Abstract
While cognitive changes and mood instability are frequent symptoms reported by menopausal women, the degree to which the decline in estrogen production is responsible is not yet clear. Several lines of evidence suggest that estrogen may produce its effects on cognition and mood through modulation of serotonergic function. To test this hypothesis, we used the tryptophan depletion (TD) paradigm to lower central serotonin levels and pharmacologically manipulated estrogen levels in healthy menopausal women. We examined the individual and combined effects of estradiol and serotonin on working memory, emotion processing and task-related brain activation. Eight healthy predominantly early postmenopausal women underwent TD or sham depletion followed by functional magnetic resonance imaging (fMRI) both before and after short-term transdermal estradiol 75-150 ug/d administration. There was an estradiol treatment by TD interaction for brain activation during performance on both the N-back Task (working memory) and Emotion Identification Task (affective processing). During the 2-back condition, TD attenuated activation prior to, but not after, estradiol treatment in the right and left dorsal lateral prefrontal and middle frontal/cingulate gyrus. During emotion identification, TD heightened activation in the orbital frontal cortex and bilateral amygdala, and this effect was attenuated by estradiol treatment. These results provide preliminary evidence that serotonergic effects directly mediate the impact of estrogen on brain activation during working memory and affective processing.
INTRODUCTION
The menopause transition is frequently accompanied by depressive symptoms and subjective declines in cognitive function (Shively & Bethea, 2004; Freeman, 2010; McVeigh 2005; Schnatz et al., 2006). Unlike the well-characterized relationship between ovarian declines in estrogen production and vasomotor and urogenital symptoms, the role of hypoestrogenism in behavioral and cognitive disturbances during the menopause is less clearly established and frankly controversial (Schmidt, et al 2000; Henderson, et al 2003; Shumaker, et al 2003; Gibbs, et al 2006; Joffe, et al 2006; Krug, et al 2006; Kok, et al 2006; Soares, et al 2006; Morrison, et al 2006, Maki, et al 2007; Epperson, et al 2007; Luetters, et al 2007; Sherwin & Henry, 2008, Resnick et al 2009; Coker et al 2010; NAMS 2010).
Estrogen has pronounced effects on numerous neurotransmitter systems (McEwen, 2002; Amin, et al 2005) neurotrophins (Scharfman, et al 2006, Suzuki, et al 2009), and brain cytoarchitecture, structure and function (Hao, et al 2006; Kramár, et al 2009), all possible mechanisms by which estrogen exerts effects on neural systems involved in mood and cognition. In this study, we focus on the estrogen-serotonin interaction for several reasons. First, estradiol modulates serotonergic function from the level of neurotransmitter synthesis and degradation (Smith et al 2004; Sanchez et al 2005) to the density of post-synaptic 5-HT type 2A (5-HT2A) receptors (Kugaya, et al 2003, Moses-Kolko, et al 2003) and sensitivity of the presynaptic 5-HT type 1A (5-HT1A) autoreceptor (Hamilton & Bethea, 2008). Second, intact serotonergic function is important for healthy mood, learning and memory (Mendelsohn, et al 2009; Robbins & Arnsten, 2009), hence the heuristic importance of investigating this hormone-neurotransmitter interaction in menopausal women. Finally, central serotonin levels can be safely manipulated in human subjects using the tryptophan depletion (TD) paradigm, thus allowing investigators to probe the relative importance of intact serotonin function on behavior (Van der Does, 2001).
TD is achieved by administering an oral load of large neutral amino acids (LNAA) excepting tryptophan (Young, 1985). In addition to stimulating protein synthesis, these LNAAs compete with tryptophan already present in the blood for passage across the blood brain barrier. This dual process essentially depletes central nervous system (CNS) tryptophan levels. Without its precursor available, brain levels of serotonin plummet (Carpenter, et al 1998). Notably, neuroimaging studies suggest that the rate of serotonin synthesis declines more rapidly and to a greater degree during TD in women compared to men (Nishizawa, et al 1997).
TD can trigger depressive symptoms in individuals with selective serotonin reuptake inhibitor (SSRI)-remitted depression (Delgado, et al 1999), but also in healthy subjects who are vulnerable to affective disturbance secondary to a positive family history, serotonin transporter genotype, or female sex (Benkelfat, et al 1994; Ellenbogen, et al 1996; Klaassen, et al 1999, Neumeister, et al 2002). This phenomenon was not observed in menopausal women whose depression had resolved with estradiol treatment (ET) alone, ET plus SSRI or SSRI alone (Epperson, et al 2007). While there appears to be a modest sex difference in biochemical and behavioral response to TD, the impact of ET on TD-induced behavioral change in women has not been elucidated.
With respect to cognitive processes in healthy humans, TD impairs verbal learning (Park, et al 1994, Schmitt, et al 2000; McAllister-Williams, et al 2002; Amin, et al 2006), paragraph recall (Amin, et al 2006), response inhibition, decision-making and processing of reward cues (Park, et al 1994, Rogers, et al 1999; 2003; Murphy, et al 2002), and improves focused attention (Schmitt, et al 2000; Gallagher, et al 2003) and verbal fluency (Schmitt, et al 2000). It is less clear whether TD negatively impacts working memory, an aspect of cognition for which there is evidence of estrogen enhancement (Harrison, et al 2004; Riedel, et al 2004; Maki, et al 2001, Resnick & Maki, 1998; Shaywitz, et al 2003; Wolf & Kirschbaum, 2002; Duff and Hampson, 2000; Phillips & Sherwin, 1992; Henderson & Sherwin, 2007; Joffe, et al 2006).
