Abstract
Neonatal hippocampal damage in rodents impairs medial prefrontal working memory functions. To examine whether similar impairment will follow the same damage in primates, adult monkeys with neonatal hippocampal lesions and sham-operated controls were trained on two working memory tasks. The Session Unique-Delayed Non-Match-to-Sample (SU-DNMS) measures maintenance of information in working memory mediated by the ventral lateral prefrontal cortex. The Object Self-Ordered Task (Obj-SO) measures monitoring of information in working memory mediated by the dorsolateral prefrontal cortex. Adult monkeys with neonatal hippocampal lesions performed as well as sham-operated controls on SU-DNMS at either the 5 or 30s delays, but were severely impaired on the Obj-SO task. These results extend the earlier findings in rodents by demonstrating that early lesions of the hippocampus in monkeys impair working memory processes known to require the integrity of the dorsolateral prefrontal cortex, while sparing lower-order working memory processes, such as recency. Although the present results suggest that the lack of functional hippocampal inputs may have altered the maturation of the dorsolateral prefrontal cortex, future studies will be needed to determine whether the nature of the observed working memory deficit is due to an absence of the hippocampus, a maldevelopment of the dorsolateral prefrontal cortex or both.
Keywords: neonatal lesion, working memory, dorsolateral prefrontal cortex, schizophrenia
Introduction
Behavioral, electrophysiological, neurochemical and ultra-structural studies in rodents have provided mounting evidence that damage to the ventral hippocampus few days after birth result in protracted and widespread brain alterations, including the prefrontal cortex (for a review see, Tseng, Chambers, & Lipska, 2009). For example, working memory impairment after neonatal hippocampal lesions, a function mediated by the prefrontal cortex, has consistently been demonstrated by a number of working memory tasks, such as the radial arm maze, delayed and discrete alternation, win-shift paradigm, set-shifting and spatial working memory (Beninger et al., 2009; Brady, 2009; Chambers, Moore, McEvoy, & Levin, 1996; Levin & Christopher, 2006; Lipska, Aultman, Verma, Weinberger, & Moghaddam, 2002; Marquis, Goulet, & Dore, 2008). Concurrently to these working memory deficits, neonatal hippocampal lesions also altered prefrontal cortical firing patterns following dopaminergic and glutamatergic stimulation (O'Donnell, Lewis, Weinberger, & Lipska, 2002; Tseng, Lewis, Lipska, & O'Donnell, 2007), decreased dopamine transporter in the ventral tegmental area and GAD-67, the rate limiting enzyme of gamma-aminobutyric acid (GABA) synthesis (Francois et al., 2009; Lipska, Lerman, Khaing, & Weinberger, 2003), as well as reduced number of interneurons and decreased spine density of pyramidal prefrontal neurons (Francois et al., 2009; Marquis et al., 2008). Thus, the rodent literature clearly established a causal link between neonatal damage to the ventral hippocampus and prefrontal cortical development, suggesting that dopamine control of prefrontal mechanisms are compromised in the absence of functional hippocampal inputs during development.
Although these studies have been extremely valuable in establishing a putative role for the hippocampus in the development of the prefrontal cortex, some of their limitations reside in the inability to make inferences on the effects of early hippocampal dysfunction on prefrontal cortex development in humans. Although rats have a defined medial prefrontal cortex, which includes rudimentary elements of the lateral prefrontal cortex, this lateral area cannot be clearly delineated from another area believed to be homologous to the anterior cingulate cortex in primates (Seamans, Lapish, & Durstewitz, 2008; Uylings, Groenewegen, & Kolb, 2003). The issue of structural homology of the prefrontal cortex between rodents and primates becomes more problematical, especially when it comes to dissociate structure-function relationships of different lateral prefrontal subregions. In this domain, studies in non-human primates offer important advantages since in both monkeys and humans working memory processes are loosely segregated within defined sectors of the lateral prefrontal cortex. Within the dorsal and ventral sectors, working memory processes are organized according to the type of processing required (process-specific) rather than according to the domain of the information being processed (material-specific) (for a review see, Curtis & D'Esposito, 2004; Owen, 2000; Petrides, 2005). Thus, simple maintenance of information in a working memory buffer is inherently dependent upon the ventrolateral prefrontal cortex (Petrides, 1995; Petrides, Alivisatos, & Frey, 2002; Stern et al., 2000). In contrast, higher order working memory processes, such as monitoring of sequential actions and of temporal order of information and manipulation of this information, is dependent upon the dorsolateral prefrontal cortex (Petrides, 1991, 1995; Petrides, Alivisatos, Evans, & Meyer, 1993; Petrides & Milner, 1982). Given this division of labor in the lateral prefrontal cortex in primates, it became critical to evaluate whether neonatal hippocampal damage in monkeys will, as in rodents, yield deficits in working memory processes mediated by the prefrontal cortex, and if yes, to determine which sector of the lateral prefrontal cortex may be more specifically impacted by the absence of functional hippocampal inputs during development. To this end, adult rhesus monkeys that had received neonatal neurotoxic lesions of the hippocampus and their sham-operated controls were tested on two tasks of working memory that tap either on functioning of the ventral sector or on functioning of the dorsal sector. The Session-Unique Delayed NonMatching-to-Sample measured maintenance of information in working memory and assessed functioning of the ventrolateral prefrontal cortex (Mishkin & Delacour, 1975; Rapp & Amaral, 1989). The Object-Self Ordered task measured monitoring of information and assessed functioning of the dorsolateral prefrontal cortex (Petrides, 1995). Preliminary data of this study were reported in abstract form (Heuer & Bachevalier, 2007).
Materials and Methods
Subjects
Eleven adult rhesus macaques (Macaca mulatta), aged 5 - 6 years and weighing between 5 to 8 kg, were used in this study. They were acquired as newborns from the breeding colony of the University of Texas, M.D. Anderson Cancer Center Science Park (Bastrop, TX) and brought to the nursery at M.D. Anderson Cancer Center where they were grouped in age- and sex-matched cohorts of 4 animals each (Goursaud & Bachevalier, 2007). They were surrogate-nursery reared according to procedures developed by Sackett and colleagues (Sackett, Ruppenthal, & Davis, 2002) that allow the development of normal growth and of species-specific social skills. Briefly, infant monkeys were individually housed but permitted social contacts with other animals in adjoining cages until one month of age. In addition, a plush surrogate of approximately 30cm in length was provided to the animals for comfort. A principal human caregiver spent roughly 6 hrs per day, 5 days/week in the nursery with the infant monkeys. On weekends, the infants were handled 2-3 times per day and received a total of 2-4 hours of social interactions with familiar experimenters. From one to nine months of age, infants received daily social interactions for 3-4 hours, 5 days/week with age/sex matched peers, and, by 1 year of age, each cohort was socially housed until approximately 3 years of age. They were then moved to the Yerkes National Primate Research Center at Emory University (Atlanta, GA) where they were paired-housed in rooms with a 12 hour light/dark cycle (7AM:7PM) until their weight required to keep them individually housed. All monkeys were fed Purina Old World Primate chow supplemented with fresh fruit, and, during behavioral testing, food was minimally restricted to sufficiently motivate the animal. Their weight was closely monitored and maintained at or above 85% of their full feed weight. Water was provided ad libitum.
All monkeys had received brain surgeries when they were infants (10-15 days post-natally) and consisted of either sham-operations (Group Neo-C, 2 males, 3 females) or neurotoxic lesions of the hippocampus (Group Neo-H-ibo, 4 males, 2 females). To bring the equal sample size in the two groups, we also added one normal female rhesus monkeys to group Neo-C. This animal (case N-7) also came from the breeding colony of the University of Texas, M.D. Anderson Cancer Center Science Park (Bastrop, TX), but was mother-reared and raised in a semi-naturalistic social group before being brought to the laboratory when she was 1.5 year of age.
