Abstract
Molecular chaperones, as the name suggests, are involved in folding, maintenance, intracellular transport, and degradation of proteins as well as in facilitating cell signaling. Heat shock protein 90 (Hsp90) is an essential eukaryotic molecular chaperone that carries out these processes in normal and cancer cells. Hsp90 function in vivo is coupled to its ability to hydrolyze ATP and this can be regulated by co-chaperones and post-translational modifications. In this review, we explore the varied roles of known post-translational modifications of cytosolic and nuclear Hsp90 (phosphorylation, acetylation, S-nitrosylation, oxidation and ubiquitination) in fine-tuning chaperone function in eukaryotes.
Keywords: Heat Shock Protein 90, Hsp82, Post-translational modification Phosphorylation, Acetylation, Oxidation, Nitrosylation
1. Introduction
Cells synthesize large amounts of protein in a very short time, in a cytosol where protein concentration at steady state approaches 300 g/l [1]. Under these conditions, many hydrophobic surfaces on proteins may be transiently exposed and the likelihood of deleterious interactions is quite high [2]. To counter this threat to cell viability, molecular chaperones have evolved to help nascent polypeptides fold correctly and multimeric protein complexes assemble productively, while minimizing the danger of protein aggregation. Heat shock protein 90 (Hsp90) is an evolutionarily conserved molecular chaperone that is involved in stabilizing and activating at least two hundred signaling proteins (clients) under normal cellular conditions [3,4,5]. An updated list of Hsp90 clients can be found at http://www.picard.ch/downloads/downloads.html. Cancer cells have co-opted Hsp90 to serve a crucial role in protecting an array of mutated and over-expressed oncoproteins from misfolding and degradation [6,7]. Thus, Hsp90 inhibitors are being actively evaluated as novel cancer therapeutics [8,9]. This review describes advances in our understanding of how cytosolic and nuclear Hsp90 is regulated by post-translational modification in lower and higher eukaryotes.
2. Hsp90 structure and the chaperone cycle
Hsp90 is found in all kingdoms except Archaea [10]. In humans, as in other eukaryotes, there are two cytosolic Hsp90 isoforms: stress-induced Hsp90α and constitutively expressed Hsp90β [11]. Hsp90 is a member of the ATPase/kinase GHKL (Gyrase, Hsp90, Histidine Kinase, MutL) superfamily – a group of proteins that are characterized by a unique ATP binding cleft [4,12]. Hsp90 is comprised of three domains: i) an N-terminal domain, containing nucleotide, co-chaperone (proteins that assist Hsp90 in modulating client activity), and drug binding sites; ii) a middle (M) domain, which provides binding sites for client proteins and other co-chaperones; iii) a C-terminal domain containing a dimerization motif and binding sites for yet other co-chaperones (Figure 1) [13,14,15]. Hsp90 proteins also have an unstructured charged-linker region of significant but variable length that connects N and M domains and provides conformational freedom to the protein [16,17].
Figure 1.
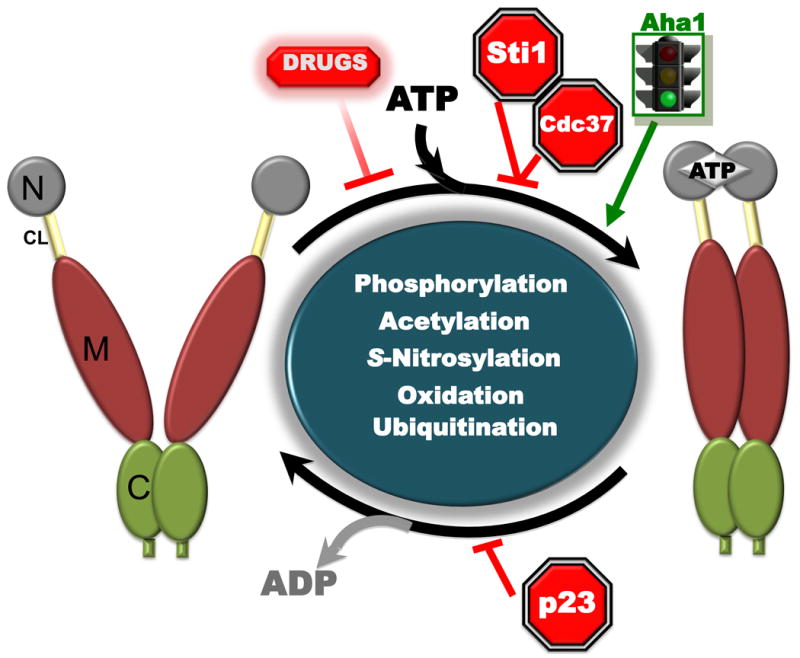
Post-translational modification of Hsp90 fine-tunes its chaperone function.
ATP binding to the N-terminal domain of Hsp90 (gray) promotes transient dimerization of the N-domains (closed conformation). Subsequent structural rearrangements establish the ’closed and twisted’ conformation capable of ATP hydrolysis. The co-chaperone Aha1 enhances Hsp90 ATPase activity by facilitating the conformational changes necessary to achieve ATPase competence, while Sti1 and Hsp90 inhibitors such as geldanamycin (GA) or radicicol (RD) exert the opposite effect by inhibiting the initial structural changes necessary for N-domain dimerization. p23 slows ATP hydrolysis at a late stage of the chaperone cycle. Domain labeling is as follows: N, N-domain (gray); CL, charged linker (yellow); M, M-domain (amber); C, C-domain (green).
Various biophysical techniques, such as X-ray crystallography, small angle X-ray scattering (SAXS) analysis, and electron microscopy have provided valuable snap shots of different Hsp90 conformations in the presence or absence of nucleotides and different co-chaperones [13,18,19,20,21,22]. From these data, investigators have proposed that Hsp90 cycles through several conformations that ultimately result in correctly orienting N and M domains to form a split ATPase. Indeed, much accumulated data support the hypothesis that Hsp90 chaperone function relies on ATP binding and hydrolysis (Figure 1), [12]. However, although all Hsp90 proteins can hydrolyze ATP, the rates of hydrolysis are low and variable from one species to another (0.0015 s–1 for human Hsp90α and 0.013 s–1 for yeast Hsp90) [19,23]. Recent studies suggest that Hsp90 rapidly samples multiple conformations comprising the chaperone cycle in the absence of nucleotides [24]. Current data suggest that ATP binding and hydrolysis subtly shift the conformational equilibrium, presumably by lowering the energy barrier between certain conformations, thus providing directionality to the Hsp90 cycle [24,25]. Hsp90 inhibitors such as radicicol (RD) or geldanamycin (GA) disrupt the chaperone cycle by replacing ATP in Hsp90's nucleotide binding pocket [26,27].
Studies using fluorescence resonance energy transfer (FRET) have revealed that nucleotides and co-chaperones (Aha1, p23 and Sti1) modulate Hsp90 conformational dynamics in real time [24,25]. Further, although the essential aspects of the Hsp90 conformational cycle are conserved from bacteria to humans, the population dynamics (which conformational states are most occupied at steady state) differ between bacteria, yeast, and human Hsp90 [19]. These data suggest that the Hsp90 cycle may be tunable and tailored to the unique intracellular environment of different organisms, or to discrete physiological states within different tissues of metazoans (such as the distinct intracellular environment that is characteristic of cancer). This fine-tuning is likely provided by complex post-translational modifications to Hsp90 itself and to other components of the chaperone machinery.
