georgetown, TX) with minimum and maximum detection limits of 0.2 ng/mL and 250 ng/mL, respectively. The intra-assay coefficient of variation (CV) was 12% and inter-assay CV was 13%. Personnel who performed the assays were blinded to all clinical data.
The following analytes were measured previously and the methods have been described: high sensitivity C-reactive protein (hsCRP)14, interleukin-18 (IL18)12, osteoprotegerin13, Tumor necrosis factor-alpha 1 receptor (TNFR1A)21, matrix metalloproteinase-9 (MMP-9)21, pulmonary surfactant protein-B (SP-B)21, monocyte chemoattractant protein (MCP-1)22, and soluble receptor for advanced glycation end products (sRAGE)23.
Statistical Analysis
Participants were divided into quartiles on the basis of MPO levels. Demographic and clinic variables were compared across MPO quartiles using the χ2 trend test for categorical variables and the Jonckheere-Terpstra test for continuous variables. Correlations between selected biomarkers and MPO were evaluated by Spearman rank correlation coefficients. Logistic regression was performed to investigate associations between MPO and prevalent CAC and AP in unadjusted models and models adjusted for traditional risk factors including age, sex, race/ethnicity, body mass index, diabetes, current smoking, hypertension, hypercholesterolemia and low HDL. Linear regression was performed to assess associations between MPO and AWT and APB in unadjusted models and models adjusting for the same traditional risk factors. Sensitivity analyses were also performed with MPO entered into the models as a log-transformed continuous variable. Testing for statistical interaction was performed for MPO × race/ethnicity for all the atherosclerosis phenotypes. Because significant race × MPO interactions were seen, stratified analyses were performed in subgroups defined by race/ethnicity. All models included only subjects with complete data available for covariates and the phenotype of interest. For all statistical testing, 2-sided probability values were reported and a probability value <0.05 was considered statistically significant. No adjustment for multiple testing was done. All analyses were performed using SAS 9.2 (Cary, NC, USA) and all box-plot figures were created using GraphPad PRISM 5.01 (La Jolla, CA, USA).
Results
Associations of MPO with Atherosclerosis Risk Factors and Biomarkers
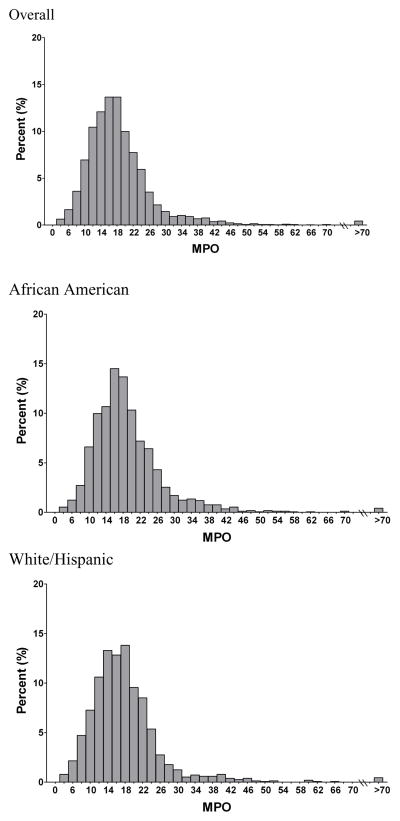
MPO levels followed a right-skewed distribution and were higher in African American (median 17.5 [13.9, 22.3] ng/mL) compared with non-African American participants (median 16.8 [12.9, 21.0] ng/mL; p<0.0001) (Figure 1). Increasing quartiles of MPO were associated with traditional risk factors such as current smoking, diabetes, low HDL higher body mass index, but not with hypertension or hypercholesterolemia (Table 1). In addition, MPO did not differ by age or sex. MPO was significantly correlated with other inflammatory biomarkers, with strong positive associations observed with TNFR 1A and SP-B (Table 1).
Figure 1.
Distribution of MPO (ng/mL)
Table 1.