Because estrogen has trophic effects on brain structures underlying important cognitive processes (Rapp, et al 2003) and potent serotonin-modulating properties, we hypothesized that estradiol administration would reverse the effects of TD on neural systems thought to underlie working memory and affective processing. Here we examined performance and brain activation in healthy, predominantly early postmenopausal women undergoing TD and sham depletions pre- and post-transdermal estradiol administration. Women underwent functional magnetic resonance imaging (fMRI) while performing a working memory (N-back Task) and an affective processing task (Emotion Identification).
METHODS
Participants
Women ages 45-60 were recruited to a university-based women's behavioral health specialty research program by posted fliers, paid advertising, word of mouth and home mailings. Nine right-handed women without history of any Axis I psychiatric or substance use disorder according to the Structured Clinical Interview for Diagnosis-DSM-IV (SCID)-Non-Patient Version were enrolled. History of first-degree relatives with a substance use disorder, but not psychiatric disorder, was allowed. Women who had menstrual cycle irregularity or were within 15 years of their final menstrual period (FMP) and had follicular stimulating hormone levels of ≥ 20 IU/ml were eligible. All subjects were required to provide documentation of having a normal PAP smear, mammogram, and breast and pelvic examinations within the previous year. Women were in good health and without history of any significant medical problems including, but not limited to, cancer, unstable hypertension, known cardiovascular disease, thromboembolic disease, neurological disorders or previous head injury. All women were without hormone use for at least twelve months prior to participation. Subjects gave written informed consent to participate in this study, which was approved by the Yale University School of Medicine Human Investigations Committee. A total of 8 women with mean ± SD age of 53.4 ± 3.9 years completed all four test days and were included in data analyses. They were paid for each test day with an additional bonus for completing all four testing sessions.
Tryptophan Depletion Procedure
This study used a double-blind, placebo-controlled, cross-over design with subjects undergoing an active and sham depletion prior to and after 3-8 weeks of transdermal estradiol 75-150 ug/d (Vielle-Dot®, Novartis, Hamilton, New Jersey). On each test day, participants presented to the General Clinical Research Center at 7:30am after an overnight fast and ingested 70 capsules containing either 31.5 gm of amino acids without tryptophan (Active Depletion) or 31.5 gm of lactose (Sham Depletion). The amino acid mixture consisted of L-isoleucine 4.2 gm, L-leucine 6.6 gm, L-lysine 4.8 gm, L-methionine 1.5 gm, L-phenylalanine 6.6 gm, L-threonine 3.0 gm and L-valine 4.8 gm. This combination of amino acids reliably lowers plasma total and free tryptophan by 71-78 % (Neumeister, et al 2004) and results in behavioral and/or brain metabolism change (Nugent, et al 2008; Neumeister, et al 2005, 2006). Blood was taken for free tryptophan analysis prior to and approximately 6 hours after consumption of the amino acid mixture. Participants remained fasting and involved in quiet, sedentary activity until their fMRI scans.
Estrogen Treatment
After completing two test days, subjects took transdermal estradiol 75-150 ug/d (Vivelle-Dot®, donated by Novartis Pharmaceuticals, Hamilton, NJ) for at least 3 weeks and then underwent their 3rd and 4th TD and fMRI test days. Dose of estrogen was adjusted from 75 ug/d to 150 ug/d after 2-3 weeks of treatment if estrogen levels were not above 50 pg/ml and at least two-fold higher than baseline. For the five women with an intact uterus, this was followed by treatment with oral micronized progesterone (Prometrium® Abbott Laboratories, Abbott Park, IL) 200 mg/d for 10 days.
Tryptophan and Hormone Assays
Free plasma tryptophan levels were determined by direct injection of 5 uL of plasma ultrafiltrate on a high-performance liquid chromatographic-fluorometric system (Anderson, et al 1981). Tryptophan levels were measured with within-assay and assay-to-assay coefficients of variation of less than 5 and 10%, respectively, and with a detection limit of less than 0.01 ug/ml.
Estradiol levels were evaluated in the morning of each test day and measured by competitive immunoassay using a chemiluminescent substrate in a commercially available kit provided by Diagnostic Productions Corporation, Los Angeles, CA. The sensitivity for this kit is 15 pg/ml and the approximate coefficient of variability at ranges observed in this study is 11-13%.
Mood Assessment
Clinician and patient ratings were performed the morning prior to and 6 hours after ingesting the amino acid or lactose capsules. Ratings consisted of the 19-item Hamilton Depression Rating Scale (HDRS; Hamilton, 1960) and the Profile of Mood States (POMS; McNair, et al 1992). The HDRS is a clinician-rated instrument widely used to assess severity of depressive symptoms. The POMS requires that subjects rate themselves with respect to 65 different adjectives, such as ‘friendly’, ‘listless’, ‘on edge,’ which are then utilized to compute scores for 6 subscales; depression, tension/anxiety, anger/hostility, fatigue, vigor, and confusion/bewilderment.