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas at Houston and Emory University and were conformed to the NIH Guide for the care and use of Laboratory Animals (HHS publication 85-23, 1985).
Neuroimaging and Surgical Procedures
Neuroimaging and surgical procedures have been described extensively in earlier reports (Goursaud & Bachevalier, 2007) and will be briefly summarized below.
Neuroimaging procedures
On the day of surgery, the infant monkeys were placed in an induction box saturated with isoflurane gas (1.0-3.0%, v/v, to effect), intubated and maintained under isoflurane gas throughout the procedure. They were then placed in a non-ferromagnetic stereotaxic apparatus (Crist Instruments, Damascus, MD) and centered in the scanner. Two types of MR images were acquired with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI) using a 5-inch surface coil. A series included a 3D T1-weighted fast spoiled gradient (FSPGR)-echo MR images (TE = 2.6 ms, TR = 10.2 ms, 25° flip angle, contiguous 1 mm sections, 12 cm FOV, 256 x 256 matrix) and the other included three series of Fluid-Attenuated Inversion Recovery (FLAIR) images (TE = 140 ms, TR = 10000 ms, inversion time (TI) = 2200 ms, contiguous 3 mm sections, 12 cm FOV, 256 × 256 matrix), offset by 1mm posteriorly. These two series of MR images were repeated 6-8 days after the surgical procedures. The pre-surgical T1 images were used to select stereotaxic coordinates for the neurotoxin injection sites for group Neo-H-ibo (Saunders, Aigner, & Frank, 1990), and the post-surgical FLAIR images were used to locate areas of high water density and matched with both the pre-surgical T1W and FLAIR images to verify the location and extent of lesions to the targeted area as well as to evaluate any inadvertent damage to adjacent structures (Malkova, Lex, Mishkin, & Saunders, 2001; Nemanic, Alvarado, Price, Jackson, & Bachevalier, 2002). Further investigation of the lesion extent was performed at approximately 1 to 1.5 year after surgery, when all animals received another scanning procedure using T1-W MR images to estimate the percent reduction of hippocampal volume.
Surgical procedures
At completion of the pre-surgical MRI, the animal was maintained under gas anesthesia, secured in the stereotaxic device, and immediately transported to the surgical suite. The scalp was disinfected with Nolvasan solution, and an intravenous drip containing 5% dextrose and 0.5% sodium chloride was infused for hydration. The animal was placed on a heating pad to maintain core temperature, and all vital signs (core temperature, pulse, blood pressure and expired CO2) were continuously monitored until the end of the procedure. Marcaine (25%, 1.5m., s.c.) was injected along the midline incision of the scalp from the occiput to a point in between the eyebrows. After skin incision, the connective tissue was displaced laterally to expose the skull in which two craniotomies were made in the left and right hemispheres just above the hippocampus followed by small slits in the dura.
For the hippocampal lesions, two 10μl Hamilton syringes attached to a Kopf electrode manipulators (David Kopf Instruments, Tujunga, CA) were used to simultaneously deliver the neurotoxin in the left and right hippocampus. A total of 2.8 - 4.2 μl ibotenic acid (Biosearch Technologies, Novato, CA, 10mg/ml in PBS, pH 7.4) was injected into 7-8 sites along the hippocampus and was intended to target the uncus and hippocampus along its entire length. The needles were slowly lowered at each injection site and 0.4 - 0.6 μl of ibotenic acid was manually injected at a rate of 0.2 μl/min. After each injection, the needles were left in place for an additional 3-min period to allow diffusion of the drug at the tip of the needle and minimize its spread in the needle track during retraction of the needles. After each injection, the needles were cleaned with cotton-tipped applicators to remove any residual neurotoxin or tissue along the needle.
The procedures for the sham lesions were identical to those used for the neurotoxic acid lesion of the hippocampus, except that no needle was lowered into the brain.
Following the completion of the surgical procedure, the incision was closed in anatomical layers and the infant was placed in an incubator to maintain core temperature. The post-operative care included a seven-day regimen of dexamethasone sodium phosphate (0.3 mg/kg i.m.) with progressive reduction of the drug to reduce swelling, Cefazolin (25 mg/kg i.m.) to minimize infection, and acetaminophen for pain management.
Lesion Assessment
Because all animals are undergoing behavioral testing, extent of lesions was estimated from the MR images (Nemanic et al., 2002). Pre- and post-surgical FLAIR images were matched to corresponding pre-surgical T1-weighted images and coronal drawings of a normal one-week-old rhesus monkey template brain (J. Bachevalier, unpublished data). Hypersignals on FLAIR MR images were identified, plotted onto corresponding coronal drawings from the template brain, and drawings were imported into a Java-based image analysis program (ImageJ®; http://rsb.info.nih.gov/ij/) to measure the surface area (in pixels2) of damage for intended targets, as well as all areas sustaining unintended damage (entorhinal and perirhinal cortex, parahippocampal cortex, and amygdala). For any given region of interest (ROI), the measured surface area of damage on each section through each hemisphere was summed and then multiplied by image thickness to calculate a total volume of damage (Gundersen & Jensen, 1987). The volume of damage was then divided by the normal volume of the ROI and multiplied by 100 to indicate a percent of the total volume damaged.
In addition, the total hippocampal volume reduction was calculated using the 1-year post-surgical T1-weighted MR images. For each animal, the coronal MR images were imported into the Java-based image analysis program (ImageJ®; http://rsb.info.nih.gov/ij/). Images containing the hippocampal formation were identified and surface area measurements were recorded for the left and right hemispheres separately. Descriptions of borders used to define the hippocampal formation on MR images have been previously described (Payne, Machado, Bliwise, & Bachevalier, 2009). Briefly, the first image posterior to the optic chiasm was used as the most anterior border in which the hippocampal formation appeared ventral to the amygdala and the tail of the lateral ventricle was often visible on the lateral and superior aspects of the hippocampal formation. The most posterior measurement for the hippocampal formation was made on the image that clearly showed the crus of the fornix emerging from the hippocampal formation. The gyrus fasciolaris and the fornix were excluded from the measurements. On all images between these two extremes, the hippocampal formation was bounded ventrally and medially by the white matter separating it from the parahippocampal gyrus, and laterally and dorsally, by the temporal horn of the lateral ventricle. Thus, the volume of the hippocampal formation included the CA fields, dentate gyrus, subicular complex and fimbria, but excluded the entorhinal, perirhinal, and parahippocampal cortices. For each hemisphere separately, the hippocampal volume (in mm3) was calculated by summing hippocampal surface areas on each image and multiplying by the distance between the images (i.e. 1 mm), using Cavalieri's principle (Gundersen & Jensen, 1987). For each animal in Group Neo-H-ibo, the hippocampal volume in each hemisphere was then compared to the averaged hippocampal volume from 3 normal male and 3 normal female monkeys of the same age (approximately 1 year of age). Percent volume reduction was then calculated using the following formula: [100-total H volume remaining/average H volume in normal subject]*100). Two trained observers measured the volume of hippocampal formation in normal animals and animals of Group Neo-H-ibo (Cronbach's alpha; p < 0.01 for all inter- and intra-observer reliabilities).
