Abstract
Yes-associated protein (YAP) is a well characterized transcriptional coactivator that interacts with various transcription factors and modulates their transcriptional activities. Phosphorylation of YAP by specific kinases regulates its cellular distribution and transcriptional activation functions. Sequestration of phosphorylated YAP in cytoplasm results in the reduction of transcription from its target genes. Since, YAP has been characterized as a coactivator of estrogen (ER) and progesterone (PR) receptors, we examined the immunohistochemical expression profile of YAP and correlation of YAP expression with that of ER and PR in normal (40 samples) and tumor breast (226 samples) from microarray tissue samples using immunohistochemistry. Here we show that YAP expression is significantly reduced in invasive carcinoma samples compared to normal breast tissues, which express high levels of YAP (YAP was positive for 45.1% of invasive carcinoma samples vs. 82.5% of normal samples p<.0001). Furthermore, our data shows that reduced expression of YAP in invasive carcinoma samples is significantly associated with ER negativity (YAP was negative for 59.9% in ER negative vs. 38.9% in ER positive invasive carcinoma samples, p=0.007) and PR negativity (YAP was negative for 60.1% in PR negative vs. 28.9% in PR positive, p=0.0004). Among invasive carcinoma samples, 42.9% were YAP, ER and PR negative, whereas only 7.5% were found to be YAP, ER and PR positive. On the contrary, 20 out of 23 (87%) normal breast tissues that were positive for ER and PR were also positive for YAP. These data suggest that YAP may act as a tumor suppressor in invasive breast carcinomas and it can also be used as a molecular marker for ER and PR negative breast tumors.
Keywords: Yes-associated protein, YAP, estrogen and progesterone receptors, breast cancers
INTRODUCTION
Yes-associated protein (YAP) is a 65 kDa proline rich phosphoprotein that contains the ‘WW domain’ and an activation domain, among other domains [1-3]. The activation domain of YAP is similar to the virion protein 16 (VP16) of herpes simplex virus type I activation domain [4]. It is a well characterized transcriptional coactivator that interacts with various transcription factors and modulates their transcriptional activities [5]. Phosphorylation of YAP by specific kinases regulates its cellular distribution and transcriptional activation functions. Akt-dependent phosphorylation of YAP at serine 127 leads to the nuclear export of YAP via its interaction with 14-3-3 [6]. This results in the sequestration of YAP in the cytoplasm, thereby reducing its ability to function as a coactivator.
YAP was originally identified as a protein binding to the SH3 domain of the Yes proto-oncogene product that belongs to the src family of protein-tyrosine kinases [1]. The gene for YAP is located on 11q22-23. It has been suggested that the 11q22-23 region is associated with both gene amplification and loss of heterozygosity (LOH) in breast cancers [7, 8]. Furthermore, it has been shown that YAP binds to the p53 family member p73 and increase the ability of p73 to induce apoptosis in response to DNA-damage suggesting that YAP may act as a tumor suppressor by activating apoptotic pathway [9]. On the contrary, other studies have suggested that YAP may act as an oncogene by stimulating cell proliferation [10]. YAP is frequently amplified in breast cancers. Furthermore, overexpression of YAP in human nontransformed mammary epithelial cells results in phenotypic alterations that are hallmarks of tumorigenic transformation [7]. To add to the confusion, one study suggests that YAP expression is decreased in breast cancer [11, 12], whereas another study suggests that YAP expression is increased in breast cancers [8]. In light of these contradictory findings, we decided to examine the expression profile of YAP in breast cancers and to resolve the inconsistencies regarding the role and the clinical significance of YAP in breast tumorigenesis.