Cognitive and Affective Task
N-back Task
The N-back Task is a working memory task that reliably activates the dorsal lateral prefrontal cortex (DLPFC) and middle frontal/cingulate gyrus (MF/CG). Enhanced activation was reported in non-human primate studies of estrogen's effects on working memory (Rapp, et al 2003; Huo, et al 2006). Subjects completed a 2-Back task that was created using an E-Prime stimulus presentation program (Psychology Software Tools, Pittsburgh, PA). There were four versions of the task in order to minimize practice effects across scans. Order was counterbalanced across subjects. Subjects were instructed to press one of two buttons to indicate whether a stimulus presented on the screen is the same or not the same as the stimulus that was presented two trials earlier (2-Back). A control condition (0-back) required the subject to press either button when a target stimulus was presented. The stimuli consisted of 4-letter non-words (e.g. “cnsy”) without vowels. The two conditions (2-Back and 0-Back) alternated in 6, 18-second blocks, which were preceded by an instructions screen for 3 seconds. Blocks consisted of 12 trials of 1500 ms each.
Emotion Identification Task
The Emotion Identification task probes emotional bias under various affective conditions and has been extensively studied in conjunction with the TD paradigm (reviewed by Mendelsohn, et al 2009). Estrogen may be associated with increased brain activation to positive affective stimuli (Amin, et al 2006), while TD is associated with bias towards negative affective stimuli (Evers, et al 2006; Klaassen, et al 2002). In addition, tasks involving emotional face identification are associated with amygdala and orbital frontal cortex (OFC) activation (Lidaka, et al 2001; Streit, et al 2003). TD has been found to modulate the OFC (Rubia, et al 2005) and enhanced serotonin transmission has been associated with attenuating the amygdala's response to negative emotional face stimuli (Del-Ben, et al 2005). Animal studies indicate that the amygdala has among the highest concentrations of estrogen receptors in the brain (Hagihara, et al 1992; Merchenthaler, et al 2004, Mitra, et al 2003), highlighting the importance of the amygdala as a region of interest in this study.
The face processing task was also created using E-Prime. Subjects were randomly presented with images of 30 individuals with angry, happy and neutral facial expressions (a total of 90 face pictures). They were instructed to choose which emotion was being expressed. Face stimuli were obtained from a set of digital images which depicted approximately 40 individuals posing several different facial expressions (Tottenham, 2002). During a variable inter-stimulus interval (0.5-16 sec) a fixation cross was randomly presented as a low level baseline. Each trial lasted 2500 ms, during which each face was presented for 150 ms followed by a choice screen lasting 2350 ms.
Image Acquisition
Brain imaging was conducted on a Siemens 3.0 Tesla Trio system. For structural whole brain images, the first scan was a sagittal localizer, followed by a T1 scan oriented in the axial-oblique dimension, parallel to the anterior commissure-posterior commissure (AC-PC) line (24 slices, RT=300 ms, matrix size = 256 × 256, FOV = 220 × 220 mm). A three dimensional high resolution spoiled gradient scan was conducted (176 slices, RT = 2530 ms, matrix size = 256 × 256, FOV = 256 × 256 mm). Functional whole-brain images were acquired using a gradient echo T2-weighted echoplanar imaging scan (RT = 1500 ms; echo delay = 30 ms; flip angle = 80°, FOV = 220 × 220 mm).
Image Analysis
BOLD time series data were analyzed with FEAT (fMRI Expert Analysis Tool) Version 5.92, part of FSL 4.0, using standard image analysis procedures including brain extraction, slice time-correction, motion correction, high pass filtering (100 seconds), spatial smoothing (6 mm FWHM, isotropic), and mean-based intensity normalization. The median functional and anatomical volumes were coregistered, and the anatomical image was transformed into standard space (2mm3 T1 MNI template). Resulting transformation parameters were later applied to statistical images and the images were resampled (2mm3) before group level analyses.
Subject-level statistical analyses were carried out using FILM (FMRIB's Improved Linear Model) with local autocorrelation correction (Smith, 2004). For the N-back task, condition events (0-back, 2-back) were modeled using a canonical hemodynamic response function. The Instruction period and motion correction parameters were included as nuisance covariates and the rest condition (fixation point) was treated as the unmodeled baseline. Similar procedures were used for Emotion Identification Task modeling the three condition events (Happy, Angry, and Neutral) with fixation as baseline. To characterize the treatment (pre vs post estrogen) by condition (sham vs active depletion) effects for the N-back task, mean percent signal change for the 2-0 back contrast was extracted from functionally defined regions of interest (ROIs) in the dorsolateral prefrontal cortex (DLPFC right and left) and medial frontal/cingulate gyrus (MF/CG). Similar procedures were applied to the Emotion Identification task using atlas based ROIs for the amygdala (right and left) and orbital frontal cortex (OFC right and left). Mean percent signal change values were exported for analysis using procedures described below.
