Abstract
Aged (20–22 months) male Fischer 344 rats were randomly assigned to sedentary (A-SED), environmentally enriched (A-ENR) or exercise (A-EX) conditions. After 10–12 weeks of differential experience, the three groups of aged rats and young sedentary controls were tested for physical and cognitive function. Spatial discrimination learning and memory consolidation, tested on the water maze, were enhanced in A-ENR compared to A-SED. A-EX exhibited improved and impaired performance on the cue and spatial task, respectively. Impaired spatial learning in A-EX was likely due to a bias in response selection associated with exercise training, as object recognition memory improved for A-EX rats. An examination of senescent hippocampal physiology revealed that enrichment and exercise reversed age-related changes in long-term depression (LTD) and long-term potentiation (LTP). Rats in the enrichment group exhibited an increase in cell excitability compared to the other two groups of aged animals. The results indicate that differential experience biased the selection of a spatial or a response strategy and factors common across the two conditions, such as increased hippocampal activity associated with locomotion, contribute to reversal of senescent synaptic plasticity.
Keywords: Aging, enrichment, exercise, hippocampus, learning and memory, synaptic plasticity, after hyperpolarization, cell excitability
1. Introduction
It is clear that initiation of treatments to increase cognitive stimulation, including environmental enrichment, can increase memory function in aging humans and animal models of normal aging and neurodegenerative disease (Frick and Fernandez, 2003; Hall, et al., 2009; Harburger, et al., 2007; Harburger, et al., 2007; Leal-Galicia, et al., 2008; Lores-Arnaiz, et al., 2006; Soffie, et al., 1999; Winocur, 1998). Exercise has also been proposed as a means for delaying age-related cognitive decline; however, the effect of exercise on cognitive decline during normal aging is still debated. A number of studies indicate no effect of exercise in humans (van Uffelen, et al., 2008) and some rodent models (Asl, et al., 2008; Barnes, et al., 1991; Hansalik, et al., 2006).
Similar cellular and molecular mechanisms have been proposed for environmental enrichment and exercise effects on hippocampal function, including increased neurogenesis, and interactions of synaptic signaling and genomic regulation (During and Cao, 2006; Mohammed, et al., 1993; Mora, et al., 2007; Nguyen and Woo, 2003; Olson, et al., 2006; Sweatt, 2009). Much of the work has focused on the dentate gyrus. In contrast, there are notable differences in the effects of these treatments on synaptic plasticity in region CA1 such that environmental enrichment, but not exercise, enhances long-term potentiation (LTP) (Artola, et al., 2006; Lange-Asschenfeldt, et al., 2007; van Praag, et al., 1999; Yang, et al., 2006; Yang, et al., 2007). The results of the current study demonstrate that differential experience influences animal’s selection of a spatial or taxon strategy. In contrast, environmental enrichment and exercise had similar effects on synaptic plasticity, promoting the induction of LTP and reducing the propensity for long-term depression (LTD). The results indicate that neural activity associated with exercise and exploring novel environments rejuvenates hippocampal neural plasticity processes. We suggest that physical activity may be useful as an adjunct to various cognitive training programs.
2. Materials and methods
2.1 Animals
Procedures involving animal subjects have been reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Male Fischer 344 rats, young (5–8 months) and aged (20–22 months) were obtained from National Institute on Aging colony at Harlan Sprague Dawley Inc through the University of Florida Animal Care and Service facility. Animals were maintained on a 12:12 hr light schedule, and provided ad lib access to water. During this period the daily food intake and weight was recorded for aged animals. Aged rats were randomly assigned to sedentary (A-SED), environmentally enriched (A-ENR) or exercise (A-EX) conditions, which were maintained for 10–12 weeks prior to behavioral testing.
2.2 Behavioral studies
Table 1 provides information on the sequence of behavioral tasks and the number of animals that were examined for each task. The total number of animals included young (n = 12), A-SED (n = 49), A-ENR (n = 22), and A-EX (n = 34). Following 10–12 weeks of differential experience a subset of animals was behaviorally characterized on the water maze (Table 1). Animals were first trained on the cue discrimination version of the water maze. Animals that did not find the platform during the final block of testing on the cue task were removed from the study (see below). This was followed three days later by spatial discrimination testing. Testing for grip strength, gridwalking, and object recognition was initiated at least one week following water maze training. A-SED (n = 10) and A-EX (n = 13) animals that had been differentially housed for 10–12 weeks, but had not been tested on the water maze, were added for testing on grip strength, gridwalking, and object recognition (Table 1). For examination of electrophysiological measures (one animal per day), differential housing conditions were maintained for at least 2–4 weeks following behavioral characterization in order to reduce the effects of behavioral testingon subsequent biological measures.