3. Post-translational modification of Hsp90 co-chaperones
3.1 Co-chaperones modulate Hsp90 activity
Co-chaperones can be generally defined as proteins that modulate the function of other chaperones [28]. Depending on its conformational state, Hsp90 normally interacts with distinct co-chaperones with diverse activities. In total, these conformation-specific multi-protein complexes comprise the functional chaperone unit, frequently referred to as the ‘Hsp90 chaperone machine’. Co-chaperones containing tetratricopeptide repeat (TPR) domains use this motif to interact with Hsp90 (e.g., p60Hop/Sti1, Chip, FKBP51 & 52, PP5/Ppt1), and they have additional domains that catalyze reactions as diverse as ubiquitin ligation, dephosphorylation, and peptidylprolyl isomerization. Non-TPR-co-chaperones include Aha1/Hch1, p23 (prostaglandin E synthase 3, PGES3)/Sba1, Sgt1, and p50/Cdc37 [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. A number of co-chaperones modulate Hsp90 ATPase activity, thereby altering the cycling rate of the chaperone machine (e.g., p50/Cdc37, p60Hop/Sti1, p23, Aha1). Many Hsp90 co-chaperones are conserved in all eukaryotes. For this reason, the single cell eukaryote baker's yeast, Saccharomyces cerevisiae, has proven to be an excellent model organism to study the function of both Hsp90 and its co-chaperones in maintaining cellular homeostatis [46,47,48,49,50,51].
3.2 Impact of co-chaperone phosphorylation on Hsp90 function
One of the challenging tasks in understanding the role of co-chaperones in controlling Hsp90 activity in normal and cancer cells is to appreciate the functional consequences of co-chaperone post-translational modifications. While this has only recently been appreciated, recent work by Vaughan and colleagues [36] emphasizes its importance. These investigators showed that co-chaperones Cdc37 and PP5/Ppt1 form complexes with Hsp90 in yeast and in human tumor cells. The protein phosphatase PP5/Ppt1 dephosphorylates the Ser-13 residue on Cdc37 in vivo, directly affecting its interaction with Hsp90 and negatively impacting the chaperoning of numerous kinase clients by the Hsp90-Cdc37 complex. Serine phosphorylation of Cdc37, mediated by casein kinase 2 (CK2), is necessary for it to chaperone numerous kinase clients including CK2 itself, Cdc28Cdc2, Ste11RAF, Kin28 and Mps1, and also for binding to Hsp90 [52,53,54]. Thus, Cdc37 phosphorylation status is likely to affect cellular sensitivity to Hsp90 inhibition. Indeed, the cellular toxicity of GA is markedly enhanced in cells expressing Cdc37-S13A [36]. Further, over-expression of PP5/Ppt1 is synthetically lethal with GA in yeast. In cancer cells, PP5 over-expression correlated with reduced Cdc37 phosphorylation, reduced Raf-1 protein (an Hsp90 client) expression, and reduced activity of the MAP kinase pathway [36].
Another example of co-chaperone post-translational modification impacting Hsp90 function is supplied by an elegant study recently published by Bansal and colleagues [55]. They showed that dimerization of Sgt1, is negatively regulated by CK2-mediated phosphorylation at Ser-361. This in turn affects kinetochore assembly, which requires a functional Sgt1/Hsp90 complex and which is essential for proper chromosome segregation during cell division in eukaryotes [55].
CK2 also phosphorylates p23, on Ser-113 and Ser-118 in vivo [56]. This is important for its prostaglandin synthase activity and is required for the formation, in this context, of a tertiary complex between p23, CK2 and Hsp90 [56]. Finally CK2 and pp90rsk can also phosphorylate murine Sti1 (mSti1) in vitro (on Ser-189), affecting mSti1 binding to Hsp90 [57]. Cdc2 is also able to phopshorylate mSti1 at Thr-198 in vitro, suggesting that mSti1 phosphorylation may play a role in cell cycle regulation [58].
The immunophilin FK506 binding protein 52 (FKBP52) binds to Hsp90 via its TPR domain and is important for chaperoning of steroid hormone receptors. CK2 phosphorylates FKBP52 on Thr-143 both in vitro and in vivo. Phosphorylation of this residue does not affect FK506 binding to FKBP52, but phosphorylated FKBP52 does not interact with Hsp90. These findings suggest that phosphorylation of FKBP52 plays a role in modulating steroid hormone receptor-mediated signal transduction [52,59].
FKBP52 phosphorylation (general, residues not specified) also plays a role in the transduction efficiency of adeno-associated virus type 2 (AAV) [60,61,62]. AAV is considered to be a potential tool for gene therapy, since it is a non-pathogenic single-stranded DNA virus that can integrate into the human genome in a site-specific manner. Its transduction efficiency is cell type dependent and relies on the synthesis of a second strand of viral DNA. Phosphorylated FKBP52 regulates this process by binding the D-sequence in the inverted terminal repeat of AAV and inhibiting synthesis of the second DNA-strand, negatively affecting transgene expression. Further examination of co-chaperone post-translational modifications is likely to uncover additional important regulatory mechanisms affecting Hsp90.
4. Post-translational modification of Hsp90
4.1. Phosphorylation
Hsp90 is subject to various post-translational modifications including phosphorylation, acetylation, S-nitrosylation, oxidation, and ubiquitination that impact chaperone function in numerous ways (Figure 2) [63]. Hsp90 is a phosphoprotein and its steady-state phosphorylation level is influenced by different cellular environments in a species-specific manner. For example, heat shock decreases the turnover of Hsp90 phosphate groups in yeast cells, while this process has the opposite effect on HeLa cell Hsp90 phosphorylation [64,65]. A number of serine, threonine, and tyrosine phosphorylation sites have been identified in Hsp90 [64,66,67,68,69,70,71,72,73,74,75,76,77]. Early work showed that Hsp90 became hyper-phosphorylated and associated less avidly with its client kinase p60v-src in NIH 3T3 cells treated with the serine/threonine phosphatase inhibitor okadaic acid, suggesting a link between Hsp90 phosphorylation and its ability to chaperone client proteins [66,78]. Supporting these data, the co-chaperone PP5/Ppt1 dephosphorylates Hsp90 in vitro and positively regulates its chaperone activity in vivo [74,75,79].
Figure 2.
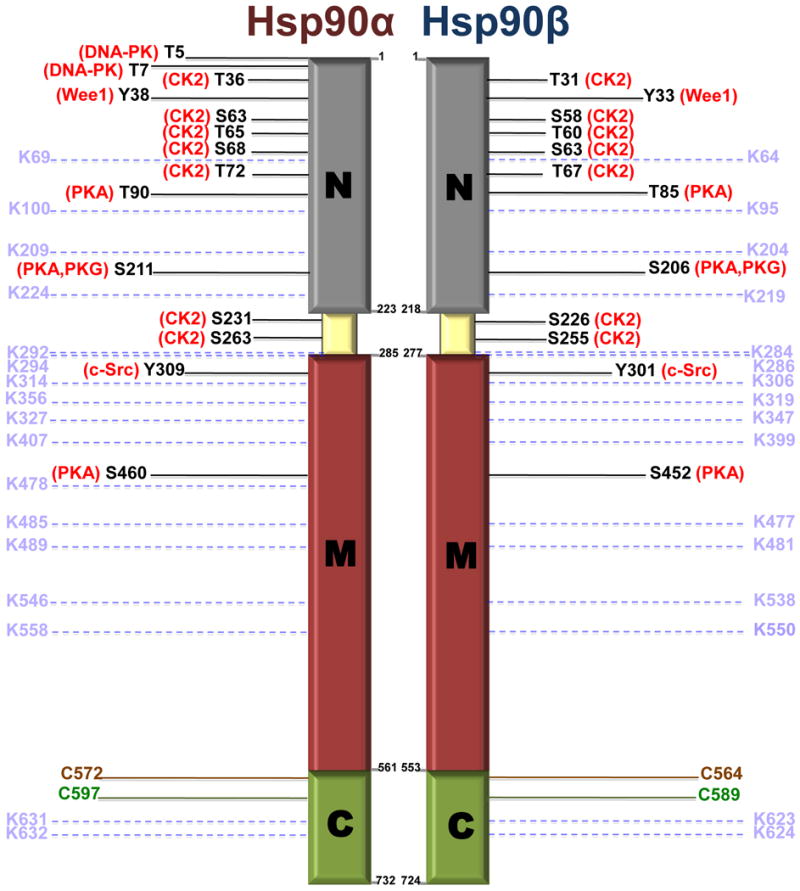
Post-translational modification sites on Hsp90.