Behavioral procedures
As part of a longitudinal developmental study, all eleven operated animals received extensive behavioral testing from infancy through the start of this experiment. This behavioral testing included measures of incidental recognition memory at 1.5, 6, 18 and 48 months (Heuer & Bachevalier, 2011; Zeamer, Heuer, & Bachevalier, 2010), non-spatial relational memory at 3 and 15 months, spatial memory at 8 and 24 months (Blue, Kazama, & Bachevalier, 2009; Glavis-Bloom, Alvarado, & Bachevalier, 2006), object discrimination reversals at 48-60 months (Glavis-Bloom, Kazama, & Bachevalier, 2008), food preference and shift in choice selection after food devaluation at 60 months, dyadic social interactions (3, 6, and 36 months), emotional reactivity to human intruder at 2 and 4.5 months and social attachment to caregiver at 9 months (Goursaud & Bachevalier, 2007). Case N-7, which joined the group at a later age, did not receive behavioral testing for the first 18 months of life, but thereafter followed the same testing schedule as the other animals. Thus, before receiving the SU-DNMS and Obj-SO tasks, she had been trained on incidental recognition memory, object discrimination reversal, and object and spatial relational memory.
Apparatus and stimuli
Monkeys were tested in a darkened room containing a white noise generator to mask extraneous noise. For both the Session-Unique Delayed NonMatching-to-Sample (SU-DNMS) and the Object Self-Ordered Task (Obj-SO), animals were transferred from their home cage to a Wisconsin General Testing Apparatus (WGTA), and positioned in front of a testing tray containing three recessed food wells (2 cm in diameter, 1 cm deep and 13 cm apart from each other).
The SU-DNMS task utilized a set of 1000 junk objects that differed in color, size and shape, although only a single pair of objects was used for any given daily test session. The Obj-SO task employed a single set of three junk objects for all daily sessions. Correct choices were rewarded with a preferred food (i.e. M&M, marshmallow, peanut etc).
Session-Unique Delayed Nonmatching-to-Sample (SU-DNMS)
The procedures for SU-DNMS are similar to those of the Trial-Unique-DNMS (Bachevalier, Beauregard, & Alvarado, 1999), except that only a single pair of objects was used on each trial of a daily session instead of using new pairs of stimuli for each trial as for the Trial-Unique DNMS task. This manipulation changes the DNMS paradigm from a purely recognition memory task to a recency memory task (Mishkin & Delacour, 1975). For each daily session, a pair of objects was selected. Each trial began with the presentation of one of the two objects covering the center well baited with a food reward. After the subject displaced the sample object and retrieved the food reward, a 5-s delay was imposed during which the view of the testing tray was obstructed by a screen. For the choice test, the sample object now unbaited was presented together with the other baited object with both objects positioned over the lateral wells of the test tray, with the position of the objects (left versus right) pseudorandomly varied to preclude the use of a spatial strategy. Training continued in this way for 30 trials presented at 30-sec intervals and both objects served as the sample or the choice in a random order. However, starting with the second trial and for all remaining daily trials, both objects had been seen and baited such that successful performance required the animal to remember the object seen during the sample phase of a given trial (i.e. the object he had seen the most recently). The following day a new pair of objects was selected for the 30 daily trials and so on for all subsequent testing days. Learning criterion was set at 27 correct choices (90%) or better on one day followed the next day by 24 correct choices (80%) or better. Training was discontinued after a maximum of 1000 trials. After reaching criterion using the 5-s delay, testing continued in the same way with a longer delay of 30 seconds. At this longer delay, animals were given 20 trials per day using a different pair of objects on each testing day until performance averaged 85% correct across two consecutive daily sessions or to a maximum of 500 trials.
Object Self-Ordered Task (Obj-SO)
The Obj-SO task was delivered according to procedures described by Petrides and colleagues (Petrides, 1991, 1995). The Obj-SO task requires the animal to choose three objects, one by one, on successive trials of a daily session. Because the positions of the objects are shuffled spatially on each trial, to solve the task, the animal cannot simply rely on the locations of each object on the tray, but rather has to make choices based on their object selections in previous trials of the daily session. Specifically, on each day, animals received three successive trials using the same set of three objects until learning criterion was met. In Trial 1, the subject was presented with the three objects covering each of the three baited wells on the testing board. After the animal displaced one of the three objects and retrieved the food reward, a 10-s delay was imposed during which the view of the testing tray was obstructed by a screen. During this delay, the locations of the objects were re-ordered on the three wells and only the two objects that were not selected on the first trial were baited. After selecting one of the two baited objects in Trial 2, a 10-s delay was again imposed during which the three objects were again re-ordered on the three wells, but this time only the object that had not been selected on the two previous trials was now baited. After selecting the last baited object on Trial 3, the daily session ended, and the next day, the three objects were again used and presented over the same three trials, but their positions on the wells varied randomly each day. Because all objects are baited in Trial 1 and the animal cannot commit an error on this trial, this first trial is not scored. If at any time during trials 2 or 3, the animal incorrectly selected the unbaited object, this was scored as a primary error and a correction procedure was given. That is, a 10-s delay was imposed during which the three objects were re-ordered on the tray and represented to the animal for choice. This correction procedure was repeated until the animal correctly selected a baited object. The number of times the correction procedure was repeated for a given trial was used as a measure of perseverative errors. Testing for the Obj-SO task continues until the animal made three or less errors on both trials 2 and 3 (85% correct) across 10 consecutive days of testing (i.e. total of 20 trials). Testing was discontinued after a maximum of 50 daily sessions (100 trials).
Results
Assessment of Lesion Extent
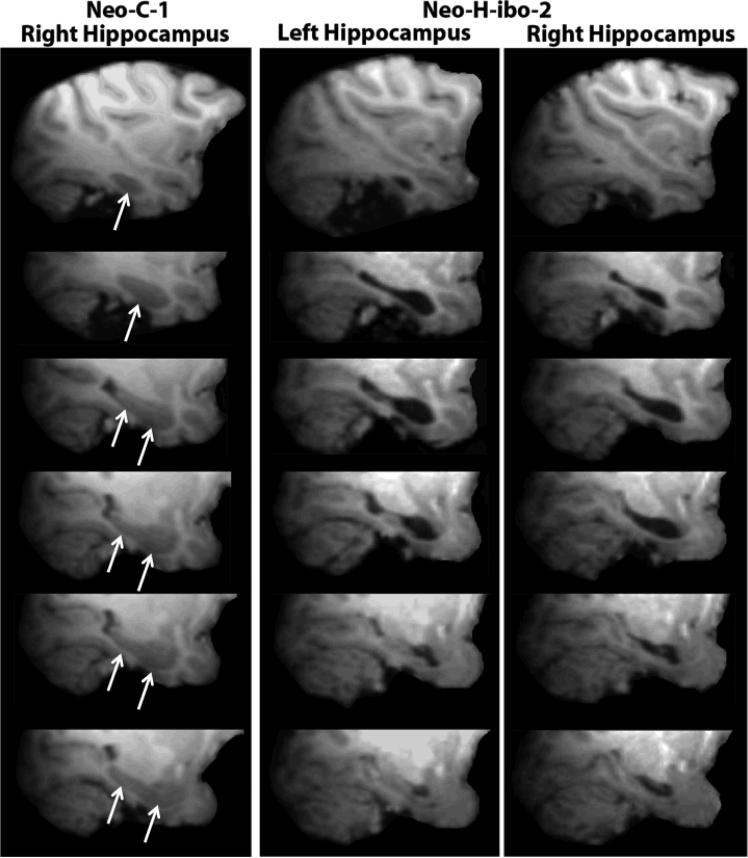
Table 1 provides an estimate of extent of hippocampal damage as well as unintended damage to adjacent structures. Two animals (Neo-H-ibo2 and Neo-H-ibo3) received extensive damage to the hippocampus bilaterally, averaging 67.6 and 87.4%, respectively. Three others cases (Neo-H-ibo-1, -4 and -5) had asymmetrical lesions with extensive damage on one hemisphere (average: 72%), but moderate damage on the other hemisphere (average: 15%). Only case Neo-H-ibo-6 had relatively small hippocampal damage on each side (7.9 and 0% for the left and right hemispheres, respectively). Unintended damage in each case was almost non-existent, except for very small damage to the posterior amygdala (from 0 to 7% bilaterally) and to areas TH/TF (from 0 to 12.1%). The percent volume reduction calculated from the one year post-lesion MRI scans (Table 2) confirmed the FLAIR MRI estimation of the hippocampal lesions, and resulted in a positive correlation between the two methods (r = 0.805, p = 0.05). Thus, four animals (Neo-H-ibo-2 to Neo-H-ibo-5) showed substantial hippocampal volume reduction, averaging 47.6 to 67% bilaterally, whereas two others (Neo-H-ibo-1 and -6) had only slight reduction of hippocampal volume, averaging 19% and 15% bilaterally, respectively. Figure 1 displays extent of hippocampal volume reduction in a representative case (Neo-H-ibo-2) as compared to a sham-operated case (Neo-C-1).