The process of breast tumorigenesis is thought to result from a “benign to malignant” progression in which the accumulation of multiple genetic changes allows evolution from normal breast epithelium through benign proliferative lesions to atypical proliferation lesions, and then to carcinoma in situ and frankly invasive tumors [13, 14]. The lesions associated with the greatest risk of invasive breast cancer are hyperplasia of usual type, atypical ductal hyperplasia, ductal carcinoma in situ and lobular carcinoma in situ [13, 14]. Clinical and epidemiological evidence suggest that estrogen (ER) and progesterone (PR) receptors are involved in human mammary gland development and tumorigenesis [15, 16]. Based on ER status, breast cancers are characterized as either ER positive (ER+) or as ER negative (ER−) [17]. The presence of ER is associated with a better prognosis both in terms of increased disease-free survival and overall survival, and predicts for response to hormonal therapies such as tamoxifen. On the other hand, absence of ER is associated with a worse prognosis than ER positive cancers and they do not respond to antiestrogens [18-21]. We decided to examine the association of YAP with that of ER and PR in breast cancer, since, as of today, their relation is unknown.
In this report, we show that YAP immunohistocemical expression is significantly reduced in invasive carcinomas as compared to normal breast tissues. Furthermore, we show that in invasive carcinoma samples, YAP negativity is correlated with ER and PR negativity and, correspondingly, in normal breast tissues. Taken together, our data suggest a strong association of YAP positivity when ER and PR are both positive in normal breast tissues, and YAP negativity when ER and PR are both negative in invasive carcinoma tissues.
METHODS
We have analyzed 226 invasive breast carcinoma microarray samples from 226 patients by immunohistochemistry to assess the expression patterns of ER, PR, and YAP. As controls, we have included 40 normal breast tissues in this study. This study was approved by the University of Miami IRB review committee.
Tissue Microarrays
Tissue Microarrays were purchased from IMGENEX (San Diego, CA) (IMH-371, IMH364 and IMT-01320). Sections were 4-5 μm thick and paraffin coated to prevent sample oxidization. There were a total of 226 invasive breast carcinoma samples along with 40 normal breast tissues.
Antibodies
Primary antibodies used for immunohistochemistry were as ER monoclonal antibody (6F11) from Novocastra Lab, PR polyclonal antibody (A0098) from Dakocytomation, and YAP polyclonal antibody (H-125) from Santa Cruz biotechnology, inc. YAP polyclonal antibody (4912) from Cell Signaling and Histone H3 antibody from Millipore. The following dilutions of the antibodies were used in this study: ER (6F11) 1:50, PR (A0098) 1:250, and YAP (H-125) 1:500 YAP (4912) 1:100 and H3 1:100. These dilutions were selected on the basis of 20-40% cellular expression of specific proteins respectively. Biotinylated secondary antibodies were used respectively as mouse monoclonal and rabbit polyclonal were purchased from Vector Labs. All antibodies were diluted in 5% normal goat serum (Vector Labs, Burlington, CA).
Hematoxylin and Eosin (H&E) Staining
Tissue microarray slides were de-waxed by placing them in incubator at 60°C for 1 hr, deparaffinized them with xylene followed by successive rehydration using 100%, 95% and 80% ethanol respectively as recommend by the manufacturer. The slides were rinsed in distilled water. The tissues were then stained with hematoxylin for 3-5 seconds. Slides were rinsed in distilled water followed by tap water. The slides were then dipped in acid ethanol and rinsed with tap water followed by distilled water. Excess water from the slides were blotted and stained with eosin for 30 seconds. The slides were dehydrated immediately using 95% and 100% ethanol and xylene. The cover slips were placed on the slides using permount.