Domain location of phosphorylated serine (S), theronine (T) and tyrosine (Y) sites for which kinases are known, acetylated lysine (K) residues (pale blue), S-nitrosylated cysteine (C) (green) and cysteine oxidation sites (brown) on human Hsp90α and Hsp90β are shown. For additional phosphorylation sites, the reader is referred to [64,76].
Tyrosine phosphorylation of Hsp90 has been shown to affect its interaction with distinct client proteins. Thus, Hsp90 tyrosine phosphorylation induced by 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) was reported to increase its association with endothelial nitric oxide (eNOS) [80]. Tyrosine phsophorylation of Hsp90 was also shown to enhance its interaction with ionotropic P2X7 receptors [69]. Geldanamycin treatment decreased both Hsp90 tyrosine phosphorylation and association with P2X7 receptors [69]. A series of recent proteomic studies have identified a large number of phosphosites on Hsp90, but the protein kinases targeting many of these sites remain unknown [81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101]. A comprehensive list of these phosphorylation sites can be found in a recent review [76].
As can be appreciated by referring to the specific examples provided below, phosphorylation of specific residues in Hsp90 can affect client protein interaction (CK2-mediated charged linker phosphorylation) and can provide a mechanism to regulate the chaperoning of different classes of client proteins by Hsp90 (CK2-mediated and Wee1-mediated N-domain phosphorylation). Certainly, additional functional consequences of Hsp90 phosphorylation will become apparent as these investigations continue.
4.1.1. Double-stranded DNA protein kinase
Elegant work by Lees-Miller and Anderson has shown that double-stranded (ds) DNA-activated protein kinase (DNA-PK) isolated from HeLa cells is able to phosphorylate in vitro two threonine residues (Thr-5 and Thr-7) found only in the N domain of human Hsp90α. Equivalent residues in mouse Hsp86 and rabbit Hsp90α are also phosphorylated by DNA-PK [102]. The physiologic significance of Hsp90α phosphorylation by DNA-PK remains to be resolved, although cellular DNA damage has been reported to alter Hsp90 phosphorylation [81,96].
4.1.2. B-Raf and Akt kinases
Functional proteomics studies have identified B-Raf and Akt, both Hsp90 clients, as able to phosphorylate Hsp90. Old and colleagues identified Hsp90α Ser-263 as one of the targets of B-Raf in melanoma cells [72]. It would be valuable to know the impact of this phosphorylation on Hsp90 chaperone function and drug binding. A similar study recently reported that the serine-threonine protein kinase Akt phosphorylates several chaperone proteins, including Hsp90α, Hsp90β, Glucose-regulated protein (Grp) 78, Grp94, Hsp70, and protein disulfide isomerase (PDI), in rat mesangial cells [103]. Mesangial cells contribute to glomerular injury through their ability to undergo proliferation and hypertrophy. Akt plays a major role in these processes and it remains to be seen whether Akt-mediated chaperone phosphorylation contributes to glomerular injury [103].
4.1.3. c-Src Kinase
While some studies suggest a negative impact of de-regulated Hsp90 phosphorylation on chaperone function, recent work has shown that phosphorylation of discrete residues on Hsp90 may stimulate chaperone activity. Duval and co-workers have shown that c-Src kinase, another Hsp90 client, phosphorylates Hsp90β Tyr-301 in response to vascular endothelial growth factor receptor-2 (VEGFR-2) activation [70]. This leads to increased association between Hsp90β and eNOS, and results in increased eNOS activity, and nitric oxide (a vasodilator) production and release from endothelial cells. Hsp90β Tyr-301 is conserved in other Hsp90 proteins (it corresponds to Tyr-309 in Hsp90α). It will be interesting to determine whether this residue in Hsp90α is also a target for c-Src, and if so what are the functional consequences of its phosphorylation.
4.1.4. Protein Kinase A (PKA)
A systematic phospho-proteomics approach has identified Hsp90β Ser-452 to be phosphorylated by PKA in vitro [104]. Interesting work by Lei and colleagues has shown that diabetes mellitus or hyperglycemia can activate PKA phosphorylation of Hsp90α at Thr-90 in rat aortic endothelial cells [105]. This was associated with Hsp90α translocation to the cell surface [105]. Diabetes mellitus and hyperglycemia both reduce the bioavailability of nitric oxide by inhibiting eNOS activity. These investigators have proposed that PKA-mediated phosphorylation of Hsp90α reduces the amount of the chaperone that is available to associate with and activate eNOS, thus contributing to reduced eNOS activity and a decline in nitric oxide production in the aortic endothelium of diabetic rats [105]. The role of PKA in Hsp90α secretion was further explored by Wang and colleagues. They have confirmed that cellular secretion of Hsp90α is stimulated by PKA-mediated phosphorylation of Thr-90 [74]. Phosphorylation-dependent regulation of Hsp90 secretion in keratinocytes and cancer cells may also play an important role in wound healing and tumor metastasis, respectively [82,106,107].
4.1.5. CK2 protein kinase
CK2 is a ubiquitous serine-threonine, acidophilic kinase whose activity depends on Hsp90 chaperone function [52,108,109]. Lees-Miller and Anderson have shown that CK2 phosphorylates two serine residues, Ser-231 and Ser-263, in the charged-linker of Hsp90α [110]. Equivalent residues in Hsp90β (Ser-226 and Ser-255) are also phosphorylated in untransformed cells but not in leukemic cells [111]. The leukemogenic kinases, Bcr-Abl, FLT3/D835Y, and Tel-PDGFRβ, all suppress constitutive phosphorylation of Hsp90β at these sites, and this leads to inhibition of apoptosome function. This is achieved by stabilization of a strong interaction between Hsp90β and apoptotic peptidase activating factor 1 (Apaf-1), which prevents cytochrome c-induced Apaf-1 oligomerization and caspase-9 recruitment. Stabilization of the Hsp90β -apoptosome interaction by suppression of Ser-226 and Ser-255 phosphorylation may contribute to chemoresistance in leukemias [111].
It would be of interest to know the impact of these serine phosphorylations on Hsp90 secretion, since these serine residues are contained within the charged-linker region, a motif that has been previously implicated in modulating Hsp90 secretion [16]. Ck2 phosphorylation of the charged linker is also important for function of the aryl hydrocarbon receptor (AhR) [78]. This is a ligand-activated transcription factor that regulates genes involved in xenobiotic metabolism. The cytosolic AhR exist as a complex with Hsp90 and hepatitis B virus X-associated protein 2 (XAP2) [78]. Phosphorylation of Hsp90α Ser-231 and Hsp90β Ser-226 and Ser-255 caused dissociation of the AhR-Hsp90 complex and destabilization of AhR protein. Mutation of these residues to non-phosphorylatable alanine increased the transcriptional activity of AhR and stabilized its interaction with Hsp90 [78].
We have recently shown that CK2 also phosphorylates a conserved threonine residue (Thr-22) in the N-domain of yeast Hsp90 both in vitro and in vivo. Thr-22 is the only threonine residue in the N-domain targeted by CK2. This residue, together with adjacent amino acids, participates in an important hydrophobic interaction with the catalytic loop in the middle domain of Hsp90. We recently showed that ATP binding and not N-domain dimerization of Hsp90 is a prerequisite for CK2-mediated phosphorylation of Thr-22 [75,76]. Mutation of this residue to a non-phosphorylatable alanine (T22A) did not affect Hsp90 ATPase activity, while the phospho-mimetic mutant (T22E) had only 40% of wild type ATPase activity. These mutants in yeast, and the equivalent mutations in human Hsp90α (T36A and T36E), affected Hsp90-dependent chaperoning of kinase (v-Src, Mpk1/Slt2, Raf-1, ErbB2 and CDK4) and non-kinase (heat shock factor 1, cystic fibrosis transmembrane conductance regulator protein, glucocorticoid receptor) clients [75,76]. In addition, Thr-22 phosphorylation status also contributes to Hsp90 inhibitor sensitivity [76]. Finally, we observed that mutation of this residue in both yeast and human Hsp90 significantly reduced interaction with the co-chaperone Aha1, and the chaperoning defects of Thr-22 Hsp90 mutants were corrected by over-expressing Aha1 [75]. Clearly, CK2 phosphorylation of serine and threonine residues in Hsp90 represents an important but complex regulatory component of chaperone function in eukaryotic cells.