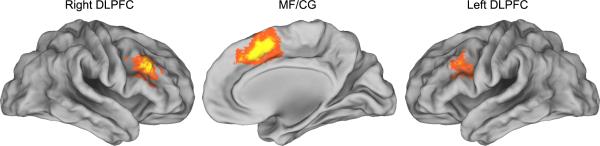
Region of interest masks for the DLPFC and MF/CG were functionally defined using the main effect of memory load (2-0 back) map derived from a whole brain repeated measures ANOVA of this sample (see Figure 1). The activation map was cluster corrected at voxel threshold of Z ≥ 4.26 and cluster probability of p<0.05 (Woolrich, 2004). These procedures produced well-defined clusters in the right DLPFC, left DLPFC, and the MF/CG consistent with our previous studies (2005; Loughead et al, 2009; Loughead et al, 2010). ROIs for the amygdala and OFC were anatomically defined using the Harvard-Oxford probabilistic atlas (Maximal Probability Threshold: 25%). ROI masks were transformed into native subject space using methods described above. Mean percent signal change values for each subject (from all ROIs described above) were entered as the dependent variable in a repeated-measures analysis of variance (ANOVA) with ROI , condition (active vs sham depletion) and treatment (pre-estrogen vs post-estrogen) as within-subject factors using SAS, version 9.2 (SAS Institute Inc., Cary, NC). The N-back and Emotion Identification tasks were tested in separate ANOVAs.
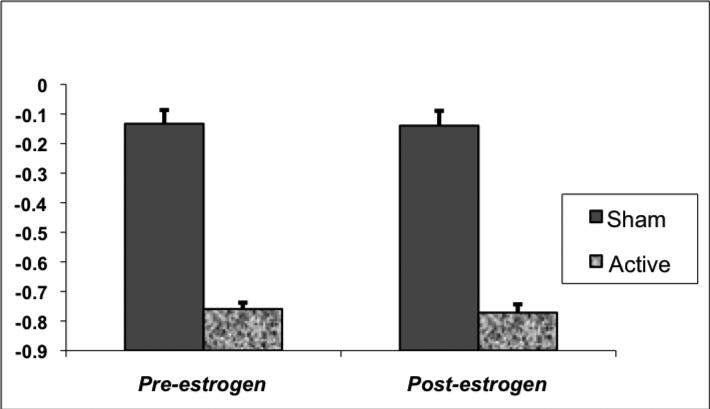
FIGURE 1. Change in Free Tryptophan Levels During Active and Sham Depletion.
Free plasma tryptophan levels decreased by 64% to 90% from pre to 6 hours pos-ingestion of an amino acid mixture without tryptophan. Free plasma tryptophan levels decreased between 5% and 26% on sham depletion test days.
Data Analysis: Behavioral Measures and Assays
Behavioral and hormone data were assessed for normality before analysis using normal probability plots and Kolmogorov-Smirnov test statistics. Variables were log-transformed as necessary. Analysis of plasma free tryptophan was performed with a linear mixed model, which included within-subjects effects of condition (active vs sham depletion) and treatment (pre-estrogen vs post-estrogen) and random subject effects. The interaction between treatment and condition was also fitted and explained by appropriate post-hoc test. The best-fitting covariance structure was selected according to information criteria. Plasma estradiol, performance on the N-back and Emotion Identification Tasks, and mood assessments were analyzed using similar models as described above, with the exception that time (PM-AM) was included as a third factor in the analysis of HDRS and POMS data. Bonferroni correction was applied within but not between hypotheses.
RESULTS
Tryptophan and Estrogen Assays
Mean number of months since FMP was 28.1 ± 10.9 months (range 12-41 months) in those women with intact uterus or hysterectomy post FMP. Final menstrual period could not be confirmed for one 55 year-old subject who had a partial hysterectomy prior to menopause, but her FSH of 106 IU/ml confirmed that she was post-menopausal at the time of study participation. One other woman had hysterectomy with bilateral oophorectomy during the menopause transition.
All subjects ingested the 70 capsules within the allotted 45-minute time frame and without vomiting or significant nausea. As expected, there was a main effect of TD on plasma free tryptophan levels (F(1, 20)=249, p<0.0001), with mean ± SD decreases of 75.9 ± 0.06% before and of 77.2 ± 0.07% after ET, but no significant interaction between estrogen treatment and TD condition (F(1, 20)=0.01, p=.95) (Figure 1). No significant changes in tryptophan levels were noted on sham test days either before (-13.3 ± 13.1%) or after (-13.9 ± 14.2%) ET.
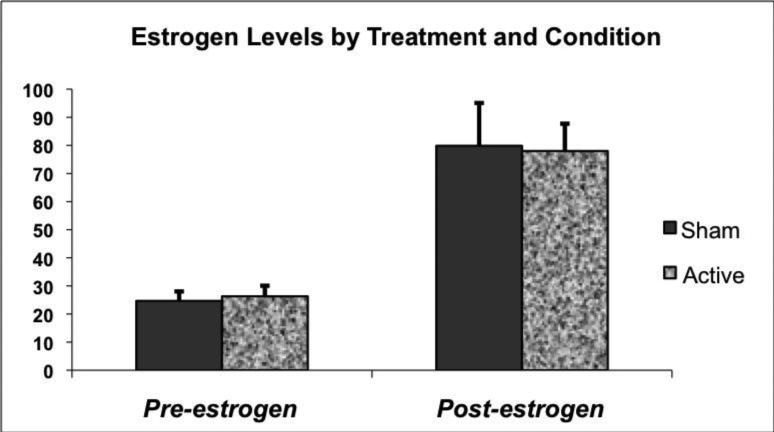
There was no significant treatment (estrogen) by condition (TD versus sham) interaction (F(1, 21)=0.05, p=0.83) in the model comparing estradiol levels. However, as expected estrogen treatment resulted in significantly increased plasma estradiol levels with mean ± SD being 26.3 ± 10.6 pg/ml during TD and 24.7 ± 9.6 pg/ml during sham depletion before estrogen treatment and 78.0 ± 27.5 pg/ml on active and 79.9 ± 43.1 pg/ml on sham tests days during treatment (F(1, 21)=25.7, p<0.0001) (Figure 2).