Immunohistochemistry Staining
Tissue microarray slides were de-waxed and rehydrated as recommend by the manufacturer. Antigen was retrieved by boiling slides in antigen unmasking buffer (Citrate buffer pH 6.0, Vector Labs) diluted (1:100 in dH2O), in a microwave oven for 10 minutes. Then slides were allowed to cool down for 30 minutes. Endigenous peroxidase activity was quenched by incubation of sections in 1% hydrogen peroxide in Methanol at room temperature for 30 minutes. The remaining of the protocol was performed in humidified chamber. Nonspecific immunoreactivity was blocked by incubating the slides with 10% normal goat serum in 1× PBS (phosphate buffer saline) for 1 hour. Slides were then incubated with the primary antibodies (diluted in 5% goat serum), overnight at 4°C, washed the slides thrice, with of 0.05% 1× PBST (Tween, phosphate buffer saline) for 10minutes/wash (10×3) at room temperature in PBS, and incubated with the appropriate Biotinylated secondary antibody (diluted in 5% goat serum). Avidin-biotin immunoperoxidase technique was used for detection of antigen –antibody complex (VECTASTAIN Elite ABC Kit and DAB Substrate Kit, 3, 3′-diaminobenzidine Vector Labs. Burlington, CA) Slides were washed with distilled running water for 10 minutes. Stained the slides with Hematoxylin (counter-stain), for 3-5 seconds and washed them quickly. (Hematoxylin QS H-3404 Vector Labs Inc. Burlington). Slides were washed with running water for 10 minutes. Dehydrate the slides successively in 70%, 95% and 100% ethanol (2 minutes each) then in xylene for three times, each for 5 minutes. Slides were mounted with Permount mounting medium (Fisher) and cover slips were applied.
Analysis of Expression and Tumor Pathology
ER and PR are present in normal breast samples and form a useful internal positive control. ER focal expression was taken as control for other sections on the same array to ensure the quality of tissue. Histological diagnosis were used to assess sections as invasive carcinoma and normal breast by analyzing sections stained with hematoxylin and eosin from representative tissue microarrays. The levels of cellular expression of ER, PR and YAP were examined by a breast pathologist, masked to pathology status, outcomes and without knowledge of other immunostaining results from the sample slides data provided by the manufacturing company. Expression intensity was scored as negative (if absent), low, moderate or high.
Statistical Analysis
Associations between each tested stained protein and tissue type were examined using the χ2(chisquare) test or the Fisher’s exact test, as appropriate. Similar analyses were conducted for associations between proteins within tissue type. P-value of 0.05 was considered statistically significant. It was assumed that protein expression depend only on tissue type. Analyses were performed using SAS 9.2 statistical software package (SAS Institute, Inc., USA, 2010).
RESULTS
Immunohistochemical expression analysis of YAP in normal breast tissues and invasive breast carcinomas
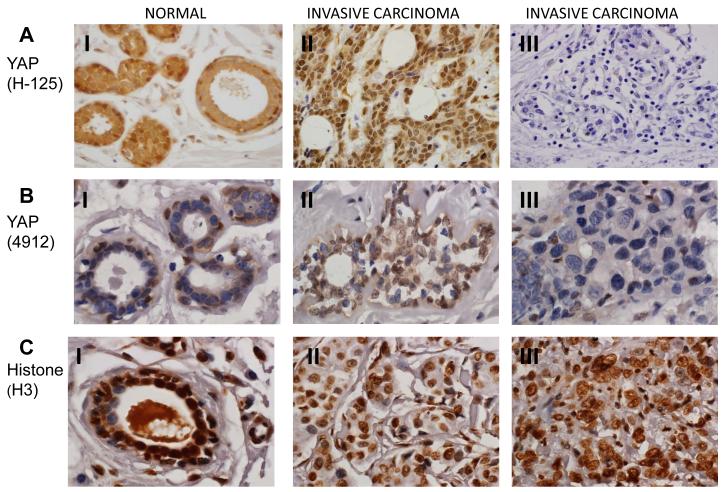
YAP is a transcriptional coactivator and it regulates the transactivation functions of various transcription factors including ER and PR [22]. Previously, the expression of YAP has been studied in breast cancers but the published data is not consistent. One study suggests that YAP expression is decreased in breast cancer, whereas another study suggests that YAP expression is increased in breast cancers [7, 12, 23]. Furthermore, the relationship between YAP, ER and PR expression is not known yet. In order to resolve the inconsistency in the expression profile of YAP and to relate YAP expression profile with that of ER and PR, we examined the expression