4.1.6. Swe1Wee1 kinase
Swe1 (Saccharomyces Wee1)/Wee1 tyrosine kinase is an Hsp90 client that regulates the G2/M cell cycle transition by phosphorylating Cdc28Cdc2 [112,113,114]. Swe1 also directly phosphorylates a conserved tyrosine residue (Tyr-24 in Hsp82 and Tyr-38 in Hsp90α) in the N-domain of yeast and human Hsp90 both in vitro and in vivo [77]. In yeast cells, Swe1-mediated phosphorylation of Hsp90 occurs in S phase of the cell cycle and causes Hsp90 to translocate from nucleus to cytoplasm, where it is polyubiquitinated and degraded by proteasomes. This is the “switching off” mechanism for this population of Hsp90, since no tyrosine phosphatase capable of dephosphorylating Tyr-24/Tyr-38 could be identified. Tyrosine 24 is among a group of residues that have been shown to form an interacting cluster in ATP-bound Hsp90 [115]. The hydrophobic interactions established by this cluster are essential for N-domain dimerization and are necessary for active site formation and for ATPase activity [115,116]. Swe1Wee1 targets Tyr-24/Tyr-38 when Hsp90 is in an “open” (N-domain undimerized) conformation, since Tyr-24/Tyr-38 is not accessible when Hsp90 assumes the “closed” (N-domain dimerized) conformation [77]. Mutation of this residue to a non-phosphorylatable amino acid reduces Hsp90 binding to Aha1 and Sba1/p23 co-chaperones. Phospho-mimetic mutation positively affects the ability of Hsp90 to chaperone a selected group of client proteins, including the kinases ErbB2, Raf-1, and Cdk4, but negatively affects GA binding to Hsp90. Deletion of Swe1 in yeast, and Wee1 silencing or pharmacologic inhibition in prostate and cervical carcinoma cells, sensitizes these cells to Hsp90 inhibitors. This suggests a novel therapeutic strategy to increase the cellular potency of Hsp90 inhibitors [77].
4.2. Acetylation
Acetylation is a post-translational modification that adds acetyl groups to proteins, usually on lysine residues. Histones are a major acetylation target and, historically, acetylating enzymes were termed histone acetylases (HAT) and deacetylating enzymes were termed histone deacetylases (HDACS). However, these enzymes are now known to be capable of modifying numerous non-histone proteins including Hsp90. Yu and colleagues were first to report Hsp90 acetylation in response to HDAC inhibitors (HDACi). These investigators showed that the HDACi depsipeptide (Romidepsin) increased steady state acetylation of Hsp90 while simultaneously destabilizing Hsp90 interaction with several client proteins, including ErbB2, Raf-1, and mutant p53 [117]. Hsp90 acetylation was also correlated with decreased binding to ATP. Others have shown that additional HDACi also cause Hsp90 hyper-acetylation [118,119].
4.2.1. Acetylases and deacetylases targeting Hsp90
p300 and HDAC6 promote acetylation and deacetylation of Hsp90, respectively [120,121,122,123]. Acetylated Hsp90 levels are increased in HDAC6-deficient mouse embryonic fibroblasts and glucocorticoid receptor function in these cells is compromised [124]. Androgen receptor, an Hsp90 client, is also down-regulated upon HDAC6 inhibition [125]. Reduction in HDAC6 expression also promotes destabilization of another Hsp90 client protein, the hypoxia-inducible transcription factor HIF-lα [126]. HDAC6 and HDAC10 have been shown to regulate Hsp90 mediated VEGF receptor regulation [127]. Finally, recent work by de Zoeten et al. has shown that pharmacologic inhibition or genetic deletion of HDAC6 increased acetylation of Foxp3 and Hsp90 both in vitro and in vivo. This in turn increased the immune suppressive activity of Foxp3+ T-regulatory (Treg) cells [128]. Foxp3+Tregs are essential to immune homeostasis, and if diminished in number or function can cause autoimmunity and allograft rejection. Therefore, specific targeting of HDAC6, or its downstream target, HSP90, can promote Treg-dependent suppression of autoimmunity and transplant rejection [128].
Although the impact of HDAC6 on Hsp90 acetylation has been extensively studied, other HDACs also are able to deacetylate the chaperone. HDAC1 has been reported to deacetylate Hsp90 in the nucleus of human breast cancer cells [129], and both HDAC1 and HDAC10 inhibit the productive Hsp90 chaperoning of VEGF receptor proteins [127]. Thus, different HDACs may alter Hsp90 acetylation status in distinct cellular locations and thus likely for distinct purposes. It is not yet clear which HDACs affect the acetylation status of individual lysine residues discussed below, nor is it known whether any redundancy exists for deacetylation of particular sites.
4.2.2. Acetylation of specific lysine residues on Hsp90
Treating SkBr3 breast cancer cells with the pan HDACi trichostatin A (TSA) caused hyperacetylation of Hsp90α at lysine 294 [130]. Interestingly, K294 acetylation can be detected even in the absence of HDACi, suggesting that a pool of Hsp90 may be constitutively acetylated on this lysine residue [130]. K294Q or K294A Hsp90α mutants (acetylated lysine mimics) displayed reduced interaction with numerous client proteins (ErbB2, p60v-Src, mutant p53, androgen receptor, Raf-1, HIF1α) and failed to associate with the co-chaperones Aha1, CHIP and FKBP52. The non-acetylated mimic mutant K294R showed an equivalent or stronger interaction with these co-chaperone and client proteins compared to wild type Hsp90 [130].
Treating human embryonic kidney 293 cells (HEK293) with the pan-HDACi Panobinostat (LBH589) led to identification of 7 additional acetylated lysine residues in Hsp90 [131]. Mutation of these lysine residues to glutamine affected the binding of Hsp90α to several co-chaperones, including CHIP, Hsp70 and p23, inhibited ATP binding, and inhibited Hsp90 chaperoning of Raf-1 [131]. Taken together, these data suggest that acetylation of Hsp90 is a dynamic, tightly regulated process that impacts multiple aspects of Hsp90 function. In general, acetylation of Hsp90 results in a lost or weakened interaction with its client proteins, leading to their instability and degradation.
What are the functional consequences of Hsp90 acetylation in yeast? When expressed on a single copy plasmid under a glycerol-3-phosphate dehydrogenase (GPD1) promoter, human Hsp90α did not optimally support yeast growth and K294R was inviable. Hsp90α-K294Q, however, was functional in yeast [130]. Hsp90α-K294R remained inviable when expressed on a single copy plasmid under a HSC82 promoter, although the difference in chaperone activity of Hsp90α wild type and K294Q mutant was less marked. In contrast, yeast expressing R or Q mutations to the equivalent residue of yeast Hsp90 (K274) are both viable (manuscript in preparation). These results suggest that acetylation of this conserved lysine residue likely arose in metazoans as a unique post-translational modification not utilized by yeast Hsp90.
4.3. S-nitrosylation
S-nitrosylation, the covalent attachment of a nitrogen monoxide group to the thiol side chain of cysteine, is a reversible post-translational modification of Hsp90, mediated by nitric oxide (NO). Martinez-Ruiz and colleagues first reported that NO causes S-nitrosylation of human HSP90α in endothelial cells, and they showed that this modification, which inhibited chaperone activity, occurred in the C-domain of Hsp90α at Cys-597 [132]. Because endothelial nitric oxide synthase (eNOS) requires association with Hsp90 for its activity, these authors proposed that NO-dependent inactivation of Hsp90 in endothelial cells functions as a regulatory feedback mechanism to control production of NO. Cys-597 and surrounding amino acids have been suggested by Colombo and colleagues to represent a conformational switch region in the Hsp90 C-domain that propagates long-range communication of structural information to Hsp90'S N-domain [116]. Recently, Retzlaff and colleagues reported that S-nitrosylation of Cys-597 inhibits Hsp90 ATPase activity [133], confirming that environmentally induced structural cues can be propagated between spatially distant domains of Hsp90. It is intriguing to speculate that some of the antitumor activity of NO may reflect NO-mediated Hsp90 inhibition in cancer cells.