Demographic and Clinical Variables Across Quartiles of MPO (n=3294)
| Variable | Q1 (n=823) | Q2 (n=824) | Q3 (n=824) | Q4 (n=823) | P trend |
|---|---|---|---|---|---|
| MPO (ng/mL) | 3.7–13.34 | 13.35–17.12 | 17.13–21.58 | 21.59–183.33 | |
| Age (years) | 44 | 43 | 43 | 44 | 0.22 |
| Male | 43% | 46% | 43% | 44% | 0.77 |
| White | 35% | 32% | 28% | 23% | <0.001 |
| African American | 46% | 51% | 52% | 57% | <0.001 |
| Hispanic | 15% | 15% | 19% | 18% | 0.03 |
| Body Mass Index (kg/m2) | 28.1 | 29.3 | 29.6 | 30.8 | <0.001 |
| Hypertension | 32% | 35% | 31% | 37% | 0.14 |
| Current smoker | 27% | 27% | 29% | 34% | <0.001 |
| Hypercholesterolemia | 13% | 13% | 13% | 13% | 0.80 |
| Low HDL | 37% | 40% | 44% | 45% | <0.001 |
| High Triglyceride | 12% | 12% | 12% | 13% | 0.45 |
| Diabetes Mellitus | 9% | 12% | 12% | 14% | 0.001 |
| TNFR1A (ng/mL) | 0.38 | 0.55 | 0.67 | 0.94 | <0.001 |
| SP-B (ng/mL) | 1.13 | 2.21 | 2.99 | 6.52 | <0.001 |
| MCP-1 (pg/ml) | 29.8 | 34.9 | 37.9 | 42.6 | <0.001 |
| Osteoprotegerin (pg/ml) | 1168 | 1182 | 1202 | 1233 | 0.001 |
| MMP9 (ng/mL) | 3.3 | 4.8 | 6.0 | 10.1 | <0.001 |
| IL18 (mg/L) | 492.7 | 501.2 | 536.9 | 556.6 | 0.002 |
| hsCRP (mg/L) | 2.4 | 2.5 | 2.7 | 4.2 | <0.001 |
| SRAGE (ng/mL) | 1.4 | 1.9 | 2.1 | 2.3 | <0.001 |
Values are medians (interquartile range) for continuous variable and proportions for categorical variables. TNFR1A, tumor necrosis factor-alpha 1 receptor; SP-B, pulmonary surfactant protein-B; MCP-1, monocyte chemoattractant protein-1; MMP9, matrix metalloproteinase-9; IL18, interleukin-18; hsCRP, high sensitivity C-reactive protein; SRAGE, soluble receptor for advanced glycation end products.
Associations of MPO with Atherosclerosis Phenotypes
MPO was not associated with CAC in either crude or adjusted analyses (Table 2). In contrast, in unadjusted models, MPO was modestly associated with all prevalent aortic atherosclerosis phenotypes, including AP, APB and AWT (Table 2). After adjustment for age, sex, race, BMI, DM, smoking, HTN, hypercholesterolemia, and low HDL, subjects in the 4th MPO quartile had a significantly higher prevalence of AP compared with subjects in the 1st MPO quartile (OR 1.41, 95% CI 1.08–1.84; Table 2). MPO levels in the 4th quartile also independently associated with AWT and APB after multivariable adjustment (p<0.05 for each; Table 2). Similar findings were observed when MPO was entered into the models as a log transformed continuous variable (Table 2). In models additionally adjusting for other inflammatory biomarkers, MPO remained associated with aortic atherosclerosis phenotypes but not coronary calcification (Supplementary Table 4). In sensitivity analyses excluding individuals with self-reported prior myocardial infarction, stroke and revascularization, all results were qualitatively similar (data not shown).
Table 2.