profile of YAP by immunohistochemistry in invasive carcinoma breast tissues (n=226) and compared it with normal breast tissues (n=40) (Fig. 1). YAP expression intensity was scored as negative (if absent), low, moderate or high. We found focal expression of YAP in 33 (82.5%) normal tissues (Table 1). In 17 out of 40 (42.5%) normal breast tissue samples, YAP expression was nuclear, whereas, in 15 (37.5%) normal breast samples, YAP was expressed in cytoplasm. Only in one sample (2.5%) YAP was expressed in both cytoplasm and nucleus. The breast samples exhibited strong expression of YAP in basal cells, (Fig. 1). Out of 226 invasive carcinoma samples, 173 (76.5%) invasive carcinoma samples exhibited either low levels of YAP expression or no expression. Whereas, 53 (23.5%) invasive carcinoma samples express moderate to high levels of YAP. Among YAP positive invasive carcinoma tumors, 60 (26.5%) tumor samples exhibited nuclear staining, 34 (15%) tumor samples exhibited cytoplasmic staining and 8 (3.5%) tumors exhibited both nuclear and cytoplsmic staining. 124 (54.9%) invasive carcinoma samples were negative for YAP expression (Table 1 and Fig. 1). In order to further confirm and to eliminate the possibility that YAP negativity in invasive carcinoma samples is not due to antibody artifact, we utilized another YAP antibody which recognizes different epitope of YAP in our immunohistochemistry assays. The results were identical with both antibodies (Fig. 1). To further validate the negativity of YAP and to confirm that it is not artifact of tissue fixation, we stained few tissue samples with histone (H3) antibody as controls. Histone, H3 antibody was able to stain all the tested tissues suggesting that negative staining for YAP is not due to issues with tissue preparation (Fig. 1). Taken together, our data suggest that YAP expression is significantly associated with tissue type. YAP is significantly less expressed in invasive carcinomatumors if compared with normal breast tissue. (YAP positivity 45.1% vs. 82.5%, respectively, p<0.0001, Table 1).
Fig. 1.
YAP immunostaining in normal and invasive carcinoma breast tissues. (60X magnification). A and B (I): Immunohistochemistry with two different YAP antibodies, H-125 (A) and 4912 (B) showing that expression of YAP is strongly positive with high level of nuclear staining in basal cells of normal breast ducts and in the cytoplasm of luminal cells. A and B (II): Representative invasive carcinoma tissues that are positive for YAP expression. A and B (III): Representative invasive carcinoma tissues that are negative for YAP expression. C (I): Immunstaining of normal breast tissue showing strong expression of histone H3. C (II): Immunostaining of histone H3 of invasive carcinoma tissues those are positive for YAP expression. C (III): Immunostaining of histone H3 of invasive carcinoma tissues those are negative for YAP expression.
Table 1.
| Tissue type |
||||||||
|---|---|---|---|---|---|---|---|---|
| IC | Normal Breast Tissues |
Total | P-value1 | |||||
| N | % | N | % | N | % | |||
| YAP status | Negative | 124 | 54.9 | 7 | 17.5 | 131 | 49.2 | <.0001 |
| Positive | 102 | 45.1 | 33 | 82.5 | 135 | 50.8 | ||
| Total | 226 | 100.0 | 40 | 100.0 | 266 | 100.0 | ||
| YAP intensity | None | 124 | 54.9 | 7 | 17.5 | 131 | 49.2 | <.0001 |
| Low | 49 | 21.7 | 19 | 47.5 | 68 | 25.6 | ||
| Moderate | 37 | 16.4 | 8 | 20.0 | 45 | 16.9 | ||
| High | 16 | 7.1 | 6 | 15.0 | 22 | 8.3 | ||
| YAP location | No staining | 124 | 54.9 | 7 | 17.5 | 131 | 49.2 | <.0001 |
| Nuclear | 60 | 26.5 | 17 | 42.5 | 77 | 28.9 | ||
| Nuclear & Cytoplasmic | 8 | 3.5 | 1 | 2.5 | 9 | 3.4 | ||
| Cytoplasmic | 34 | 15.0 | 15 | 37.5 | 49 | 18.4 | ||
Chi-square or Fisher’s exact test when appropriate.