4.4. Oxidation and Ubiquitination
Oxidative stress has been shown to post-translationally modify Hsp90. For example, treating human breast cancer MDA-MB-231 cells with tubocapsenolide A (TA), a novel withanolide, leads to a transient increase in reactive oxygen species and a decrease in intracellular glutathione content [134]. Consequently, this causes a direct thiol oxidation of Hsp90. TA treatment is correlated with proteasome-dependent degradation of the Hsp90 clients Cdk4, cyclin D1, Raf-1, Akt, and mutant p53, suggesting that TA-induced thiol oxidation of Hsp90 inhibits its chaperone function [134]. Oxidative stress can also cause lipid peroxidation which leads to accumulation of thiol-reactive α,β-unsaturated aldehydes, including 4-hydroxy-2-nonenal (4-HNE) and 4-oxo-2-nonenal (4-ONE). 4-HNE targets Cys-572 of Hsp90 and inhibits its ability to chaperone clients [135].
Lysines are also post-translational modified by ubiquitination. The photodynamic signal transduction inhibitor hypericin was shown to increase ubiquitination of Hsp90 [136]. As a consequence, Hsp90 chaperone function was inhibited and the client proteins Raf-1, mutant p53, Cdk4, and Plk were dissociated from Hsp90 complexes and degraded via a proteasome-independent pathway [136]. The cancer chemotherapeutic drug Taxotere (docetaxel) inhibits VEGF-induced human umbilical vein endothelial cell (HUVEC) migration in vitro at concentrations substantially lower than required to cause cell cycle arrest or apoptosis. Taxotere also promotes the ubiquitination and subsequent proteasomal degradation of Hsp90 in HUVECs. Taxotere prevents VEGF-induced phosphorylation of focal adhesion kinase, Akt, and eNOS, all of which are Hsp90 clients [137]. This drug also blocks the VEGF-induced increase in eNOS activity, which depends on Hsp90 interaction. Thus, Hsp90 inhibition may contribute to the antiangiogenic activity of Taxotere [137]. Finally, as mentioned earlier in this review, Swe1 phosphorylation of Hsp90 in yeast signals Hsp90 polyubiquitination and subsequent degradation by cytoplasmic proteasomes. These data highlight the possibility of cross-talk between two distinct post-translational modifications of Hsp90 and their impact on Hsp90 function [77].
5. Concluding Remarks
Post-translational modifications of Hsp90 make complex contributions to the regulation of chaperone function since they influence multiple regulatory factors, including ATP binding/hydrolysis and co-chaperone binding. Nevertheless, several general conclusions can be made. (1) The extent of Hsp90 post-translational modifications is greater in metazoans compared to single cell eukaryotes, suggesting that they provide an additional layer of regulation to Hsp90 as its client repertoire increased during evolution. (2) Sites that undergo post-translational modification are scattered throughout all domains of Hsp90, including the charged linker. (3) Numerous Hsp90 phosphorylations are catalyzed by kinase clients of the chaperone, suggesting that client-mediated phosphorylation may regulate Hsp90 chaperone activity. This hypothesis is supported by the recent observation that substrate binding to Hsp90 can drive conformational changes in the chaperone [138]. Current data certainly suggest that some post-translational modifications mediated by Hsp90 clients themselves (e.g., phosphorylation and S-nitrosylation) may provide a feedback mechanism to regulate client (and Hsp90) activity.
Many questions concerning post-translational modification of Hsp90 remain unanswered. (1) The kinases, phosphatases, HATs, and HDACs responsible for regulating post-translational modification of many sites on Hsp90 remain to be identified. (2) Development of phosphorylation or acetylation site-specific antibodies to Hsp90 lags far behind the identification of modified sites but will be absolutely necessary to study the impact of individual Hsp90 post-translational modifications on subcellular localization and tissue specificity. (3) Understanding the impact of post-translational modification of Hsp90 (or co-chaperones) on sensitivity to Hsp90 inhibitors is likely to provide novel strategies to improve drug sensitivity, and also to suggest possible mechanisms of acquired drug resistance. (4) Finally, further explication of the cross-talk between various Hsp90 post-translational modifications will greatly add to our knowledge of the multilayered control mechanisms higher eukaryotes have adopted to fine-tune Hsp90 function in a client-, cell-, and tissue-specific manner.
Research Highlights.
Heat shock protein 90 (Hsp90) is evolutionarily conserved
Hsp90 activates and maintains numerous signaling networks in normal & cancer cells
Hsp90 chaperone function is regulated by post-translational modifications of Hsp90 and various co-chaperones
Hsp90 post-translational modifications include phosphorylation, acetylation, S-nitrosylation, oxidation, and ubiquitination
Acknowledgments
We are grateful to our colleagues Shinji Tsutsumi, Wanping Xu, Kristin Beebe, Bradley Scroggins, and our collaborators Jane Trepel, Laurence H. Pearl, Peter W. Piper, Chris Prodromou, Johannes Buchner, Matthias Mayer, Brian Blagg, William G. Stetler-Stevenson, Giorgio Colombo, Barry Panaretou, Dimitra Bourboulia, Min-Jung Lee, Giulia Morra, Sunmin Lee, Alison Donnelly, Cara Vaughan, and Andrew Truman for their scientific contributions and stimulating discussions. Our research in this field benefits from the support of the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin J. Chaperonin function--effects of crowding and confinement. J Mol Recognit. 2004;17:465–472. doi: 10.1002/jmr.707. [DOI] [PubMed] [Google Scholar]
- 2.Kampinga HH. Chaperones in preventing protein denaturation in living cells and protecting against cellular stress. Handb Exp Pharmacol. 2006:1–42. doi: 10.1007/3-540-29717-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 4.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 6.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 8.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Alarcon SV, Lee S, Lee MJ, Giaccone G, Neckers L, Trepel JB. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9:1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Large AT, Goldberg MD, Lund PA. Chaperones and protein folding in the archaea. Biochem Soc Trans. 2009;37:46–51. doi: 10.1042/BST0370046. [DOI] [PubMed] [Google Scholar]
- 11.Grad I, Cederroth CR, Walicki J, Grey C, Barluenga S, Winssinger N, De Massy B, Nef S, Picard D. The molecular chaperone Hsp90alpha is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One. 2010;5:e15770. doi: 10.1371/journal.pone.0015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 13.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3:301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- 15.Ratzke C, Mickler M, Hellenkamp B, Buchner J, Hugel T. Dynamics of heat shock protein 90 C-terminal dimerization is an important part of its conformational cycle. Proc Natl Acad Sci U S A. 2010;107:16101–16106. doi: 10.1073/pnas.1000916107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsutsumi S, Mollapour M, Graf C, Lee CT, Scroggins BT, Xu W, Haslerova L, Hessling M, Konstantinova AA, Trepel JB, Panaretou B, Buchner J, Mayer MP, Prodromou C, Neckers L. Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat Struct Mol Biol. 2009;16:1141–1147. doi: 10.1038/nsmb.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hainzl O, Lapina MC, Buchner J, Richter K. The charged linker region is an important regulator of Hsp90 function. J Biol Chem. 2009;284:22559–22567. doi: 10.1074/jbc.M109.031658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan CK, Gohlke U, Sobott F, Good VM, Ali MM, Prodromou C, Robinson CV, Saibil HR, Pearl LH. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 2006;23:697–707. doi: 10.1016/j.molcel.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32:631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiau AK, Harris SF, Southworth DR, Agard DA. Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127:329–340. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin SH, Ventouras LA, Lobbezoo B, Jackson SE. Independent ATPase activity of Hsp90 subunits creates a flexible assembly platform. J Mol Biol. 2004;344:813–826. doi: 10.1016/j.jmb.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 22.Meyer P, Prodromou C, Liao C, Hu B, Roe SM, Vaughan CK, Vlasic I, Panaretou B, Piper PW, Pearl LH. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 2004;23:1402–1410. doi: 10.1038/sj.emboj.7600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krukenberg KA, Bottcher UM, Southworth DR, Agard DA. Grp94, the endoplasmic reticulum Hsp90, has a similar solution conformation to cytosolic Hsp90 in the absence of nucleotide. Protein Sci. 2009;18:1815–1827. doi: 10.1002/pro.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mickler M, Hessling M, Ratzke C, Buchner J, Hugel T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat Struct Mol Biol. 2009;16:281–286. doi: 10.1038/nsmb.1557. [DOI] [PubMed] [Google Scholar]