Table 1.
Lesion Extent as estimated via the FLAIR MR images
| Group |
Intended Damage |
Unintended Damage |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects |
Hippocampus |
Amygdala |
TH/TF |
|||||||||
| Neo-H-ibo | L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% |
| Neo-H-ibo-1 | 63.8 | 2.9 | 33.2 | 1.8 | 14.0 | 0.0 | 7.0 | 0.0 | 3.1 | 0.5 | 1.8 | 0.0 |
| Neo-H-ibo-2 | 54.4 | 80.9 | 67.6 | 44.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.4 | 2.7 | 12.1 | 0.6 |
| Neo-H-ibo-3 | 78.5 | 96.3 | 87.4 | 75.6 | 1.7 | 0.0 | 0.8 | 0.0 | 6.1 | 5.5 | 5.8 | 0.3 |
| Neo-H-ibo-4 | 20.3 | 67.3 | 43.8 | 13.6 | 0.0 | 4.7 | 2.4 | 0.0 | 15.3 | 0.0 | 7.6 | 0.0 |
| Neo-H-ibo-5 | 20.7 | 84.0 | 52.6 | 17.5 | 0.0 | 4.9 | 2.5 | 0.0 | 6.1 | 4.0 | 5.1 | 0.2 |
| Neo-H-ibo-6 | 7.9 | 0.0 | 3.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mean | 40.9 | 55.2 | 48.0 | 25.4 | 2.6 | 1.6 | 2.1 | 0.0 | 8.6 | 2.1 | 5.4 | 0.1 |
Note: L% percent damage in the left hemisphere; R%: percent damage in the right hemisphe; X% average damage to both hemispheres; W%: weighted average damage to both hemispheres (W% = L% × R%)/100; weighted index as defined by Hodos and Bobko (1984)). TH/TF: ventral cortical area of the temporal lobe as defined by von Bonin and Bailey (1947).
Table 2.
Volumetric Reduction of the Hippocampus
| Group |
Volume Reduction |
||
|---|---|---|---|
| Subjects |
Hippocampus |
||
| Neo-H-ibo | L% | R% | X% |
| Neo-H-ibo-1 | 27.6 | 10.6 | 19.1 |
| Neo-H-ibo-2 | 61.1 | 72.8 | 67.0 |
| Neo-H-ibo-3 | 54.7 | 47.8 | 51.3 |
| Neo-H-ibo-4 | 33.6 | 61.6 | 47.6 |
| Neo-H-ibo-5 | 49.1 | 64.0 | 56.6 |
| Neo-H-ibo-6 | 21.3 | 8.3 | 14.8 |
| Mean | 41.2 | 44.1 | 42.7 |
L% = Percent reduction of hippocampal volume in left hemisphere, R% = Percent reduction of hippocampal volume in right hemisphere, X% = Average percent reduction in both hemispheres.
Figure 1.
Left column illustrates sagittal T1-weighed images depicting the hippocampus of the right hemisphere (white arrows) in a sham-operated control monkey (Neo-C-1). The middle and right columns illustrate sagittal images of the left and right hippocampus respectively in a case with neonatal hippocampal lesion (Neo-H-ibo-2). Notice the empty ventricles in both hemispheres.
Session-Unique Delayed Nonmatching-to-Sample (SU-DNMS)
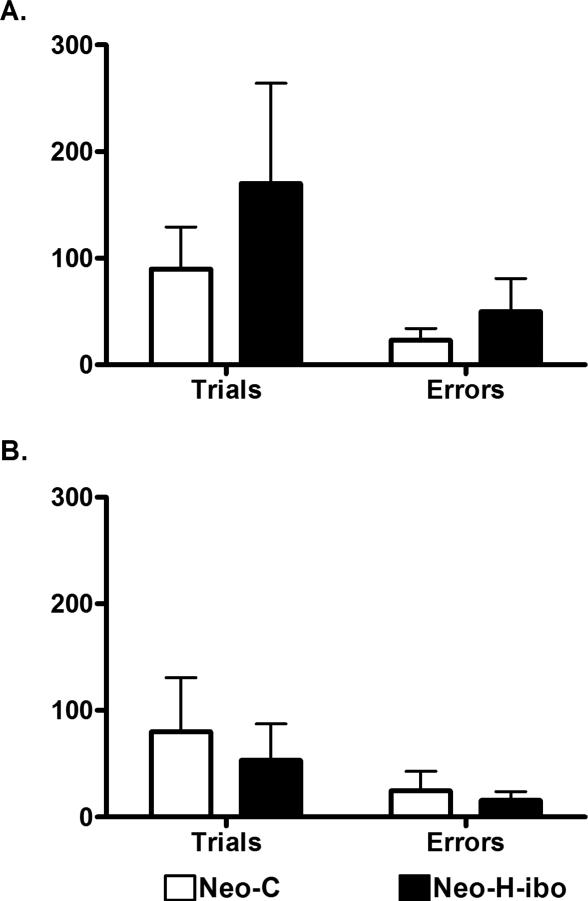
Number of trials and errors to reach learning criterion at the 5-sec and 30-sec delays by each animal are shown in Table 3. All animals successfully completed SU-DNMS at both the 5-sec and 30-sec delays well within the learning criterion limits of 1000 and 500 trials, respectively. Animals with neonatal hippocampal lesions took slightly longer to acquire the SU-DNMS at the 5-s delay, averaging 170 trials (50 errors) as compared to sham-operated controls (90 trials and 23 errors), whereas animals with neonatal hippocampal lesions reached criterion slightly faster at the 30-s delay (53 trials and 16 errors) than sham-operated controls (80 trials and 25 errors). Analysis of Variance (2 Groups × 2 delays) with repeated measures for the last factor revealed no significant effects of Group on trials and errors to criterion [F(1,10) = 0.204, p = 0.661 and F(1,10) = 0.199, p = 0.665, respectively]. In addition, the factor Delay was not reliable for both trials and errors [F(1,10) = 1.210, p = 0.315 and F(1,10) = 0.731, p = 0.412, respectively] as was the interaction between the two factors [F(1,10 = 0.794, p= 0.394 and F(1,10) = 0.871, p = 0.373, respectively]. Thus, as shown in Figure 2, animals with neonatal hippocampal lesions performed similarly to sham-operated controls at both delays. Because hippocampal lesion extent varied greatly between animals and could have masked a potential impairment on the task, correlations were performed between extent of lesions and scores at the two delays separately. However, no correlation between the number of trials to criterion and lesion size reached significance at either the 5 second [r = 0.011, p = 0.984] or 30 second [r = 0.359, p = 0.485] delay.
Table 3.