IC: Invasive Carcinomas
Immunohistochemical expression analysis of ER and PR in normal breast tissues and invasive breast carcinomas in conjuction to analysis of YAP expression
Since YAP is a coactivator of ER and PR [22], after establishing the expression profile of YAP in invasive carcinoma tumors, we wanted to examine the expression profile of ER and PR in normal breast tissues and invasive carcinoma tumors. All the samples previously analyzed for YAP expression, were now analyzed also for ER and PR expression. Out of 226 invasive carcinoma samples, 172 (76.1%) were ER negative while 54 (23.9%) were ER positive (Table 2). In contrast, all of the normal samples (100%) were ER positive (Table 2 and Fig. 2A). ER is present in normal breast samples and can therefore be used as internal positive control, to ensure the quality of the sections. The proportion of ER negative breast cancers was significantly higher in invasive carcinomas compared to tissues without diseases (p<0.0001) (Table 2).
Table 2.
| Tissue type |
||||||||
|---|---|---|---|---|---|---|---|---|
| IC | Normal Breast Tissues |
Total | P-value1 | |||||
| N | % | N | % | N | % | |||
| ER status | Negative | 172 | 76.1 | - | - | 172 | 64.7 | <.0001 |
| Positive | 54 | 23.9 | 40 | 100.0 | 94 | 35.3 | ||
| Total | 226 | 100.0 | 40 | 100.0 | 266 | 100.0 | ||
| PR status | Negative | 188 | 83.2 | 17 | 42.5 | 205 | 77.1 | <.0001 |
| Positive | 38 | 16.8 | 23 | 57.5 | 61 | 22.9 | ||
| Total | 226 | 100.0 | 40 | 100.0 | 266 | 100.0 | ||
Chi-square or Fisher’s exact test when appropriate.
IC: Invasive Carcinomas
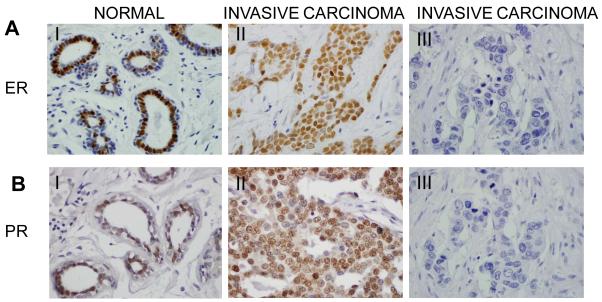
Fig. 2.
ER and PR immunostaining in normal and breast carcinoma. (60X magnification). A and B. Immunostaining was performed to detect the expression of ER (A) or PR (B) in normal and invasive carcinoma tissues. I): Strong positive and nuclear focal expression of ER (A) or PR (B) was observed in cells of normal ducts. II): Representative invasive breast carcinoma that is positive for ER (A) or PR (B) expression. III): Representative invasive breast carcinoma that is negative for ER (A) or PR (B) expression.
Since it has been shown that PR is an ER-responsive gene [24], we set out to examine the expression profile of PR in these human breast tissues. Our data suggest that 188 (83.2%) invasive carcinoma samples were PR negative while 38 (16.8%) were PR positive. In contrast, 17 (42.5%) normal breast samples were PR negative while 23 (57.5%) were PR positive. The proportion of PR negative breast cancers were significantly higher in invasive carcinoma compared to tissues without diseases (p<0.0001). Overall, ER negativity and PR negativity was significantly associated with invasive carcinomas, if compared to normal breast tissues (76.1% vs. 0%, p<.0001) and (83.2% vs. 42.5%, p<.0001) respectively (Fig. 2B and Table 2).
Correlation of YAP expression with ER and PR status in invasive carcinoma breast cancers
In order to examine the association of YAP with ER status in breast cancer, we analyzed the YAP status (negativity or positivity), in relation to ER status both in invasive carcinomas and normal breast samples. Among 172 (76.1%) ER negative invasive carcinoma samples, 103 (59.9%) were YAP negative; in contrast, among 54 (23.9%) ER positive invasive carcinoma samples, 21 (38.9%) were YAP negative (Table 3). The YAP negativity difference in invasive carcinomas, 59.9% vs. 38.8%, was statistically significant at p=0.007. On the contrary, normal breast tissues were more likely to be YAP positive (82.5% YAP positive). Overall, our data show that in invasive carcinoma samples, YAP negativity is associated with ER negativity.