Associations of MPO With Atherosclerosis Phenotypes
| Prevalent CAC (n=2502) | Prevalent Plaque (n=2287) | APB (n=2287) | AWT (n=2300) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | Beta coeff | P value | Beta coeff | P value | |
| Age (years) | 1.15 (1.13, 1.17) | 1.08 (1.07, 1.09) | 0.07 | <0.01 | 0.01 | <0.01 |
| Male | 1.05 (0.82, 1.33) | 1.12 (0.93, 1.36) | 0.17 | 0.02 | 0.15 | <0.01 |
| African American | 0.92 (0.71, 1.2) | 0.89 (0.72, 1.11) | −0.08 | 0.35 | 0.003 | 0.80 |
| Body Mass Index (kg/m2) | 1.02 (1, 1.1) | 0.97 (0.95, 0.98) | −0.03 | <0.01 | 0.001 | 0.34 |
| Hypertension | 2.73 (2.12, 3.51) | 1.44 (1.15, 1.79) | 0.33 | <0.01 | 0.06 | <0.01 |
| Current smoker | 1.99 (1.45, 2.75) | 2.13 (1.73, 2.62) | 0.69 | <0.01 | 0.12 | <0.01 |
| Hypercholesterolemia | 1.59 (1.24, 2.05) | 1.4 (1.06, 1.84) | 0.34 | <0.01 | 0.04 | 0.01 |
| Low HDL | 1.57 (1.17, 2.1) | 1.15 (0.94, 1.4) | 0.16 | 0.03 | 0.07 | <0.01 |
| Diabetes Mellitus | 3.31 (2.59, 4.23) | 1.4 (1.03, 1.91) | 0.28 | 0.01 | 0.05 | <0.01 |
| Univariable Model | ||||||
| Log_MPO | 1.01 (0.92, 1.11) | 1.06 (0.97, 1.15) | 0.06 | 0.13 | 0.04 | 0.03 |
| Q1 | Reference | Reference | Reference | Reference | ||
| Q2 | 0.95 (0.73, 1.25) | 0.96 (0.76, 1.22) | −0.12 | 0.30 | −0.02 | 0.32 |
| Q3 | 0.89 (0.68, 1.17) | 0.95 (0.75, 1.21) | −0.08 | 0.51 | 0.01 | 0.78 |
| Q4 | 1.02 (0.78, 1.33) | 1.29 (1.02, 1.63) | 0.24 | 0.04 | 0.05 | 0.02 |
| Model Adjusted for Traditional Risk Factors | ||||||
| Log_MPO | 0.98 (0.87, 1.1) | 1.1 (1.003, 1.21) | 0.07 | 0.04 | 0.02 | 0.01 |
| Q1 | Reference | Reference | Reference | Reference | ||
| Q2 | 0.86 (0.62, 1.18) | 1.03 (0.79, 1.34) | −0.08 | 0.45 | −0.02 | 0.25 |
| Q3 | 0.84 (0.61, 1.17) | 0.99 (0.76, 1.3) | −0.06 | 0.52 | 0.004 | 0.83 |
| Q4 | 0.84 (0.61, 1.17) | 1.41(1.08, 1.84) | 0.23 | 0.02 | 0.04 | 0.03 |
CAC, coronary artery calcification; APB, aorta plaque burden; AWT, aorta wall thickness.
Race-Specific Association of MPO with Atherosclerosis Phenotypes
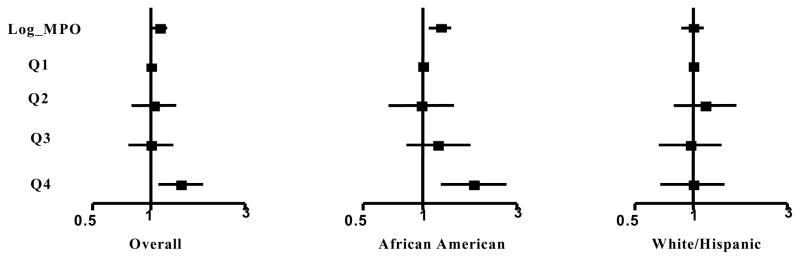
Significant interactions were found between race/ethnicity and MPO quartile regarding AP prevalence (pinteraction=0.038) and APB (pinteraction=0.022) but not AWT (pinteraction=0.22). Among African American participants, MPO was significantly associated with prevalent AP (OR 1.81 in quartile 4 versus quartile 1, 95% CI 1.23–2.65, Figure 2) as well as APB and AWT (p<0.01 for each, Table 3) in the fully adjusted models. In contrast, among White or Hispanic participants, there was no association between MPO and prevalent AP (Figure 2), APB or AWT (Table 3). Similar findings were observed when MPO was entered into the stratified analyses as a log-transformed continuous variable (Figure 2, Table 3). Findings were unchanged when White and Hispanic participants were analyzed as separate subgroups.