Session-Unique Delayed NonMatching-to-Sample
| Subjects |
5-Sec Delay |
30-Sec Delay |
||
|---|---|---|---|---|
| T | E | T | E | |
| Neo-C | ||||
| Neo-C-1 | 0 | 0 | 0 | 0 |
| Neo-C-3 | 150 | 35 | 320 | 114 |
| Neo-C-4 | 240 | 68 | 80 | 16 |
| Neo-C-5 | 120 | 30 | 0 | 0 |
| Neo-C-6 | 0 | 0 | 0 | 0 |
| N-7 | 30 | 6 | 80 | 18 |
| Mean | 90.0 | 23.2 | 80.0 | 24.7 |
| Neo-H-ibo | ||||
| Neo-H-ibo-1 | 0 | 0 | 220 | 55 |
| Neo-H-ibo-2 | 30 | 4 | 40 | 11 |
| Neo-H-ibo-3 | 570 | 190 | 40 | 17 |
| Neo-H-ibo-4 | 60 | 9 | 20 | 10 |
| Neo-H-ibo-5 | 330 | 91 | 0 | 0 |
| Neo-H-ibo-6 | 30 | 5 | 0 | 0 |
| Mean | 170.0 | 49.8 | 53.3 | 15.5 |
Scores are the number of trials (T) or of errors (E) prior to reaching criterion at each of the two delays for animals with neonatal hippocampal lesions (Neo-H-ibo) and sham-operated controls (Neo-C).
Figure 2.
Mean trials and errors to criterion in the SU-DNMS at the 5-sec delay (A) and the 30-sec delay (B) for sham-operated controls (Neo-C) and monkeys with neonatal hippocampal lesions (Neo-H-ibo).
Object Self-Ordered Task (Obj-SO)
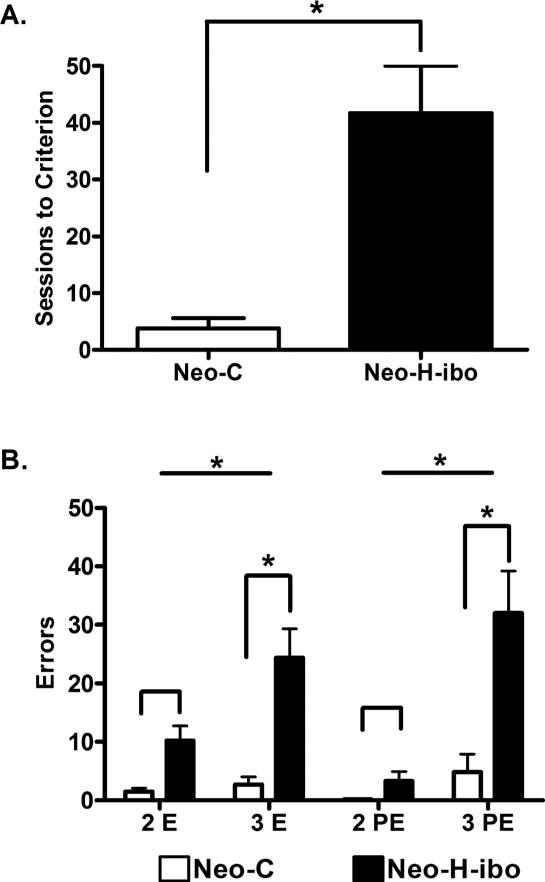
Table 4 provides the number of daily sessions as well as the number of errors and perseverative errors for Trials 2 and 3 of the Obj-SO for each animal. All control animals reached criterion in an average of 5 daily sessions. By contrast, all of the animals with neonatal hippocampal lesion, but one (Neo-H-ibo-3), failed to reach criterion within the limit of testing, averaging 44 daily sessions, resulting in a significant group difference [U(1,9) = 4.50, p = 0.05; see Figure 3A]. The impairment in learning the Obj-SO task in Group Neo-Hibo was also reflected in the increased number of primary errors on Trial 2 and Trial 3 (average: 10.2 and 24.3 errors, respectively) as compared to Group Neo-C (average: 1.5 and 2.7 errors, respectively). A repeated measures ANOVA on errors (2 Groups X 2 Trials) resulted in a significant Group Effect [F(1, 10) = 16.23, p = .002], a significant effect of Trial [F(1,10) = 22.487, 0.001], and a significant interaction between the two factors [F(1,10) = 16.16, p = 0.002]. As shown in Figure 3B, this interaction indicated that, although both groups made more errors on Trial 3 than on Trial 2, this difference was significant for Group Neo-Hibo (U = 5.5, p = .04), but not for Group Neo-C (U = 14.5, p > .05). A similar pattern of results emerged for number of perseverative errors on Trial 2 and Trial 3 [Group effect: F(1, 10) = 11.41, p = .007; Trial effect: F(1, 10) = 23.97, p = .001; Group X Trial: F(1, 10) = 12.43, p = .005; see Figure 3B), with Group Neo-Hibo perseverating more on Trial 3 than on Trial 2 (U = 5.5, p = .04) but not Group Neo-C (U = 14.0, p > .05). Finally, it is interesting to note that case Neo-H-ibo-3, which learned the task immediately and performed as well as controls on the Obj-SO task (see Table 4), had the most extended hippocampal damage (87%) as shown on FLAIR MR images. However, the correlations between the Total Errors on Obj-SO and lesion extent did not reach significance [r = -0.422, p = 0.491].
Table 4.
Object Self-Ordered Task
| Subjects |
Sessions |
Errors by Trial |
Total Errors |
||||
|---|---|---|---|---|---|---|---|
| Trial 2 Error | Trial 2 PE | Trial 3 Error | Trial 3 PE | Errors | PE | ||
| Neo-C | |||||||
| Neo-C-1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neo-C-3 | 11 | 2 | 0 | 8 | 16 | 10 | 16 |
| Neo-C-4 | 6 | 2 | 0 | 5 | 13 | 7 | 13 |
| Neo-C-5 | 5 | 4 | 1 | 3 | 0 | 7 | 1 |
| Neo-C-6 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| N-7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean | 3.8 | 1.5 | 0.2 | 2.7 | 4.8 | 4.2 | 5.0 |
| Neo-H-ibo | |||||||
| Neo-H-ibo-1 | 50* | 8 | 1 | 27 | 28 | 35 | 29 |
| Neo-H-ibo-2 | 50* | 13 | 11 | 33 | 52 | 46 | 63 |
| Neo-H-ibo-3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neo-H-ibo-4 | 50* | 15 | 3 | 28 | 39 | 43 | 42 |
| Neo-H-ibo-5 | 50* | 17 | 3 | 32 | 39 | 49 | 42 |
| Neo-H-ibo-6 | 50* | 8 | 2 | 26 | 34 | 34 | 36 |
| Mean | 44.1 | 10.2 | 3.3 | 24.3 | 32 | 34.5 | 35.3 |
Scores are the number of sessions to reach criterion, the number of primary errors on Trials 2 and 3 and the number of perseverative errors (PE) on Trials 2 and 3. Total errors and total perseverative errors are also given.
indicates failure to reach criterion within the 50-session limit.
Figure 3.
Mean number of sessions to reach criterion on the Obj-SO task is shown in A. Number of Trials 2 and Trials 3 errors and perseverative errors are shown in B for sham-operated controls (Neo-C) and monkeys with neonatal lesions of the hippocampus (Neo-H-ibo). * p = 0.05.
Discussion
The present experiment demonstrates that neonatal lesions of the hippocampus in monkeys impair working memory processes. Specifically, the findings revealed that neonatal hippocampal damage spared recency memory, i.e. the ability to indicate whether a familiar stimulus has been recently seen mediated by the ventral lateral prefrontal cortex. By contrast, the same damage profoundly altered the animals’ ability to monitor their choices from a set of three visual stimuli, known to be mediated by the dorsolateral prefrontal cortex. Thus, the data suggest for the first time that neonatal damage to the hippocampus impacts working memory processes known to be mediated by the dorsolateral prefrontal cortex.