Table 3.
| YAP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tissue type | Marker | Positive | Negative | Total | ||||
| N | % | N | % | N | % | P-value1 | ||
| ER | ||||||||
| Positive | 33 | 61.1 | 21 | 38.9 | 54 | 100.0 | 0.007 | |
| IC | Negative | 69 | 40.1 | 103 | 59.9 | 172 | 100.0 | |
| Total | 102 | 45.1 | 124 | 54.9 | 226 | 100.0 | ||
|
| ||||||||
|
Normal
Breast Tissues |
Positive | 33 | 82.5 | 7 | 17.5 | 40 | 100.0 | NA |
|
| ||||||||
| PR | ||||||||
| IC | Positive | 27 | 71.1 | 11 | 28.9 | 38 | 100.0 | 0.0004 |
| Negative | 75 | 39.9 | 113 | 60.1 | 188 | 100.0 | ||
| Total | 102 | 45.1 | 124 | 54.9 | 226 | 100.0 | ||
|
| ||||||||
|
Normal
Breast Tissues |
Positive | 20 | 87.0 | 3 | 13.0 | 23 | 100.0 | 0.432 |
| Negative | 13 | 76.5 | 4 | 23.5 | 17 | 100.0 | ||
| Total | 33 | 82.5 | 7 | 17.5 | 40 | 100.0 | ||
Chi-square or Fisher’s exact test when appropriate.
IC: Invasive Carcinomas
A similar analysis was carried out to examine the association of YAP with PR status in breast cancer. Among 188 (83.2%) PR negative invasive carcinoma samples, 113 (60.1%) were YAP negative; in contrast, among 38 (16.8%) PR positive invasive carcinoma samples, 11 (28.9%) were YAP negative. The YAP negativity difference in invasive carcinoma, 60.1% vs. 28.9%, was statistically significant at p=0.0004 (Table 3). Again, and as expected, normal breast tissues were more likely to be YAP positive (82.5% YAP positive). However, there was no significant association between PR negativity and YAP negativity in normal breast tissues (YAP negativity 13% in PR negative vs. 23.5% in PR positive, p=0.432). Our data show that in invasive carcinoma samples, YAP negativity is associated, as ER negativity, with PR negativity.
Correlation among the three proteins in invasive carcinoma breast cancers
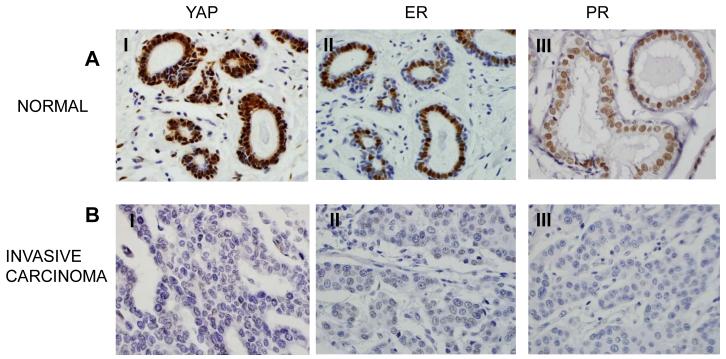
Finally, we examined the association of YAP with ER and PR simultaneously (Table 4 and Fig. 3). Among invasive carcinoma samples, 97 (42.9%) samples were triple negative for ER, PR and YAP, and 17 (7.5%) were triple positive for ER, PR and YAP. 59 (26.1%) were ER and PR negative and YAP positive and 5 (2.2%) were ER and PR positive and YAP negative. Triple negative status for ER, PR and YAP is significantly associated with invasive carcinoma (p<0.0001) (not shown). Overall, our data suggest a strong correlation of ER and PR expression with YAP expression in normal breast tissues and, vice versa, ER and PR absence with YAP absence in invasive carcinoma breast tumors. Consequently, our data suggest that YAP can be utilized as a predictive marker for ER and PR negative for invasive carcinoma breast tumors.