Figure 2.
Aortic Plaque Prevalence According to MPO Levels, Stratified by Race/Ethnic Group. All models adjusted for traditional risk factors (age, sex, race, BMI, DM, smoking, HTN, hypercholesterolemia, and low HDL). P-value for interaction of MPO quartile × race= 0.038.
Table 3.
Race-specific Association of MPO with Aortic Plaque Burden (APB) and Aortic Wall Thickness (AWT) After Adjustment for Traditional Risk Factors
| APB | AWT | |||||||
|---|---|---|---|---|---|---|---|---|
| African American (n=1097) | White or Hispanics (n=1192) | African American (n=1102) | White or Hispanics (n=1198) | |||||
| Beta coeff | P value | Beta coeff | P value | Beta coeff | P value | Beta coeff | P value | |
| Age (years) | 0.06 | <0.01 | 0.07 | <0.01 | 0.01 | <0.01 | 0.01 | <0.01 |
| Male | 0.09 | 0.39 | 0.22 | 0.03 | 0.13 | <0.01 | 0.16 | <0.01 |
| Body Mass Index (kg/m2) | −0.03 | <0.01 | −0.03 | <0.01 | 0.001 | 0.58 | 0.001 | 0.34 |
| Hypertension | 0.40 | <0.01 | 0.22 | 0.07 | 0.07 | <0.01 | 0.04 | 0.05 |
| Current smoker | 0.58 | <0.01 | 0.82 | <0.01 | 0.11 | <0.01 | 0.12 | <0.01 |
| Hypercholesterolemia | 0.20 | 0.16 | 0.50 | <0.01 | 0.01 | 0.74 | 0.08 | <0.01 |
| Low HDL | 0.01 | 0.95 | 0.30 | <0.01 | 0.05 | 0.01 | 0.08 | <0.01 |
| Diabetes Mellitus | 0.23 | 0.11 | 0.40 | 0.03 | 0.03 | 0.19 | 0.07 | 0.03 |
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | −0.06 | 0.67 | −0.03 | 0.81 | 0.02 | 0.33 | −0.05 | 0.02 |
| Q3 | 0.17 | 0.24 | −0.19 | 0.18 | 0.04 | 0.11 | −0.05 | 0.02 |
| Q4 | 0.42 | <0.01 | −0.06 | 0.70 | 0.07 | <0.01 | −0.01 | 0.73 |
| log_MPO (1 stdev) | 0.16 | <0.01 | −0.02 | 0.73 | 0.02 | <0.01 | 0.01 | 0.41 |
APB, aorta plaque burden; AWT, aorta wall thickness.
Discussion
In this large population-based study, higher MPO levels associated with African American race and with multiple traditional atherosclerosis risk factors. In the overall population, MPO was modestly associated with prevalent aortic plaque, aortic plaque burden and aortic wall thickness after adjusting for traditional risk factors, but not with coronary artery calcification. We found a significant interaction by race, such that MPO independently associated with aortic atherosclerosis in African Americans but not in White or Hispanic participants. These findings suggest that the relationships between MPO and subclinical atherosclerosis may vary across different vascular beds and are modified by race/ethnicity.