Maintenance of information in working memory buffer
In the SU-DNMS task, measuring the ability to maintain mental representations in a memory buffer, animals with neonatal lesions of the hippocampus displayed normal performance even at the long delay of 30 seconds. This finding parallels the sparing of recency judgments after damage to the hippocampus that included adjacent cortical areas, in adult monkeys even when long delays are used (Diamond, Zola-Morgan, & Squire, 1989). In addition, intact maintenance of information in working memory was also observed in patient HM with large damage to the medial temporal lobe (Drachman & Arbit, 1966) as well as in more recent patients with damage mostly restricted to the hippocampus (Jeneson, Mauldin, & Squire, 2010). Interestingly, all these findings contrast with the impairment in recency judgments found after transection of the fornix in adult monkeys (Charles, Gaffan, & Buckley, 2004). This divergence is likely related to differences in lesion type and recency memory task used. First, it is well-known that fornix transection, which disconnects the hippocampus from the thalamus and septal area, may have cognitive effects that are sometimes opposite to those produced by direct hippocampal lesions. Second, the recency task used by Charles and colleagues was in fact more complex than the one we used in this study. The animals were presented with lists of 5 objects and then to discriminate which of the two objects of the list had been seen the most recently. However, these recency judgments were also intermixed with discriminations between an object of the current list and an object that had been encountered in a previous list. Thus, the working memory task did not only measure recency judgments, but also monitoring of the objects seen in previous lists. This task is more reminiscent of a serial order task in which the monkeys with neonatal hippocampal lesions of the present study seemed to be severely impaired (see preliminary results in (Heuer & Bachevalier, 2008). Overall, the data suggest that maintenance of information in working memory, which is required in a simple recency task (SU-DNMS), is spared by damage to the hippocampus, whether the damage occurs in adulthood or in infancy. Furthermore, given that damage to ventrolateral prefrontal cortex, but not the dorsolateral prefrontal cortex, impairs performance on delayed matching-to-sample with a single pair, a task similar to the recency memory task used in the present experiment (Mishkin & Manning, 1978; Passingham, 1975) the present data demonstrate that the neonatal hippocampal lesions did not impact on the functioning of the ventrolateral prefrontal cortex, at least not in the domain of recency memory.
Monitoring of information in working memory
By contrast, in the Obj-SO task, all animals with neonatal hippocampal lesions but one (case Neo-Hibo-3) failed to reach the learning criterion, indicating severe impairment in working memory tasks measuring the ability to monitor self-ordered sequences of responses. Because the position of the three objects on the tray varied randomly on each trial, the impairment in the Obj-SO task cannot be attributed to an inability to use either spatial or motor coding of the response to the stimuli, but rather it resulted from an inability to monitor self-generated choices from a set of stimuli. The different outcomes of the neonatal hippocampal lesions in the SU-DNMS versus Obj-SO tasks could be explained by different memory load required in the two tasks, i.e. in the SU-DNMS animals had to remember two objects, whereas in the Obj-SO they had to remember three objects. Earlier studies had already demonstrated that monkeys with hippocampal lesions could remember one place but not two (Angeli, Murray, & Mishkin, 1993). But, this conclusion seems unlikely given that the same animals with neonatal hippocampal lesions could remember 5 objects or 2 locations as efficiently as their sham-operated controls (Heuer & Bachevalier, 2011). Rather, because animals with neonatal hippocampal lesions were as profoundly impaired as adult monkeys with mid-dorsolateral prefrontal lesions (Petrides, 1995), although in both studies the experimental animals were unimpaired in recognition memory and recency judgments (Heuer & Bachevalier, 2011; Petrides, 1995), the dissociable outcomes of neonatal hippocampal lesions on the two tasks suggest that these early hippocampal lesions have impacted the development of the ventrolateral and dorsolateral prefrontal cortex differently. Thus, given that ventrolateral prefrontal cortex impaired recognition memory and recency memory (Mishkin and Manning, 1978; Passingham, 1975; Kowalska et al., 1991), whereas dorsolateral prefrontal lesions spared recognition memory and recency memory but impaired the monitoring of self-ordered of responses (Petrides, 1995), the neonatal hippocampal lesions may have resulted in greater developmental alterations of the dorsolateral prefrontal cortex than of the ventrolateral prefrontal cortex.
Before discussing the mechanisms by which the hippocampus may have impacted the prefrontal cortex during development, two issues need to be addressed. The first is the normal performance of case Neo-H-ibo-3 (Table 3) in the self-ordered task. This good performance is puzzling given that this animal has one of the most extended hippocampal lesions (87% damage or 53% volume reduction) of the group and showed an impairment in incidental recognition memory tasks known to be hippocampal-dependent as did the other animals of Group Neo-Hibo (Zeamer et al., 2010). One possible explanation may relate to the use of alternative strategies that this animal may have developed to solve the task, as alluded to in earlier reports (Kimble and Pribram 1963; Waxler and Rosvold 1970). For example, good performance on the Obj-SO task may be achieved by selecting the three objects in the same specific order across multiple days that could have resulted in learning a specific and inflexible sequence of objects. We thus re-investigated object selection across each testing day for Case Neo-Hibo-3 and found no evidence that this animal had developed such a strategy to solve the task. Thus, whatever reasons may have led case Neo-Hibo-3 to perform normally in the self-ordered task, it is clear that the five other animals of the group were severely impacted and were not even able to solve the task, indicating that neonatal hippocampal lesions significantly impacted monitoring working memory processes. The second issue that needs to be considered is the effects of rearing conditions on brain development, and more specifically on the protracted development of the prefrontal cortex. As indicated in the Methods, our animals were surrogate nursery-peer reared according to earlier procedures that had demonstrated normal development of species-specific social skills (Sackett et al., 2002). However, as compared to mother-reared monkeys in a semi-naturalistic social group, monkeys raised in social isolation in a nursery showed reduced prefrontal cortical white matter volumes, reduced volume of the corpus callosum and were impaired on the Trial Unique Delayed Non-Match-to-Sample, a measure of recognition memory (Sanchez, Hearn, Do, Rilling, & Herndon, 1998). There are several reasons to suggest that rearing conditions might not be the explanation for the poor performance of animals with neonatal hippocampal lesions in the self-ordered task. First, one of the control animals in this study (case N7) had been mother-reared in a social group and performed in the Obj-SO task as well as other sham-operated controls that had been peer-reared. So, at the very least, our rearing conditions did not significantly alter performance of the sham-operated animals, indicating a normal functioning of the dorsolateral prefrontal cortex in this control group. Nevertheless, one could argue that the nursery-rearing conditions associated with a brain lesion may yield a more serious outcome. This also seems unlikely given that, as compared to the nursery-reared animals in the Sanchez study (Sanchez et al., 1998) that showed severe recognition memory loss, all animals in the present study, sham-operated controls or those with neonatal hippocampal lesions alike, performed well in tasks measuring both recognition and recency memory and their performance on the object recognition memory task did not differ from adult animals that had been mother-reared (Heuer & Bachevalier, 2011; this study). Thus, although we could not entirely rule out an effect of rearing conditions on performance of animals with neonatal hippocampal lesions in the self-ordered task, we believe that this effect has to be minor given the similar performance of these animals in many other cognitive tasks as mother-reared monkeys with adult-onset hippocampal lesions.