FIGURE 2. Estradiol Levels Pre and Post Estrogen Treatment.
Estradiol levels increased with estrogen administration but did not differ significantly between active and sham depletion test days after estrogen administration.
Mood Assessment
Mean ± SD HDRS scores were all within the asymptomatic range of 1 ± 1 to the highest of 2 ± 3. Both HDRS and POMS subscale scores remained stable in all subjects across all test days. There was no significant treatment by condition interaction for the HDRS or POMS subscales (all p>0.05) (Data not shown).
Task Performance
Reaction time during the 2-Back task on TD (695 ± 100 msec) and Sham (712 ± 56 msec) test days before and after ET (TD; 681 ± 128 msec and Sham; 681 ± 71 msec) did not differ (interaction: F(1, 21)=0.14, p=0.71). Similarly, there was no significant interaction between ET and condition with respect to errors made during performance of the 2-back (F(1, 21)=0.01, p=0.95) or the 0-back (F(1, 21)=0.09, p=0.76) blocks. Reaction time (F(1, 19)=2.49, p=0.13) and number of errors (F(1, 19)=3.11, p=0.1) during Emotion Identification Task performance were similar between treatments across conditions.
N-back Region of Interest Analysis
The repeated measures ANOVA of mean percent signal change showed a main effect of condition (F(1,83)=5.30, p=0.024), indicating reduced activation in the working memory regions for active depletion (vs sham). There was no significant main effect of treatment (F(1,83)=0.92, p=0.34) or ROI (F(2,83)=0.92, p=0.34). However a significant condition × treatment interaction was observed [F(1,83)=5.22, p=.025], indicating that under the pre-estrogen treatment, mean percent signal change was reduced by active depletion (vs sham) but post-treatment, TD did not modulate BOLD signal. The other 2-way interactions were removed from the model (as well as the 3-way interaction) as none were statistically significant. Although the ROI by condition by treatment ANOVA showed a significant ME (condition) and a two way interaction (condition by treatment), subsequent analyses in the individual ROIs revealed no significant effects (Figure-4).
Figure 4.
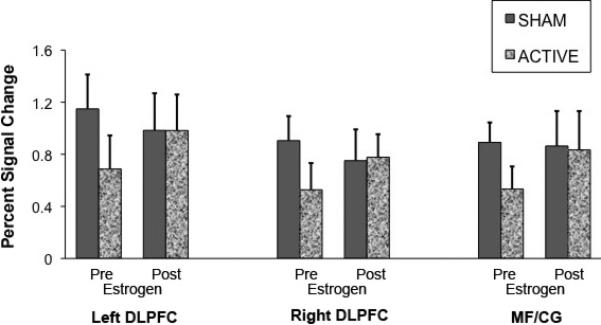
Mean percent signal change for the 2-back minus 0-back contrast calculated from functionally defined dorsolateral prefrontal cortex (DLPFC) and medal frontal/cingulate gyrus (MF/CG) regions of interest.
Emotion Identification Region of Interest Analysis
For the Face task, the repeated measures ANOVA on the mean percent signal change data showed a significant main effect of condition (F(1,114)=14.53, p=0.0002, indicating increased activation for the active depletion (vs sham) condition in the orbital frontal cortex and bilateral amygdala. There was a significant main effect of treatment [F(1,114)=11.26, p=0.001], indicating reduced activation on estradiol treatment in both regions compared to pre treatment. There was no significant main effect of region (F(3,114)=0.24, p=.872 ). The condition × treatment two-way interaction was found to be significant (F(1,114)=7.07, p=.009). In contrast to N-back, the Face task pre-treatment session showed increased mean percent signal change under active depletion and, as above, estrogen treatment negated TD effect on BOLD signal.
As with the N-back task, other 2-way and 3-way interactions were removed from the model as none were statistically significant. The ROI by condition by treatment ANOVA showed significant main effects (condition, treatment) and a two way interaction (condition by treatment), however subsequent analyses in the individual ROIs revealed no significant effects (Figure-5).
Figure 5.
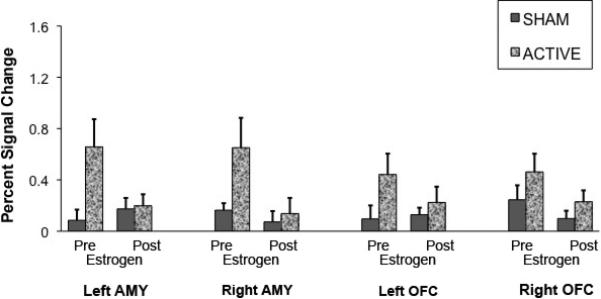
Mean percent signal change for the Emotion Identification task effect calculated from anatomically defined Amygdala and Orbital Frontal Cortex.
DISCUSSION
This study is unique in that inclusion of estrogen treatment with the TD paradigm allows for the investigation of individual and interactive effects of estrogen and serotonin on brain activation and behavior. To our knowledge this is the first study to examine brain activation during TD in subjects undergoing both affective and cognitive processing tasks. That TD had a significant impact on brain activation during performance of both tasks in a group of postmenopausal women suggests that intact serotonergic function is crucial to the underlying neural networks mediating these behaviors. The direction of TD's effect on BOLD signal differed between tasks, providing evidence that the role of serotonin in affective and cognitive processes is disparate.