Biology of MPO
MPO is an enzyme found in neutrophils, monocytes and macrophages and plays a crucial role in immune function, producing oxidant species and free radicals that defend against bacterial pathogens.1 MPO and its byproducts may also cause damage to the endothelial cells, contributing to plaque formation and instability. Several biochemical pathways have been proposed to explain the pathogenic role of MPO in atherosclerosis. MPO acts as an enzymatic catalyst and initiates low density lipoprotein (LDL) peroxidation leading to enhanced LDL uptake and contributing to foam cell development and plaque formation.2 MPO also selectively oxidizes the apoA1 component of HDL in atherosclerotic lesions, inhibiting ABCA1-mediated cholesterol efflux from macrophages, one of the principal anti-atherosclerotic mechanisms of HDL. In addition, MPO consumes nitric oxide, leading to vasoconstriction and promoting endothelial damage.24
Several in vivo models provide further support for a role of MPO in atherosclerosis development. Over-expression of the human MPO gene in mice susceptible to atherosclerosis led to a 2.3-fold larger area of aortic atherosclerosis compared with wild type mice without the MPO transgene.3 In rabbits, MPO activity was increased 3-fold in atherosclerotic compared to normal blood vessels.4 In human studies, MPO and products of MPO-catalyzed oxidation reactions have been identified in human atherosclerotic lesions.5, 6
MPO as a Biomarker of Atherosclerosis
Although a number of prior studies have reported associations between MPO and the extent of coronary atherosclerosis, these studies focused largely on symptomatic populations with a high burden of disease, contrasted with our population-based sample. In patients presenting with chest pain as well as those with ACS, some prospective studies have confirmed an independent association between higher concentrations of MPO and subsequent adverse coronary events.7, 10, In previous cross sectional studies, MPO levels have also shown a gradient across coronary disease phenotypes, with lowest levels in patients without coronary artery disease (CAD), intermediate levels in patients with stable CAD and highest levels in patients with acute coronary syndromes (ACS).8, 9 In contrast, other studies have not confirmed associations of MPO with stable coronary artery disease and or myocardial infarction.25
Little is known about the role of MPO as a biomarker of non-coronary vascular disease. One prior study, which enrolled 931 individuals with a strong family history of hypertension, reported that higher MPO levels associated with lower ankle-brachial index and with clinical PVD after adjustment for traditional risk factors.26 In this study, similar associations were seen in African American and non-African American participants. Additional studies correlating MPO with carotid stenosis measured by duplex ultrasound have been performed, but these were limited to Caucasian subjects or patients with familial hypercholesterolemia.27, 28 To our knowledge, no prior study has correlated MPO with coronary and aortic atherosclerosis in the general population.
Differential Associations of MPO with Atherosclerosis Phenotypes
We report independent associations between MPO and measures of aortic atherosclerosis, but no association with coronary calcification. These discordant observations may reflect biological differences in the role of MPO in different vascular beds, or alternatively may reflect the role of MPO in different stages of atherosclerosis. While coronary calcification is specific for coronary atherosclerosis, calcification is a relatively late manifestation of atherosclerosis, and is more common in stable as compared with vulnerable plaques. Thus, while we observed no association of MPO with calcified plaque, we cannot exclude associations with noncalcified coronary plaque. Although one prior study showed that MPO levels increased according to CAC categories, the increments were marginal, and the association was attenuated after adjustment for risk factors.29 In contrast to coronary calcification, the aortic measures, and in particular AWT, may represent an earlier phase of atherosclerosis.19 MPO may contribute more importantly toward earlier than later stages of atherosclerosis, a hypothesis consistent with the known role of MPO in foam cell development.2
An alternative hypothesis is that MPO might function differently in non-coronary versus coronary vascular beds. Although risk factors are generally similar between PVD and CAD, some notable differences exist. For example, smoking has a greater impact on non-coronary than coronary vascular disease. In our study, MPO associated with smoking exposure, as well as smoking-related biomarkers such as SP-B. We speculate that the effect of MPO in the peripheral vasculature could represent a pro-inflammatory effect of tobacco exposure, a hypothesis that requires direct testing for confirmation.
Associations of other inflammatory biomarkers with coronary and aortic atherosclerosis phenotypes have previously been reported from the Dallas Heart Study, and several additional analyses were performed for the present study. For example, in risk-factor adjusted models, IL18 and hsCRP were not associated with either coronary or peripheral atherosclerosis,12,14 while osteoprotegerin significantly associated with atherosclerosis in both vascular beds.13 MCP-1 had previously been reported to have associations with CAC that attenuated after adjustment for risk factors; 22 new analyses show similar attenuation of associations of MCP-1 with AP and AWT after adjustment (data not shown). Several other biomarkers have shown discordant associations with coronary calcification and aortic atherosclerosis in multivariable models. SP-B 21 and MMP-930 were only associated with aortic atherosclerosis while sRAGE (new analyses for aortic measurements, data not shown) associated only with coronary calcification.23 In aggregate, these findings suggest that some inflammatory pathways may play distinct roles in the coronary and peripheral vascular beds. Further elucidation of the specific roles of these inflammatory contributors to atherosclerosis may help to identify novel treatment approaches.