The critical question that needs to be addressed is whether the monitoring working memory deficits are due to the hippocampal damage itself or to a maldevelopment of the dorsolateral prefrontal cortex or both. There exist only few studies that have assessed the effects of adult-onset hippocampal lesions on working memory processes and none have used the Obj-SO task. Thus, monkeys with nonselective hippocampal lesions were impaired in variations of delayed response paradigms, although individual differences were noted with few monkeys performing as well as normal controls (Mahut, 1971; Waxler & Rosvold, 1970). Only one previous study in non-human primates used a self-ordered working memory task to assess prefrontal functioning after nonselective hippocampal lesions (Kimble & Pribram, 1963). In this study, monkeys were presented with two numbers “1” on a computer screen and had to touch each number in turn, one after the other, to receive a reward. Nonselective hippocampal lesions resulted in a significant impairment, suggesting that the hippocampus participates in the monitoring of self-ordered acts. Again, the authors reported individual differences in that one of four of the operated monkeys performed as well as the normal controls, although the hippocampal lesion extent of the four monkeys was similar. This is in fact reminiscent to the observation of the present study (see above) in which one of the five monkeys learned the task normally. These individual differences suggest that whatever process is served by the hippocampus and also necessary to solve self-ordered monitoring tasks might be utilized by some animals but not others (see also Waxler and Rosvold 1970 for a similar conclusion). Thus, although the self-order task in the Kimble and Pribram (1963) study was less complex than the one used here, and the hippocampal lesions were much larger, including medial temporal cortical areas, the data suggest that the hippocampus in primates may be essential to subserve monitoring information held in working memory. Further studies will be necessary to establish whether the impairment the Obj-SO task could also be found following selective hippocampal lesions and if so to determine whether or not the cognitive processes of the Obj-So task mediated by the hippocampus differ in any way from those mediated from by the prefrontal cortex. As one of the critical differences between the SU-DNMS and Obj-SO tasks is to monitor self-behavior on successive trials, one might speculate that there could be substantial overlap in the roles of the hippocampus and prefrontal cortex in the Obj-SO task. To answer this question additional empirical studies will be needed.
In sum, the monitoring working memory deficits following neonatal hippocampal lesions may have resulted from the absence of the hippocampus, a maldevelopment of the dorsolateral prefrontal cortex or both.
Hippocampal-Prefrontal interactions during development
The results of this study indicate that the early damage to the hippocampus may have disrupted the normal functioning of the dorsolateral prefrontal cortex and support the notion that the hippocampus is a critical regulator of prefrontal working memory function. They also parallel a large body of studies in the rodent literature that has consistently shown that the integrity of the developing prefrontal cortex is compromised following early damage to the hippocampus (Chambers et al., 1996; Levin & Christopher, 2006; Lipska, 2004; Lipska et al., 2002). Such a functional connectivity between the two regions has also been reported in schizophrenic patients who demonstrate early pathology in both the dorsolateral prefrontal cortex and hippocampus and are impaired on an n-back task that has similar cognitive demands to the self-ordered task (Goto, Yang, & Otani, 2010; Jansma, Ramsey, van der Wee, & Kahn, 2004; Meyer-Lindenberg et al., 2001; Nelson, Saykin, Flashman, & Riordan, 1998). In addition, Meyer-Lindenberg and colleagues (2005) have shown a disruption of the functional connectivity between the hippocampus and dorsolateral prefrontal cortex in schizophrenic patients while performing an n-back task.
The likelihood of the involvement of the dorsolateral prefrontal cortex in the impairment found on the Obj-SO task after neonatal hippocampal lesions is further supported by the presence of direct anatomical connections between the dorsolateral prefrontal cortex and the hippocampus (Goldman-Rakic, Selemon, & Schwartz, 1984; Morris, Pandya, & Petrides, 1999), which do not exist between the ventrolateral prefrontal cortex and hippocampus. Functional connectivity between the dorsolateral prefrontal cortex and hippocampus could also involve indirect connections with the posterior parietal cortex (Belger et al., 1998; Kim et al., 2003) and the medial dorsal thalamic nucleus (Isseroff, Rosvold, Galkin, & Goldman-Rakic, 1982), both regions known to be critical for the regulation of working memory. It is also interesting to note that neonatal hippocampal lesions in monkeys alter resting state metabolism in the retrosplenial cortex (Machado, Snyder, Cherry, Lavenex, & Amaral, 2008) and result in reduced volume of the posterior mid-body and isthmus of the corpus callosum carrying fibers from both the retrosplenial and parietal cortex to the hippocampus (Cirilli, Payne, & Bachevalier, 2009).
Although there exist no empirical studies in monkeys that have assessed the development of the prefrontal cortex after neonatal hippocampal lesions, results from our laboratory have already demonstrated that large medial temporal lobe lesions, including the hippocampus, in infant monkeys yielded alterations of dorsolateral prefrontal cortex morphology (Chlan-Fourney, Webster, Felleman, & Bachevalier, 2000; Chlan-Fourney, Webster, Jung, & Bachevalier, 2003) and functioning (Bertolino et al., 1997; Saunders, Kolachana, Bachevalier, & Weinberger, 1998). None of these changes occurred in other cortical areas and/or in adult monkeys that had received the same medial temporal lobe lesions in adulthood. Further neuroimaging and neuroanatomical investigations of monkeys of the current study will provide direct evidence on whether these early hippocampal lesions have impacted the functioning of the prefrontal cortex.
Conclusions
These results, taken together, demonstrate for the first time that selective neonatal hippocampal lesions selectively impaired self-ordered, monitoring working memory processes. Several important questions remain to be addressed. The first is the extent to which the deficit pertains to the damage to the hippocampus or to a maldevelopment of the dorsolateral prefrontal cortex or both? The second is what specific morphological and functional changes in the dorsolateral prefrontal cortex result from an absence of functional hippocampal inputs to the prefrontal cortex during maturation? The third is by which neural pathways and brain mechanisms the hippocampus may interact with the dorsolateral prefrontal cortex?
Acknowledgements
This work was supported by grants from NIMH (MH-58846), NICHD (HD-35471), the Yerkes Base Grant NIH RR00165, and the Center for Behavioral Neuroscience grant NSF IBN-9876754 to JB, as well as from a NIMH (T32-MH0732505) predoctoral fellowship to EH. We would like to thank Heather Banta for her assistance in testing the animals in this study. Eric Heuer is now an Assistant Professor of Psychology at the University of Hawaii at Hilo.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne.