The individual effect of estrogen treatment on brain activation during performance of the Emotion Identification Task was significant, while there was only a modest effect of estrogen during performance of the N-back Task. An intriguing finding with respect to estrogen is that hormone treatment resulted in reversal of the effects of TD on brain activation during both tasks. Although preliminary in nature, these data provide novel evidence of an interactive effect of estrogen and serotonin in verbal working memory and processing of emotional faces. The direction of the estrogen-serotonin interaction differed between tasks, suggesting specificity of the interaction to the type of cognitive or affective task. Our failure to demonstrate a significant effect of either TD or estrogen administration on task performance is possibly due to power but is also consistent with findings from other studies (Dumas, et al. 2010; Persad, et al. 2009; Joffe, et al. 2006; Allen, et al. 2006; Shaywitz, et al. 1999).
Previous studies of the effect of TD on brain activation during working memory tasks have been inconsistent, with some finding a reduction in activation (Cerasa, et al 2008) while others finding no significant effect (Gallagher, et al 2003; Allen, et al 2006). The effects of estrogen alone in the right and left DLPFC and MF/AC was not impressive, yet non-human primate studies suggest estrogen-enhanced DLPFC function and performance in ovariectomized female rhesus macaques performing a DLPFC-dependent task (Hao, et al 2006). In addition, another study using PET receptor imaging before and after a dose and duration of estrogen similar to that used in this study showed a significant increase in 5HT-2A receptor density in a number of brain regions, including the DLPFC (Kugaya, et al 2001; Moses-Kolko, et al 2004). Including a sham depletion condition was essential in discovering the difference between the impact of TD before estrogen treatment versus during estrogen treatment, and revealed clear evidence that both estrogen and serotonin contribute to verbal working memory. That effects of TD were seen prior to but not during estrogen administration suggests that serotonin plays a primary role in working memory, while estrogen has a moderating role by supporting healthy serotonergic function. This finding has clinical relevance considering that the use of estrogen treatment to promote working memory in healthy aging women is controversial, with some studies indicating a benefit (Duff, et al. 2000; Maki, et al. 2001; Berent-Spillson, et al. 2010) while others not (Reviewed by Lithaby, et al. 2010). These data imply that estrogen's beneficial effects on working memory may be limited to mid-aged and aging women with reduced serotonin function.
Our finding that women experienced an accentuation of amygdala and OFC activation with TD during Emotion Identification is consistent with previous fMRI studies of affective processing during TD (Cools, et al 2005; Fusar-Poli, et al 2007; van der Veen, et al 2007; Williams, et al 2007). Similar to our study, three of the above studies found no observable impact of TD on performance of emotion identification tasks in healthy volunteers (Cools, et al 2005; Fusar-Poli, et al 2007; van der Veen, et al 2007). Three studies, which did not incorporate functional imaging, found that TD impaired recognition of fearful faces (Harmer, et al 2003; Marsh, et al 2006; Merens, et al 2008). This finding was limited to individuals who were ‘at-risk’ for depression as a result of having had a previous episode of depression (Merens, et al 2008) or being heterozygous for the short, and less efficient, allele of the serotonin transporter gene (Marsh, et al 2006). Subjects in the present study could be considered affectively resilient as they have come to mid-life with no history of depression or anxiety disorders, both twice as common in reproductive aged women compared to their male counterparts.
Several studies indicate that baseline serotonin function may impact an individual's behavioral or neural response to TD. Genetic variation in the gene encoding monoamine oxidase A, the enzyme for serotonin metabolism, predicts the degree of brain activation in the ventrolateral prefrontal cortex during performance of the N-back Task (Cerasa, et al 2008). Individuals heterozygous for the short allele of the serotonin transporter showed improved memory and attention (Roiser, et al, 2007), but impaired fear face emotion recognition (Marsh, et al 2006) during TD compared to those heterozygous for the long allele. Individual characteristics such as threat sensitivity (Cools, et al 2005) and gender (Lee, et al 2002) may also contribute to TD-induced changes in brain activation during emotion processing.
While the paradigm employed in this study is unique and the findings are of interest, cautious interpretation is warranted for several reasons. First, the sample size is small making it impossible to consider individual variables such as genotype or psychological sensitivities. Notwithstanding this limitation, all of our participants were postmenopausal, without previous personal or family history of psychiatric disorders and in good general health. Given the study paradigm, 8 subjects represents 32 day-long procedures culminating in an fMRI study. That there are multiple within-subject data points and each subject serves as her own control eliminates sampling error and limits, if not entirely negates, problems related to sample size. Although a placebo control group was not included in this study and would have been ideal, 4 different versions of each task were used and presented in randomized order across test days. The stability of performance across conditions and treatment suggests that our methods may have limited practice effects, although a placebo control would be needed to confirm our assertion of estradiol effects. Finally, time since FMP varied considerably in this small group. There is growing evidence that time since FMP may impact the effects of estradiol on brain function and a considerably larger sample would be required to include this variable as a covariate in our study design.