Race-specific Associations Between MPO and Atherosclerosis
Based on National Health and Nutrition Examination Survey in 1999–2000, the prevalence of PVD in African Americans was 7.9%, but only 4.4% in white and 3.0% in Hispanics, demonstrating an almost 3-fold excess PVD prevalence in African Americans after adjustment for age and sex.11 Another prior study reported that African American race was a significant predictor of a lower ankle-brachial index and presence of PVD after adjusting for age and other traditional risk factors.31 These epidemiological observations suggest that differences in traditional risk factors do not fully explain racial disparities in PVD and that novel risk factors may play a role. In the present study, we found that MPO levels were higher in African Americans than whites, and that MPO was significantly associated with aortic atherosclerosis only in African Americans. This observation, which requires confirmation in additional studies, suggests that differences in inflammatory pathways may contribute to disparities in PVD between African Americans and other race/ethnicity groups. MPO may be a modifiable target for PVD prevention or treatment, particularly in African Americans, who have the highest burden of PVD.
Study Limitations
The associations of MPO with peripheral atherosclerosis measures, although statistically significant and independent of traditional risk factors, were quantitatively modest. Associations of this magnitude do not suggest that MPO will have a role in clinical practice to identify individuals with or at risk for aortic atherosclerosis.32 The cross-sectional nature of this study prevents establishment of any cause-effect relationships, and thus our findings should be considered hypothesis generating. Finally, although reproducibility studies have been performed for CAC and AWT, such studies have not yet been performed for aortic plaque measurements.
Conclusion
In a large and multi-ethnic population, MPO was independently associated with aortic atherosclerosis but not coronary atherosclerosis. These associations were restricted to African Americans, a finding that suggests differences in inflammation may in part explain ethnic disparities in peripheral vascular disease.
Supplementary Material
Footnotes
Disclosure:
Dr. de Lemos has received grant support from Roche Diagnostics and Alere, and consulting income from Tethys Biomedical. Dr. McGuire has received consulting income from Tethys Biomedical.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277:46116–46122. doi: 10.1074/jbc.M209124200. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Cheng Y, Ji R, Zhang C. Novel model of inflammatory neointima formation reveals a potential role of myeloperoxidase in neointimal hyperplasia. Am J Physiol Heart Circ Physiol. 2006;291:H3087–3093. doi: 10.1152/ajpheart.00412.2006. [DOI] [PubMed] [Google Scholar]
- 4.Ronald JA, Chen JW, Chen Y, Hamilton AM, Rodriguez E, Reynolds F, Hegele RA, Rogers KA, Querol M, Bogdanov A, Weissleder R, Rutt BK. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation. 2009;120:592–599. doi: 10.1161/CIRCULATIONAHA.108.813998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazen SL, Heinecke JW. 3-chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 8.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 9.Ndrepepa G, Braun S, Mehilli J, von Beckerath N, Schomig A, Kastrati A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur J Clin Invest. 2008;38:90–96. doi: 10.1111/j.1365-2362.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 10.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the united states: Results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 12.Zirlik A, Abdullah SM, Gerdes N, MacFarlane L, Schonbeck U, Khera A, McGuire DK, Vega GL, Grundy S, Libby P, de Lemos JA. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: Results from the dallas heart study. Arterioscler Thromb Vasc Biol. 2007;27:2043–2049. doi: 10.1161/ATVBAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 13.Abedin M, Omland T, Ueland T, Khera A, Aukrust P, Murphy SA, Jain T, Gruntmanis U, McGuire DK, de Lemos JA. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the dallas heart study) Am J Cardiol. 2007;99:513–518. doi: 10.1016/j.amjcard.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 14.