Table 4.
| Tissue type |
||||||||
|---|---|---|---|---|---|---|---|---|
| ER | PR | YAP | IC | Normal Breast Tissues |
Total | |||
| N | % | N | % | N | % | |||
| Pos | Pos | Pos | 17 | 7.5 | 20 | 50.0 | 37 | 13.9 |
| Pos | Pos | Neg | 5 | 2.2 | 3 | 7.5 | 8 | 3.0 |
| Pos | Neg | Pos | 16 | 7.1 | 13 | 32.5 | 29 | 10.9 |
| Pos | Neg | Neg | 16 | 7.1 | 4 | 10.0 | 20 | 7.5 |
|
| ||||||||
| Neg | Pos | Pos | 10 | 4.4 | - | - | 10 | 3.8 |
| Neg | Pos | Neg | 6 | 2.7 | - | - | 6 | 2.3 |
| Neg | Neg | Pos | 59 | 26.1 | - | - | 59 | 22.2 |
| Neg | Neg | Neg | 97 | 42.9 | - | - | 97 | 36.5 |
|
| ||||||||
| Total | 226 | 100.0 | 40 | 100.0 | 266 | 100.0 | ||
IC: Invasive Carcinomas
Fig. 3.
Immunohistochemistry analysis of YAP, ER and PR in breast tumor. (60X magnification) A: Strong correlation of ER (II), PR (III) with YAP (I) for its positivity was observed in normal breast tissues. B: YAP (I) negativity is associated with ER (II) and PR (III) negativity in invasive breast tumors.
DISCUSSION
Coactivators are proteins that modulate the functions of activators (or transcription factor), as steroid hormone receptors including that of ER and PR [25, 26]. In the specific case of ER and PR coactivators, they are also involved in mammary gland development and breast tumorigenesis [15]. YAP has been characterized as a coactivator of several transcription factors including p73 [9]. Additionally, YAP has been shown to be involved both in apoptotic and proliferation pathways [27]. YAP gene has been shown to be located on a locus which is associated with both gene amplification and LOH in breast cancers. One study suggests that YAP expression is decreased in breast cancer and based on this data it has been suggested that YAP may act as a tumor suppressor [12]. In contrast, another study suggests that YAP may act as an oncoprotein in breast cancer and this conclusion was drawn based on the observation that YAP expression is increased in certain breast cancers [7, 10, 23]. In order to clarify these discrepancies, we initially examined the expression of YAP in normal breast tissues and invasive carcinoma’s of the breast. Then, we compared the expression profile of YAP between normal and tumor tissues. Our data suggest that YAP expression is significantly reduced in invasive breast carcinomas compared to normal breast tissues. Therefore, lost or reduced expression of YAP can be utilized as a molecular marker for staging of breast cancer. On a speculative note, the findings of this study support the hypothesis that YAP may act as a tumor suppressor in invasive breast carcinomas.
Breast cancers are usually classified either as ER+/PR+ or as ER−/PR−. The ER+/PR+ tumors have better prognosis both in terms of increased disease-free survival and overall survival, and are usually treated with antihormonal therapies [18-21]. On the contrary, ER−/PR− tumors are aggressive and fail to respond to current therapies. In this study, we wanted to test the association between the expression of YAP with that of ER and PR. We show that YAP negativity is associated with ER and PR negativity. These data suggest that YAP may act be a tumor suppressor protein in invasive breast carcinomas and YAP negativity in conjunctions with ER and PR negativity can be used as a molecular marker for ER− and PR− breast tumors. Since, ER−/PR− tumors are aggressive and are hard to treat with current available therapies, our data suggest that up regulation of YAP expression in ER− and PR− tumors may have therapeutic potential and small molecules that enhance YAP expression in ER− and PR− invasive breast carcinomas can be utilized as a treatment option to reduce malignancies.