- 25.Hessling M, Richter K, Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol. 2009;16:287–293. doi: 10.1038/nsmb.1565. [DOI] [PubMed] [Google Scholar]
- 26.Neckers L. Using natural product inhibitors to validate Hsp90 as a molecular target in cancer. Curr Top Med Chem. 2006;6:1163–1171. doi: 10.2174/156802606777811979. [DOI] [PubMed] [Google Scholar]
- 27.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 28.Caplan AJ. What is a co-chaperone? Cell Stress Chaperones. 2003;8:105–107. doi: 10.1379/1466-1268(2003)008<0105:wiac>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbas-Terki T, Briand PA, Donze O, Picard D. The Hsp90 co-chaperones Cdc37 and Sti1 interact physically and genetically. Biol Chem. 2002;383:1335–1342. doi: 10.1515/BC.2002.152. [DOI] [PubMed] [Google Scholar]
- 30.Chang HC, Nathan DF, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter K, Muschler P, Hainzl O, Reinstein J, Buchner J. Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the atpase cycle. J Biol Chem. 2003;278:10328–10333. doi: 10.1074/jbc.M213094200. [DOI] [PubMed] [Google Scholar]
- 32.Song Y, Masison DC. Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing protein Sti1 (Hop1) J Biol Chem. 2005;280:34178–34185. doi: 10.1074/jbc.M505420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee P, Shabbir A, Cardozo C, Caplan AJ. Sti1 and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase. Mol Biol Cell. 2004;15:1785–1792. doi: 10.1091/mbc.E03-07-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLean M, Picard D. Cdc37 goes beyond Hsp90 and kinases. Cell Stress Chaperones. 2003;8:114–119. doi: 10.1379/1466-1268(2003)008<0114:cgbhak>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siligardi G, Panaretou B, Meyer P, Singh S, Woolfson DN, Piper PW, Pearl LH, Prodromou C. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J Biol Chem. 2002;277:20151–20159. doi: 10.1074/jbc.M201287200. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan CK, Mollapour M, Smith JR, Truman A, Hu B, Good VM, Panaretou B, Neckers L, Clarke PA, Workman P, Piper PW, Prodromou C, Pearl LH. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell. 2008;31:886–895. doi: 10.1016/j.molcel.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin SH, Sobott F, Yao ZP, Zhang W, Nielsen PR, Grossmann JG, Laue ED, Robinson CV, Jackson SE. The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J Mol Biol. 2006;356:746–758. doi: 10.1016/j.jmb.2005.11.085. [DOI] [PubMed] [Google Scholar]
- 38.Picard D. Intracellular dynamics of the Hsp90 co-chaperone p23 is dictated by Hsp90. Exp Cell Res. 2006;312:198–204. doi: 10.1016/j.yexcr.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan WP, Owen BA, Toft DO. The influence of ATP and p23 on the conformation of hsp90. J Biol Chem. 2002;277:45942–45948. doi: 10.1074/jbc.M207754200. [DOI] [PubMed] [Google Scholar]
- 40.Lotz GP, Lin H, Harst A, Obermann WM. Aha1 binds to the middle domain of Hsp90, contributes to client protein activation, and stimulates the ATPase activity of the molecular chaperone. J Biol Chem. 2003;278:17228–17235. doi: 10.1074/jbc.M212761200. [DOI] [PubMed] [Google Scholar]
- 41.Meyer P, Prodromou C, Liao C, Hu B, Mark Roe S, Vaughan CK, Vlasic I, Panaretou B, Piper PW, Pearl LH. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 2004;23:511–519. doi: 10.1038/sj.emboj.7600060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, Millson SH, Clarke PA, Naaby-Hansen S, Stein R, Cramer R, Mollapour M, Workman P, Piper PW, Pearl LH, Prodromou C. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol Cell. 2002;10:1307–1318. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 43.Johnson JL, Halas A, Flom G. Nucleotide-dependent interaction of Saccharomyces cerevisiae Hsp90 with the cochaperone proteins Sti1, Cpr6, and Sba1. Mol Cell Biol. 2007;27:768–776. doi: 10.1128/MCB.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayr C, Richter K, Lilie H, Buchner J. Cpr6 and Cpr7, two closely related Hsp90-associated immunophilins from Saccharomyces cerevisiae, differ in their functional properties. J Biol Chem. 2000;275:34140–34146. doi: 10.1074/jbc.M005251200. [DOI] [PubMed] [Google Scholar]
- 45.Catlett MG, Kaplan KB. Sgt1p is a unique co-chaperone that acts as a client adaptor to link Hsp90 to Skp1p. J Biol Chem. 2006;281:33739–33748. doi: 10.1074/jbc.M603847200. [DOI] [PubMed] [Google Scholar]
- 46.Caplan AJ, Mandal AK, Theodoraki MA. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 2007;17:87–92. doi: 10.1016/j.tcb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Boter M, Li K, Kadota Y, Panaretou B, Prodromou C, Shirasu K, Pearl LH. Structural and functional coupling of Hsp90- and Sgt1-centred multi-protein complexes. EMBO J. 2008;27:2789–2798. doi: 10.1038/emboj.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millson SH, Nuttall JM, Mollapour M, Piper PW. The Hsp90/Cdc37p chaperone system is a determinant of molybdate resistance in Saccharomyces cerevisiae. Yeast. 2009 doi: 10.1002/yea.1670. [DOI] [PubMed] [Google Scholar]
- 49.Vaughan CK, Piper PW, Pearl LH, Prodromou C. A common conformationally coupled ATPase mechanism for yeast and human cytoplasmic HSP90s. FEBS J. 2009;276:199–209. doi: 10.1111/j.1742-4658.2008.06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forafonov F, Toogun OA, Grad I, Suslova E, Freeman BC, Picard D. p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol Cell Biol. 2008;28:3446–3456. doi: 10.1128/MCB.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson JL, Brown C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperones. 2009;14:83–94. doi: 10.1007/s12192-008-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyata Y. Protein kinase CK2 in health and disease: CK2: the kinase controlling the Hsp90 chaperone machinery. Cell Mol Life Sci. 2009;66:1840–1849. doi: 10.1007/s00018-009-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao J, Prince T, Hartson SD, Matts RL. Phosphorylation of serine 13 is required for the proper function of the Hsp90 co-chaperone, Cdc37. J Biol Chem. 2003;278:38117–38120. doi: 10.1074/jbc.C300330200. [DOI] [PubMed] [Google Scholar]
- 54.Bandhakavi S, McCann RO, Hanna DE, Glover CV. A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J Biol Chem. 2003;278:2829–2836. doi: 10.1074/jbc.M206662200. [DOI] [PubMed] [Google Scholar]
- 55.Bansal PK, Mishra A, High AA, Abdulle R, Kitagawa K. Sgt1 dimerization is negatively regulated by protein kinase CK2-mediated phosphorylation at Ser361. J Biol Chem. 2009;284:18692–18698. doi: 10.1074/jbc.M109.012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi T, Nakatani Y, Tanioka T, Tsujimoto M, Nakajo S, Nakaya K, Murakami M, Kudo I. Regulation of cytosolic prostaglandin E synthase by phosphorylation. Biochem J. 2004;381:59–69. doi: 10.1042/BJ20040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lassle M, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, murine protein mSTI1. Characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876. [DOI] [PubMed] [Google Scholar]