In summary, this is the first study to examine the effects of TD and estrogen administration on cognitive and affective tasks and brain activation in postmenopausal women. These data provide preliminary evidence that serotonin is important for verbal working memory and face emotion identification and that estrogen supports serotonin function under conditions of TD. That estrogen reverses the effects of acute TD on brain activation suggests that estrogen bolsters serotonergic functioning under conditions of reduced tryptophan and serotonin concentrations. Because serotonin reduction with tryptophan depletion is both dramatic and acute, the generalizability to slower and more chronic reductions in serotonin function seen with normal and pathological aging in humans is admittedly limited. Complementary work may be necessary in preclinical models.
Figure 3. N-Back Brain Rendering Highlights Regions of Interest.
N-back working memory task activation. Bilateral dorsolateral prefrontal cortex (DLPFC) and medal frontal/cingulate gyrus (MF/CG) regions identified by the main effect of working memory load (2 back vs. 0 back) in a whole brain repeated measures ANOVA (p<0.05, corrected). Brain rendering performed with Caret (Van Essen, 2001)
Acknowledgements
The authors wish to acknowledge and thank Novartis for the generous donation of the Vivelle dot® estradiol patches used in this study.
Funding Sources:
This study was funded in part by the National Institute on Aging R01AG03041 (Epperson), KO2MH73090 (Epperson), Women's Health Research at Yale (Epperson) and an Investigator Initiated Grant from Eli Lilly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suggested Reviewers:
Cynthia Bethea, Ph.D. Oregon Health and Science University
Linda Carpenter, M.D. Brown
Julie Dumas, Ph.D. University of Vermont
Natalie Rasgon, Ph.D. Stanford University
Conflict of Interest:
Dr. C. Neill Epperson has been a consultant to Eli Lilly and received research grant support for her research from Eli Lilly and Shire. Dr. Epperson or her dependent owns shares in Johnson and Johnson.
Dr. Gur declares that over the past three years he has received compensation as a consultant to Johnson and Johnson. He is or has been the recipient of Investigator Initiated Research Grants from Merck and from AstraZeneca.
All remaining authors have no conflicts of interest to report.
REFERENCES
- Allen PP, Cleare AJ, Lee F, Fusar-Poli P, Tunstall N, Fu CH, Brammer MJ, McGuire PK. Effect of acute tryptophan depletion on pre-frontal engagement. Psychopharmacology (Berl) 2006;187(4):486–97. doi: 10.1007/s00213-006-0444-x. [DOI] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- Amin Z, Gueorguieva R, Cappiello A, Czarkowski KA, Stiklus S, Anderson GM, Naftolin F, Epperson CN. Estradiol and tryptophan depletion interact to modulate cognition in menopausal women. Neuropsychopharmacology. 2006;31(11):2489–97. doi: 10.1038/sj.npp.1301114. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51(9):687–97. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30(2):212–38. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, Frey KA, Zubieta JK, Smith YR. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010;17(4):692–9. doi: 10.1097/gme.0b013e3181cc49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ. Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology. 1998;19(1):26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS). J Steroid Biochem Mol Biol. 2010;118(4-5):304–10. doi: 10.1016/j.jsbmb.2009.11.007. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Maki PM, Murphy DG. Reversibility of the effects of acute ovarian hormone suppression on verbal memory and prefrontal function in pre-menopausal women. Psychoneuroendocrinology. 2008;33(10):1426–31. doi: 10.1016/j.psyneuen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry. 1999;46(2):212–20. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38(4):262–76. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010;58(5):929–35. doi: 10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Young SN, Dean P, Palmour RM, Benkelfat C. Mood response to acute tryptophan depletion in healthy volunteers: sex differences and temporal stability. Neuropsychopharmacology. 1996;15(5):465–74. doi: 10.1016/S0893-133X(96)00056-5. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Amin Z, Naftolin F, et al. The resistance to depressive relapse in menopausal women undergoing tryptophan depletion: Preliminary findings. J Psychopharmacol. 2007;21:414–20. doi: 10.1177/0269881106067330. [DOI] [PubMed] [Google Scholar]
- Freeman EW. Associations of depression with the transition to menopause. Menopause. Jun 6, 2010. [Epub ahead of print] [DOI] [PubMed]
- Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447–453. doi: 10.1016/j.maturitas.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Massey AE, Young AH, McAllister-Williams RH. Effects of acute tryptophan depletion on executive function in healthy male volunteers. BMC Psychiatry. 2003;4:3–10. doi: 10.1186/1471-244X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Preclinical data relating to estrogen's effects on cognitive performance. In: Rasgon N, editor. Effects of Estrogen on Brain Function. Johns Hopkins University Press; Baltimore, MD: 2006. pp. 9–45. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. Neurosci. 2006;26(9):2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Olver JS, Norman TR, Burrows GD, Wesnes KA, Nathan PJ. Selective effects of acute serotonin and catecholamine depletion on memory in healthy women. J Psychopharmacol. 2004;18(1):32–40. doi: 10.1177/0269881104040225. [DOI] [PubMed] [Google Scholar]
- Henderson JA, Bethea CL. Differential effects of ovarian steroids and raloxifene on serotonin 1A and 2C receptor protein expression in macaques. Endocrine. 2008;33(3):285–293. doi: 10.1007/s12020-008-9087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60:1369–1371. doi: 10.1212/01.wnl.0000059413.75888.be. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14(3 Pt 2):572–579. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–22. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Klaassen T, Riedel WJ, van Someren A, Deutz NE, Honig A, van Praag HM. Mood effects of 24-hour tryptophan depletion in healthy first-degree relatives of patients with affective disorders. Biol Psychiatry. 1999;46(4):489–497. doi: 10.1016/s0006-3223(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29(41):12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R, Born J, Rasch B. A 3-day estrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology. 2006;31:965–975. doi: 10.1016/j.psyneuen.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Kok HS, Kuh D, Cooper R, et al. Cogntive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23(4):589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2008;23(1):CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14(8):820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Luetters C, Huang MH, Seeman T, et al. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women's health across the nation (SWAN). Journal of Women's Health. 2007;16:331–344. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158(2):227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- Maki PM. The timing of estrogen therapy after ovariectomy: implications for neurocognitive function. Nat Clin Pract Endocrinol Metab. 2008;4(9):494–495. doi: 10.1038/ncpendmet0901. [DOI] [PubMed] [Google Scholar]
- Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: A randomized, double-blind trial. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- McAllister-Williams RH, Massey AE, Rugg MD. Effects of tryptophan depletion on brain potential correlates of episodic memory retrieval. Psychopharmacology. 2002;160(4):434–442. doi: 10.1007/s00213-001-0996-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McNair ML, Lorr M, Droppleman LF. POMS Manual: Profile of Mood States. Edits; San Diego, CA: 1992. [Google Scholar]
- McVeigh C. Perimenopause: more than hot flushes and night sweats for some Australian women. J Obstet Gynecol Neonatal Nurs. 2005;34(1):21–27. doi: 10.1177/0884217504272801. [DOI] [PubMed] [Google Scholar]
- Mendelsohn D, Riedel WJ, Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev. 2009;33(6):926–52. doi: 10.1016/j.neubiorev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26(41):10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MF, Kallan MJ, Ten Have T, Katz I, Tweedy K, Battistini M. Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biol Psychiatry. 2004;55(4):406–412. doi: 10.1016/j.biopsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Berga SL, Greer PJ, Smith G, Cidis Meltzer C, Drevets WC. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2004;80(3):554–559. doi: 10.1016/s0015-0282(03)00973-7. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163(1):42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, Praschak-Rieder N, Zach J, de Zwaan M, Bondy B, Ackenheil M, Kasper S. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatr. 2002;59(7):613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61(8):765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Yuan P, Young TA, Bonne O, Luckenbaugh DA, Charney DS, Manji H. Effects of tryptophan depletion on serum levels of brain-derived neurotrophic factor in unmedicated patients with remitted depression and healthy subjects. Am J Psychiatry. 2005;162(4):805–807. doi: 10.1176/appi.ajp.162.4.805. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63(9):978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94(10):5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Menopause Society Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Neumeister A, Goldman D, Herscovitch P, Charney DS, Drevets WC. Serotonin transporter genotype and depressive phenotype determination by discriminant analysis of glucose metabolism under acute tryptophan depletion. Neuroimage. 2008;43(4):764–774. doi: 10.1016/j.neuroimage.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad CC, Zubieta JK, Love T, Wang H, Tkaczyk A, Smith YR. Enhanced neuroactivation during verbal memory processing in postmenopausal women receiving short-term hormone therapy. Fertil Steril. 2009;92(1):197–204. doi: 10.1016/j.fertnstert.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33(3-4):575–588. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17(5):485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann N Y Acad Sci. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI study. Neurology. 2009;72:135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, Deutz NE, van Someren A, van Praag HM. Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology (Berl) 1999;141(4):362–369. doi: 10.1007/s002130050845. [DOI] [PubMed] [Google Scholar]
- Riedel WJ. Cognitive changes after acute tryptophan depletion: what can they tell us? Psychol Med. 2004;34(1):3–8. doi: 10.1017/s0033291703008924. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, Hopwood A, Wallace C, Deakin JF, Sahakian BJ, Robbins TW. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999;146(4):482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28(1):153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Naftolin F, Zelterman D, Marchione KE, Holahan JM, Palter SF, Shaywitz BA. Better oral reading and short-term memory in midlife, postmenopausal women taking estrogen. Menopause. 2003;10(5):420–426. doi: 10.1097/01.GME.0000060241.02837.29. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27(4):415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Neiman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: A preliminary report. Am J. Obstet Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Jorissen BL, Sobczak S, van Boxtel MP, Hogervorst E, Deutz NE, Riedel WJ. Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J Psychopharmacol. 2000;14(1):21–29. doi: 10.1177/026988110001400102. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. Journal of Clinical Endocrinology & Metabolism. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13(4):345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Frontiers in Neuroendocrinology. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. ILAR J. 2004;5(2):189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29(11):2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Schnatz PF, Serra J, O'Sullivan DM, Sorosky JI. Menopausal symptoms in Hispanic women and the role of socioeconomic factors. Obstet Gynecol Surv. 2006;61(3):187–193. doi: 10.1097/01.ogx.0000201923.84932.90. [DOI] [PubMed] [Google Scholar]
- Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- Soares CN, Arsenio H, Joffe H, Bankier B, Cassano P, Petrillo LF, Cohen LS. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause. 2006;13(5):780–786. doi: 10.1097/01.gme.0000240633.46300.fa. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30(2):201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;41:1359–1378. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does A. The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disorders. 2001;64:107–119. doi: 10.1016/s0165-0327(00)00209-3. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41(3):259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 1985;87(2):173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]