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr, Grundy SM, de Lemos JA. Race and gender differences in c-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The dallas heart study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Jain T, Peshock R, McGuire DK, Willett D, Yu Z, Vega GL, Guerra R, Hobbs HH, Grundy SM. African americans and caucasians have a similar prevalence of coronary calcium in the dallas heart study. J Am Coll Cardiol. 2004;44:1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 17.Rohatgi A, Ayers CR, Khera A, McGuire DK, Das SR, Matulevicius S, Timaran CH, Rosero EB, de Lemos JA. The association between peptidoglycan recognition protein-1 and coronary and peripheral atherosclerosis: Observations from the dallas heart study. Atherosclerosis. 2009;203:569–575. doi: 10.1016/j.atherosclerosis.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Jaffer FA, O'Donnell CJ, Larson MG, Chan SK, Kissinger KV, Kupka MJ, Salton C, Botnar RM, Levy D, Manning WJ. Age and sex distribution of subclinical aortic atherosclerosis: A magnetic resonance imaging examination of the framingham heart study. Arterioscler Thromb Vasc Biol. 2002;22:849–854. doi: 10.1161/01.atv.0000012662.29622.00. [DOI] [PubMed] [Google Scholar]
- 19.Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, Timaran CH. Sex, race, and age distributions of mean aortic wall thickness in a multiethnic population-based sample. J Vasc Surg. 2011;53:950–957. doi: 10.1016/j.jvs.2010.10.073. [DOI] [PubMed] [Google Scholar]
- 20.Rosero EB, Peshock RM, Khera A, Clagett GP, Lo H, Timaran C. Agreement between methods of measurement of mean aortic wall thickness by mri. J Magn Reson Imaging. 2009;29:576–582. doi: 10.1002/jmri.21697. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen AB, Rohatgi A, Garcia CK, Ayers CR, Das SR, Lakoski S, Berry J, Khera A, McGuire DK, de Lemos JA. Interactions between smoking, pulmonary surfactant protein b and atherosclerosis in the general population: the dallas health study. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.111.228692. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey JB, de Lemos JA, Cipollone F, Ayers CR, Rohatgi A, Morrow DA, Khera A, McGuire DK. Association between circulating soluble receptor for advanced glycation end products and atherosclerosis: Observations from the dallas heart study. Diabetes Care. 2009;32:1218–1220. doi: 10.2337/dc09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 25.Apple FS, Smith SW, Pearce LA, Murakami MM. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2009;55:93–100. doi: 10.1373/clinchem.2008.102905. [DOI] [PubMed] [Google Scholar]
- 26.Ali Z, Sarcia P, Mosley TH, Jr, Kondragunta V, Kullo IJ. Association of serum myeloperoxidase with the ankle-brachial index and peripheral arterial disease. Vasc Med. 2009;14:215–220. doi: 10.1177/1358863X08101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Exner M, Minar E, Mlekusch W, Sabeti S, Amighi J, Lalouschek W, Maurer G, Bieglmayer C, Kieweg H, Wagner O, Schillinger M. Myeloperoxidase predicts progression of carotid stenosis in states of low high-density lipoprotein cholesterol. J Am Coll Cardiol. 2006;47:2212–2218. doi: 10.1016/j.jacc.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 28.Meuwese MC, Trip MD, van Wissen S, van Miert JN, Kastelein JJ, Stroes ES. Myeloperoxidase levels are not associated with carotid atherosclerosis progression in patients with familial hypercholesterolemia. Atherosclerosis. 2008;197:916–921. doi: 10.1016/j.atherosclerosis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Wong ND, Gransar H, Narula J, Shaw L, Moon JH, Miranda-Peats R, Rozanski A, Hayes SW, Thomson LE, Friedman JD, Berman DS. Myeloperoxidase, subclinical atherosclerosis, and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2:1093–1099. doi: 10.1016/j.jcmg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Grodin JLPT, Rohatgi A, Khera A, Banks K, Ayers CR, de Lemos JA, Das SR. Matrix metalloproteinase-9 is independently associated with aortic wall thickness: From the dallas heart study [abstract] Circulation. 2010:A18342. [Google Scholar]
- 31.Kullo IJ, Bailey KR, Kardia SL, Mosley TH, Jr, Boerwinkle E, Turner ST. Ethnic differences in peripheral arterial disease in the nhlbi genetic epidemiology network of arteriopathy (genoa) study. Vasc Med. 2003;8:237–242. doi: 10.1191/1358863x03vm511oa. [DOI] [PubMed] [Google Scholar]
- 32.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115:949–952. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.