- 58.Longshaw VM, Dirr HW, Blatch GL, Lassle M. The in vitro phosphorylation of the co-chaperone mSTI1 by cell cycle kinases substantiates a predicted casein kinase II-p34cdc2-NLS (CcN) motif. Biol Chem. 2000;381:1133–1138. doi: 10.1515/BC.2000.139. [DOI] [PubMed] [Google Scholar]
- 59.Miyata Y, Chambraud B, Radanyi C, Leclerc J, Lebeau MC, Renoir JM, Shirai R, Catelli MG, Yahara I, Baulieu EE. Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: regulation of HSP90-binding activity of FKBP52. Proc Natl Acad Sci U S A. 1997;94:14500–14505. doi: 10.1073/pnas.94.26.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong L, Qing K, Si Y, Chen L, Tan M, Srivastava A. Heat-shock treatment-mediated increase in transduction by recombinant adeno-associated virus 2 vectors is independent of the cellular heat-shock protein 90. J Biol Chem. 2004;279:12714–12723. doi: 10.1074/jbc.M310548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao W, Zhong L, Wu J, Chen L, Qing K, Weigel-Kelley KA, Larsen SH, Shou W, Warrington KH, Jr, Srivastava A. Role of cellular FKBP52 protein in intracellular trafficking of recombinant adeno-associated virus 2 vectors. Virology. 2006;353:283–293. doi: 10.1016/j.virol.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scroggins BT, Neckers L. Post-translational modification of heat shock protein 90: impact on chaperone function. Expert Opin Drug Discov. 2007;2:1403–1414. doi: 10.1517/17460441.2.10.1403. [DOI] [PubMed] [Google Scholar]
- 64.Mollapour M, Tsutsumi S, Neckers L. Hsp90 phosphorylation, Wee1 and the cell cycle. Cell Cycle. 2010;9:2310–2316. doi: 10.4161/cc.9.12.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Legagneux V, Morange M, Bensaude O. Heat shock increases turnover of 90 kDa heat shock protein phosphate groups in HeLa cells. FEBS Lett. 1991;291:359–362. doi: 10.1016/0014-5793(91)81320-8. [DOI] [PubMed] [Google Scholar]
- 66.Mimnaugh EG, Worland PJ, Whitesell L, Neckers LM. Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J Biol Chem. 1995;270:28654–28659. doi: 10.1074/jbc.270.48.28654. [DOI] [PubMed] [Google Scholar]
- 67.Garnier C, Lafitte D, Jorgensen TJ, Jensen ON, Briand C, Peyrot V. Phosphorylation and oligomerization states of native pig brain HSP90 studied by mass spectrometry. Eur J Biochem. 2001;268:2402–2407. doi: 10.1046/j.1432-1327.2001.02121.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhao YG, Gilmore R, Leone G, Coffey MC, Weber B, Lee PW. Hsp90 phosphorylation is linked to its chaperoning function. Assembly of the reovirus cell attachment protein. J Biol Chem. 2001;276:32822–32827. doi: 10.1074/jbc.M105562200. [DOI] [PubMed] [Google Scholar]
- 69.Adinolfi E, Kim M, Young MT, Di Virgilio F, Surprenant A. Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem. 2003;278:37344–37351. doi: 10.1074/jbc.M301508200. [DOI] [PubMed] [Google Scholar]
- 70.Duval M, Le Boeuf F, Huot J, Gratton JP. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol Biol Cell. 2007;18:4659–4668. doi: 10.1091/mbc.E07-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyata Y, Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992;267:7042–7047. [PubMed] [Google Scholar]
- 72.Old WM, Shabb JB, Houel S, Wang H, Couts KL, Yen CY, Litman ES, Croy CH, Meyer-Arendt K, Miranda JG, Brown RA, Witze ES, Schweppe RE, Resing KA, Ahn NG. Functional proteomics identifies targets of phosphorylation by B-Raf signaling in melanoma. Mol Cell. 2009;34:115–131. doi: 10.1016/j.molcel.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rose DW, Wettenhall RE, Kudlicki W, Kramer G, Hardesty B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry. 1987;26:6583–6587. doi: 10.1021/bi00395a003. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo Y. The regulatory mechanism of Hsp90{alpha} secretion and its function in tumor malignancy. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mollapour M, Tsutsumi S, Truman AW, Xu W, Vaughan CK, Beebe K, Konstantinova A, Vourganti S, Panaretou B, Piper PW, Trepel JB, Prodromou C, Pearl LH, Neckers L. Threonine 22 phosphorylation attenuates hsp90 interaction with cochaperones and affects its chaperone activity. Mol Cell. 2011;41:672–681. doi: 10.1016/j.molcel.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mollapour M, Tsutsumi S, Kim YS, Trepel J, Neckers L. Casein kinase 2 phosphorylation of Hsp90 threonine 22 modulates chaperone function and drug sensitivity. Oncotarget. 2011 doi: 10.18632/oncotarget.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mollapour M, Tsutsumi S, Donnelly AC, Beebe K, Tokita MJ, Lee MJ, Lee S, Morra G, Bourboulia D, Scroggins BT, Colombo G, Blagg BS, Panaretou B, Stetler-Stevenson WG, Trepel JB, Piper PW, Prodromou C, Pearl LH, Neckers L. Swe1Wee1-dependent tyrosine phosphorylation of Hsp90 regulates distinct facets of chaperone function. Mol Cell. 2010;37:333–343. doi: 10.1016/j.molcel.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogiso H, Kagi N, Matsumoto E, Nishimoto M, Arai R, Shirouzu M, Mimura J, Fujii-Kuriyama Y, Yokoyama S. Phosphorylation analysis of 90 kDa heat shock protein within the cytosolic arylhydrocarbon receptor complex. Biochemistry. 2004;43:15510–15519. doi: 10.1021/bi048736m. [DOI] [PubMed] [Google Scholar]
- 79.Wandinger SK, Suhre MH, Wegele H, Buchner J. The phosphatase Ppt1 is a dedicated regulator of the molecular chaperone Hsp90. EMBO J. 2006;25:367–376. doi: 10.1038/sj.emboj.7600930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 81.Bennetzen MV, Larsen DH, Bunkenborg J, Bartek J, Lukas J, Andersen JS. Site-specific phosphorylation dynamics of the nuclear proteome during the DNA damage response. Mol Cell Proteomics. 2010;9:1314–1323. doi: 10.1074/mcp.M900616-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci U S A. 2008;105:13793–13798. doi: 10.1073/pnas.0806216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choudhary C, Olsen JV, Brandts C, Cox J, Reddy PN, Bohmer FD, Gerke V, Schmidt-Arras DE, Berdel WE, Muller-Tidow C, Mann M, Serve H. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell. 2009;36:326–339. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 84.Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, Possemato A, Nardone J, Innocenti G, Wetzel R, Wang Y, MacNeill J, Mitchell J, Gygi SP, Rush J, Polakiewicz RD, Comb MJ. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 86.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J, Kornhauser J, Sprott K, Zhou J, Possemato A, Ren JM, Hornbeck P, Cantley LC, Gygi SP, Rush J, Comb MJ. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3:ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 89.Wisniewski JR, Nagaraj N, Zougman A, Gnad F, Mann M. Brain phosphoproteome obtained by a FASP-based method reveals plasma membrane protein topology. J Proteome Res. 2010;9:3280–3289. doi: 10.1021/pr1002214. [DOI] [PubMed] [Google Scholar]
- 90.Han G, Ye M, Liu H, Song C, Sun D, Wu Y, Jiang X, Chen R, Wang C, Wang L, Zou H. Phosphoproteome analysis of human liver tissue by long-gradient nanoflow LC coupled with multiple stage MS analysis. Electrophoresis. 2010;31:1080–1089. doi: 10.1002/elps.200900493. [DOI] [PubMed] [Google Scholar]
- 91.Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci U S A. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffert JD, Wang G, Pisitkun T, Shen RF, Knepper MA. An automated platform for analysis of phosphoproteomic datasets: application to kidney collecting duct phosphoproteins. J Proteome Res. 2007;6:3501–3508. doi: 10.1021/PR0701153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xia Q, Cheng D, Duong DM, Gearing M, Lah JJ, Levey AI, Peng J. Phosphoproteomic analysis of human brain by calcium phosphate precipitation and mass spectrometry. J Proteome Res. 2008;7:2845–2851. doi: 10.1021/pr8000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mayya V, Lundgren DH, Hwang SI, Rezaul K, Wu L, Eng JK, Rodionov V, Han DK. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal. 2009;2:ra46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- 96.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 97.Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res. 2008;7:311–318. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