In summary, our data indicate a reduction or loss of YAP expression in invasive breast carcinomas, in particular in ER−/PR− invasive carcinomas suggesting that YAP may act as a tumor suppressor. Furthermore, it suggests that up regulation of YAP expression in ER− and PR− negative tumors may have therapeutic potential. It also point to the possible use of YAP as a biomarker for breast cancers at different stages or with different genetic abnormalities.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ali Muhammed Saeed and Laura Buffa for critically reading the manuscript. This research was supported by the NIH grant (R01DK079217-3) to Z. N.
REFERENCES
- [1].Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–2152. [PubMed] [Google Scholar]
- [2].Sudol M, Recinos CC, Abraczinskas J, Humbert J, Farooq A. WW or WoW: the WW domains in a union of bliss. IUBMB Life. 2005;57:773–778. doi: 10.1080/15216540500389039. [DOI] [PubMed] [Google Scholar]
- [3].Sudol M, Sliwa K, Russo T. Functions of WW domains in the nucleus. FEBS Lett. 2001;490:190–195. doi: 10.1016/s0014-5793(01)02122-6. [DOI] [PubMed] [Google Scholar]
- [4].Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. Embo J. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sudol M, Hunter T. NeW wrinkles for an old domain. Cell. 2000;103:1001–1004. doi: 10.1016/s0092-8674(00)00203-8. [DOI] [PubMed] [Google Scholar]
- [6].Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- [7].Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Strano S, Munarriz E, Rossi M, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- [10].Zhao B, Ye X, Yu JD, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & Development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ehsanian R, Brown M, Lu H, et al. YAP dysregulation by phosphorylation or DeltaNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene. 2010;29:6160–6171. doi: 10.1038/onc.2010.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- [13].Allred DC, Wu Y, Mao S, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–378. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- [14].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [15].Horwitz K, Clarke C. Estrogens and progestins in mammary development and neoplasia. Introduction. J Mammary Gland Biol Neoplasia. 1998;3:1–2. doi: 10.1023/a:1018716732135. [DOI] [PubMed] [Google Scholar]
- [16].Naccarato AG, Viacava P, Vignati S, et al. Bio-morphological events in the development of the human female mammary gland from fetal age to puberty. Virchows Arch. 2000;436:431–438. doi: 10.1007/s004280050470. [DOI] [PubMed] [Google Scholar]
- [17].Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006;19:617–621. doi: 10.1038/modpathol.3800570. [DOI] [PubMed] [Google Scholar]
- [18].Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- [19].Clark GM, McGuire WL. Steroid receptors and other prognostic factors in primary breast cancer. Semin Oncol. 1988;15:20–25. [PubMed] [Google Scholar]
- [20].Clarke RB, Anderson E, Howell A. Steroid receptors in human breast cancer. Trends Endocrinol Metab. 2004;15:316–323. doi: 10.1016/j.tem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- [21].Putti TC, El-Rehim DM, Rakha EA, et al. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol. 2005;18:26–35. doi: 10.1038/modpathol.3800255. [DOI] [PubMed] [Google Scholar]
- [22].Dhananjayan SC, Ramamoorthy S, Khan OY, et al. WW domain binding protein-2, an E6-associated protein interacting protein, acts as a coactivator of estrogen and progesterone receptors. Mol Endocrinol. 2006;20:2343–2354. doi: 10.1210/me.2005-0533. [DOI] [PubMed] [Google Scholar]
- [23].Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Khan OY, Fu G, Ismail A, et al. Multifunction steroid receptor coactivator, E6-associated protein, is involved in development of the prostate gland. Mol Endocrinol. 2006;20:544–559. doi: 10.1210/me.2005-0110. [DOI] [PubMed] [Google Scholar]
- [26].McKenna NJ, Xu J, Nawaz Z, Tsai SY, Tsai MJ, O’Malley BW. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J Steroid Biochem Mol Biol. 1999;69:3–12. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- [27].Bertini E, Oka T, Sudol M, Strano S, Blandino G. YAP: at the crossroad between transformation and tumor suppression. Cell Cycle. 2009;8:49–57. doi: 10.4161/cc.8.1.7259. [DOI] [PubMed] [Google Scholar]