References
- Angeli SJ, Murray EA, Mishkin M. Hippocampectomized monkeys can remember one place but not two. Neuropsychologia. 1993;31(10):1021–1030. doi: 10.1016/0028-3932(93)90030-4. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M, Alvarado MC. Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience. 1999;113(6):1127–1151. doi: 10.1037//0735-7044.113.6.1127. [DOI] [PubMed] [Google Scholar]
- Belger A, Puce A, Krystal JH, Gore JC, Goldman-Rakic P, McCarthy G. Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Human Brain Mapping. 1998;6(1):14–32. doi: 10.1002/(SICI)1097-0193(1998)6:1<14::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Tuerke KJ, Forsyth JK, Giles A, Xue L, Boegman RJ, Jhamandas K. Neonatal ventral hippocampal lesions in male and female rats: effects on water maze, locomotor activity, plus-maze and prefrontal cortical GABA and glutamate release in adulthood. Behavioural Brain Research. 2009;202(2):198–209. doi: 10.1016/j.bbr.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, Weinberger DR. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporolimbic lesions: a proton magnetic resonance spectroscopic imaging study. Cerebral Cortex. 1997;7(8):740–748. doi: 10.1093/cercor/7.8.740. [DOI] [PubMed] [Google Scholar]
- Blue SN, Kazama AM, Bachevalier J. Neuroscience Meeting Planner. Society for Neuroscience Online, 98.7; Washington DC: 2009. The normal development of object-place association memory is altered by neonatal hippocampal lesions in rhesus monkeys. [Google Scholar]
- Brady AM. Neonatal ventral hippocampal lesions disrupt set-shifting ability in adult rats. Behavioural Brain Research. 2009;205(1):294–298. doi: 10.1016/j.bbr.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15(6):587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Charles DP, Gaffan D, Buckley MJ. Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. Journal of Neuroscience. 2004;24(8):2037–2044. doi: 10.1523/JNEUROSCI.3796-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlan-Fourney J, Webster MJ, Felleman DJ, Bachevalier J. Neuroscience Meeting Planner. Society for Neuroscience Online, 228.18; Washington DC: 2000. Neonatal medial temporal lobe lesions alter the distribution of tyrosine hydroxylase immunoreactive varicosities in the macaque prefrontal cortex. [Google Scholar]
- Chlan-Fourney J, Webster MJ, Jung J, Bachevalier J. Neuroscience Meeting Planner. Society for Neuroscience Online, 315.9; Washington DC: 2003. Neonatal medial temporal lobe lesions decrease GABAergic interneuron densities in macaque prefrontal cortex: Implications for schizophrenia and Autism. [Google Scholar]
- Cirilli L, Payne C, Bachevalier J. Neuroscience Meeting Planner. Society for Neuroscience Online, 536.20; Washington DC: 2009. Neonatal hippocampal and amygdala lesions alter the development of the corpus callosum: An MRI study in adult monkeys. [Google Scholar]
- Curtis CE, D'Esposito M. The effects of prefrontal lesions on working memory performance and theory. Cogn Affect Behav Neurosci. 2004;4(4):528–539. doi: 10.3758/cabn.4.4.528. [DOI] [PubMed] [Google Scholar]
- Diamond A, Zola-Morgan S, Squire LR. Successful performance by monkeys with lesions of the hippocampal formation on AB and object retrieval, two tasks that mark developmental changes in human infants. Behavioral Neuroscience. 1989;103(3):526–537. doi: 10.1037//0735-7044.103.3.526. [DOI] [PubMed] [Google Scholar]
- Drachman DA, Arbit J. Memory and the hippocampal complex. II. Is memory a multiple process? Arch Neurol. 1966;15(1):52–61. doi: 10.1001/archneur.1966.00470130056005. [DOI] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12(8):1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado MC, Bachevalier J. Neuroscience Meeting Planner. Society for Neuroscience Online, 574.11; Washington DC: 2006. Neonatal hippocampal damage impairs specific place/food associations in adult macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavis-Bloom C, Kazama A, Bachevalier J. Neuroscience Meeting Planner. Society for Neuroscience Online, 791.1; Washington DC: 2008. Paradoxical facilitation of stimulus-reward association learining in rhesus macaques with neonatal hippocampal lesions. [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biological Psychiatry. 2010;67(3):199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Goursaud AP, Bachevalier J. Social attachment in juvenile monkeys wth neonatal lesion of the hippocampus, amygdala and orbitofrontal cortex. Behavioural Brain Research. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. Journal of Microscopy. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Modulation of working memory by neonatal lesions of the hippocampus in the rhesus macaque. Society for Neuroscience Abstracts Online; Washington DC: 2007. 305.17, On Line. [Google Scholar]
- Heuer E, Bachevalier J. Modulation of serial order memory following neonatal lesions of the hippocampus in the rhesus macaque. Society for Neuroscience Abstracts Online; Washington DC: 2008. 791.2, On Line. [Google Scholar]
- Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behavioral Neuroscience. 2011;125(2):137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Bobko P. A weighted index of bilateral brain lesions. Journal of Neuroscience Methods. 1984;12(1):43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Isseroff A, Rosvold HE, Galkin TW, Goldman-Rakic PS. Spatial memory impairments following damage to the mediodorsal nucleus of the thalamus in rhesus monkeys. Brain Research. 1982;232(1):97–113. doi: 10.1016/0006-8993(82)90613-8. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophrenia Research. 2004;68(2-3):159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Squire LR. Intact working memory for relational information after medial temporal lobe damage. Journal of Neuroscience. 2010;30(41):13624–13629. doi: 10.1523/JNEUROSCI.2895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS, Lee DS, Lee MC. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a[15(O)]H2O PET study. American Journal of Psychiatry. 2003;160(5):919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Pribram KH. Hippocampectomy and behavior sequences. Science. 1963;139:824–825. doi: 10.1126/science.139.3557.824. [DOI] [PubMed] [Google Scholar]
- Kowalska DM, Bachevalier J, Mishkin M. The role of the inferior prefrontal convexity in performance of delayed nonmatching-to-sample. Neuropsychologia. 1991;29:583–600. doi: 10.1016/0028-3932(91)90012-w. [DOI] [PubMed] [Google Scholar]
- Levin ED, Christopher NC. Effects of clozapine on memory function in the rat neonatal hippocampal lesion model of schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30(2):223–229. doi: 10.1016/j.pnpbp.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. Journal of Psychiatry & Neuroscience. 2004;29(4):282–286. [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27(1):47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. European Journal of Neuroscience. 2003;18(11):3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imaging study. Neuroimage. 2008;39(2):832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahut H. Spatial and object reversal learning in monkeys with partial temporal lobe ablations. Neuropsychologia. 1971;9(4):409–424. doi: 10.1016/0028-3932(71)90005-4. [DOI] [PubMed] [Google Scholar]
- Malkova L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys.[erratum appears in Hippocampus 2001;11(5):614]. Hippocampus. 2001;11(4):361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Marquis JP, Goulet S, Dore FY. Neonatal ventral hippocampus lesions disrupt extra-dimensional shift and alter dendritic spine density in the medial prefrontal cortex of juvenile rats. Neurobiol Learn Mem. 2008;90(2):339–346. doi: 10.1016/j.nlm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Delacour J. An analysis of short-term visual memory in the monkey. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1(4):326–334. doi: 10.1037//0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Manning FJ. Non-spatial memory after selective prefrontal lesions in monkeys. Brain Research. 1978;143(2):313–323. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. Journal of Comparative Neurology. 1999;407(2):183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study.[see comment]. Archives of General Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. Journal of Neuroscience Methods. 2002;121(2):199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cerebral Cortex. 2002;12(9):975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Owen AM. The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Experimental Brain Research. 2000;133(1):33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- Passingham R. Delayed matching after selective prefrontal lesions in monkeys (Macaca mulatta). Brain Research. 1975;92(1):89–102. doi: 10.1016/0006-8993(75)90529-6. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2009 doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Monitoring of selections of visual stimuli and the primate frontal cortex. Proceedings Biological Sciences/The Royal Society. 1991;246(1317):293–298. doi: 10.1098/rspb.1991.0157. [DOI] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. Journal of Neuroscience. 1995;15(1 Pt 1):359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(3):873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, midventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(8):5649–5654. doi: 10.1073/pnas.072092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9(10):3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett GP, Ruppenthal GC, Davis AE. Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtailed monkeys (Macaca nemestrina). Am J Primatol. 2002;56(3):165–183. doi: 10.1002/ajp.1072. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812(1-2):38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Experimental Brain Research. 1990;81(2):443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Kolachana BS, Bachevalier J, Weinberger DR. Neonatal lesions of the medial temporal lobe disrupt prefrontal cortical regulation of striatal dopamine. Nature. 1998;393(6681):169–171. doi: 10.1038/30245. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14(2-3):249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Stern CE, Owen AM, Tracey I, Look RB, Rosen BR, Petrides M. Activity in ventrolateral and mid-dorsolateral prefrontal cortex during nonspatial visual working memory processing: evidence from functional magnetic resonance imaging. Neuroimage. 2000;11(5 Pt 1):392–399. doi: 10.1006/nimg.2000.0569. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioural Brain Research. 2009;204(2):295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biological Psychiatry. 2007;62(7):730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146(1-2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The Neocortex of Macaca mulatta. The University of Illinois Press; Urbana: 1947. [Google Scholar]
- Waxler M, Rosvold HE. Delayed alternation in monkeys after removal of the hippocampus. Neuropsychologia. 1970;8(2):137–146. doi: 10.1016/0028-3932(70)90001-1. [DOI] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of Neuroscience. 2010;30(27):9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]