- 98.Jorgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, Larsen B, Wilkinson DG, Linding R, Pawson T. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [DOI] [PubMed] [Google Scholar]
- 99.Bonnette PC, Robinson BS, Silva JC, Stokes MP, Brosius AD, Baumann A, Buckbinder L. Phosphoproteomic characterization of PYK2 signaling pathways involved in osteogenesis. J Proteomics. 2010;73:1306–1320. doi: 10.1016/j.jprot.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Albuquerque CP, Smolka MB, Payne SH, Bafna V, Eng J, Zhou H. A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol Cell Proteomics. 2008;7:1389–1396. doi: 10.1074/mcp.M700468-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molina H, Horn DM, Tang N, Mathivanan S, Pandey A. Global proteomic profiling of phosphopeptides using electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci U S A. 2007;104:2199–2204. doi: 10.1073/pnas.0611217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lees-Miller SP, Anderson CW. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem. 1989;264:17275–17280. [PubMed] [Google Scholar]
- 103.Barati MT, Rane MJ, Klein JB, McLeish KR. A proteomic screen identified stress-induced chaperone proteins as targets of Akt phosphorylation in mesangial cells. J Proteome Res. 2006;5:1636–1646. doi: 10.1021/pr0502469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang SY, Tsai ML, Chen GY, Wu CJ, Chen SH. A systematic MS-based approach for identifying in vitro substrates of PKA and PKG in rat uteri. J Proteome Res. 2007;6:2674–2684. doi: 10.1021/pr070134c. [DOI] [PubMed] [Google Scholar]
- 105.Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90 alpha impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007;282:9364–9371. doi: 10.1074/jbc.M608985200. [DOI] [PubMed] [Google Scholar]
- 106.Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, Han YP, Woodley DT, Li W. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol. 2008;28:3344–3358. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsutsumi S, Scroggins B, Koga F, Lee MJ, Trepel J, Felts S, Carreras C, Neckers L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2008;27:2478–2487. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruzzene M, Pinna LA. Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta. 2010;1804:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 109.Dougherty JJ, Rabideau DA, Iannotti AM, Sullivan WP, Toft DO. Identification of the 90 kDa substrate of rat liver type II casein kinase with the heat shock protein which binds steroid receptors. Biochim Biophys Acta. 1987;927:74–80. doi: 10.1016/0167-4889(87)90067-x. [DOI] [PubMed] [Google Scholar]
- 110.Lees-Miller SP, Anderson CW. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem. 1989;264:2431–2437. [PubMed] [Google Scholar]
- 111.Kurokawa M, Zhao C, Reya T, Kornbluth S. Inhibition of apoptosome formation by suppression of Hsp90beta phosphorylation in tyrosine kinase-induced leukemias. Mol Cell Biol. 2008;28:5494–5506. doi: 10.1128/MCB.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harvey SL, Kellogg DR. Conservation of mechanisms controlling entry into mitosis: budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr Biol. 2003;13:264–275. doi: 10.1016/s0960-9822(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 113.Lew DJ. The morphogenesis checkpoint: how yeast cells watch their figures. Curr Opin Cell Biol. 2003;15:648–653. doi: 10.1016/j.ceb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Booher RN, Deshaies RJ, Kirschner MW. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cunningham CN, Krukenberg KA, Agard DA. Intra- and intermonomer interactions are required to synergistically facilitate ATP hydrolysis in Hsp90. J Biol Chem. 2008;283:21170–21178. doi: 10.1074/jbc.M800046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morra G, Verkhivker G, Colombo G. Modeling signal propagation mechanisms and ligand-based conformational dynamics of the Hsp90 molecular chaperone full-length dimer. PLoS Comput Biol. 2009;5:e1000323. doi: 10.1371/journal.pcbi.1000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 118.Nimmanapalli R, Fuino L, Bali P, Gasparetto M, Glozak M, Tao J, Moscinski L, Smith C, Wu J, Jove R, Atadja P, Bhalla K. Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res. 2003;63:5126–5135. [PubMed] [Google Scholar]
- 119.Nimmanapalli R, Fuino L, Stobaugh C, Richon V, Bhalla K. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Blood. 2003;101:3236–3239. doi: 10.1182/blood-2002-08-2675. [DOI] [PubMed] [Google Scholar]
- 120.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 121.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 122.Kekatpure VD, Dannenberg AJ, Subbaramaiah K. HDAC6 modulates Hsp90 chaperone activity and regulates activation of aryl hydrocarbon receptor signaling. J Biol Chem. 2009;284:7436–7445. doi: 10.1074/jbc.M808999200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Murphy PJ, Morishima Y, Kovacs JJ, Yao TP, Pratt WB. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J Biol Chem. 2005;280:33792–33799. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, Matthias P. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ai J, Wang Y, Dar JA, Liu J, Liu L, Nelson JB, Wang Z. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol Endocrinol. 2009;23:1963–1972. doi: 10.1210/me.2009-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang D, Li J, Costa M, Gao J, Huang C. JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 2010;70:813–823. doi: 10.1158/0008-5472.CAN-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park JH, Kim SH, Choi MC, Lee J, Oh DY, Im SA, Bang YJ, Kim TY. Class II histone deacetylases play pivotal roles in heat shock protein 90-mediated proteasomal degradation of vascular endothelial growth factor receptors. Biochem Biophys Res Commun. 2008;368:318–322. doi: 10.1016/j.bbrc.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 128.de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, Hancock WW. Histone deacetylase 6 and heat shock protein 90 control the functions of foxp3+ T-regulatory cells. Mol Cell Biol. 2011;31:2066–2078. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou Q, Agoston AT, Atadja P, Nelson WG, Davidson NE. Inhibition of histone deacetylases promotes ubiquitin-dependent proteasomal degradation of DNA methyltransferase 1 in human breast cancer cells. Mol Cancer Res. 2008;6:873–883. doi: 10.1158/1541-7786.MCR-07-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Y, Rao R, Shen J, Tang Y, Fiskus W, Nechtman J, Atadja P, Bhalla K. Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res. 2008;68:4833–4842. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martinez-Ruiz A, Villanueva L, Gonzalez de Orduna C, Lopez-Ferrer D, Higueras MA, Tarin C, Rodriguez-Crespo I, Vazquez J, Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Retzlaff M, Stahl M, Eberl HC, Lagleder S, Beck J, Kessler H, Buchner J. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep. 2009;10:1147–1153. doi: 10.1038/embor.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen WY, Chang FR, Huang ZY, Chen JH, Wu YC, Wu CC. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J Biol Chem. 2008;283:17184–17193. doi: 10.1074/jbc.M709447200. [DOI] [PubMed] [Google Scholar]
- 135.Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 136.Blank M, Mandel M, Keisari Y, Meruelo D, Lavie G. Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism for mitotic cell death in cancer cells induced with hypericin. Cancer Res. 2003;63:8241–8247. [PubMed] [Google Scholar]
- 137.Murtagh J, Lu H, Schwartz EL. Taxotere-induced inhibition of human endothelial cell migration is a result of heat shock protein 90 degradation. Cancer Res. 2006;66:8192–8199. doi: 10.1158/0008-5472.CAN-06-0748. [DOI] [PubMed] [Google Scholar]
- 138.Street TO, Lavery LA, Agard DA. Substrate binding drives large-scale conformational changes in the hsp90 molecular chaperone. Mol Cell. 2011;42:96–105. doi: 10.1016/j.molcel.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