Table 1.
Table 1 illustrates the total number of animals employed (TOTAL) and the number in each task. Animals in the grip strength, gridwalk, and object recognition tasks included animals tested on the spatial task and additional A-SED (n = 10) and A-EX (n = 13) animals.
| Young | A-SED | A-ENR | A-EX | |
|---|---|---|---|---|
| TOTAL | 12 | 49 | 22 | 34 |
| Cue Discrimination | 12 | 39 | 22 | 21 |
| Removed | 0 | 8 | 3 | 3 |
| Spatial Acquisition | 12 | 31 | 19 | 18 |
| Spatial Retention | 11 | 18 | 12 | 4 |
| Grip strength | 6 | 29+10 | 16 | 18+13 |
| Gridwalk | 12 | 21 | ||
| Object Recognition | 6 | 14 | ||
Young (n = 12) and aged rats in the A-SED condition (n = 49) were individually housed with ad lib access to food and water. Food intake was recorded daily and body weight was recorded weekly. Food intake and changes in body weight of A-SED rats over the 12 weeks were used as a baseline for adjusting conditions of A-EX rats.
Animals in the A-EX group were assigned to one of two different exercise conditions: calorie restriction-exercise (n = 18) and conditioned (n = 16). Rats exhibit a decrease in their running activity during aging and a slightfood restriction (8–10%) preserves running behavior in aged rats (Cui, et al., 2009; Holloszy, et al., 1985). Calorie restriction-exercise rats were individually housed in cages equipped with activity wheels (1.068 m circumference, Fisher Scientific, Pittsburgh, PA). Each wheel was equipped with a magnetic switch and counter to record the number of wheel revolutions. Rats had free access to water and received food pellets equal to approximately 90% that of the ad libitum food intake of sedentary, age-matched rats. The number of wheel revolutions was recorded daily and body weight was recorded weekly. Throughout the duration of the study, food intake of restricted animals was adjusted each week based on food intake of the ad libitum group during the previous week. The expected ad libitum weight of calorie restriction-exercise rats was calculated according to the initial ad libitum weight and age-related weight changes in the A-SED group. Food intake was increased if the animal lost more than 10% of the adjusted body weight.
A second set of exercise rats was conditioned to use a running wheel to obtain food pellets. Conditioned-running wheel cages (model H10–38R, Coulbourn Instruments, Allentown, PA) were monitored by a computer program (Graphic State Notation Version 3.02, Coulbourn Instruments, Allentown, PA) to deliver food pellets (45 mg) via an attached feeder, following a specified number of wheel revolutions. Initially the criterion was set at one pellet / revolution and was increased as the animals learn to run on the wheel in order to obtain the food pellets. Wheel revolutions were recorded daily and body weight was recorded weekly. The criterio distance for a food pellet was reduced if the animal exhibited a decrease of more than 10% of the expected ad libitum weight. During the final eight weeks of training, most animals were running ~3–4 meters for each pellet.
In accord with our previous research, the goal of the enrichment procedure was to alter the environment in order to make available opportunities to perform the widest possible range of behaviors that depend on the hippocampus, and to limit these behaviors for animals in the control or sedentary condition (Foster and Dumas, 2001; Foster, et al., 1996; Gagne, et al., 1998; Kumar and Foster, 2007). Animals in the A-ENR group (n = 22) were pair housed. Each day, rats were placed in one of several novel environments (e.g. empty water maze, large wooden box or large wire cage) for approximately 2–3 hr. The environments contained an array of three dimensional objects, rat chow, and a water bottle. The environments and the objects in each environment were rotated each day.
2.3 Water Maze
Methods employed to assess sensory-motor and memory deficits on the water maze have been published previously (Carter, et al., 2009; Foster and Kumar, 2007; Foster, et al., 2003). Animals were trained in a black tank, 1.7 m in diameter, positioned in a well-lit room containing (when appropriate) an assortment of 2- and 3-dimensional cues. Water (27±2° C) was maintained at a level approximately 8 cm below the surface of the tank. Behavioral data was acquired with a tracking system and included cumulative path-length and latency to escape to the platform (12 cm diameter) during training trials.
Rats were first trained on the cue discrimination version of the water escape task. Animals were habituated to the pool by allowing 30 sec free swim and 4 trials to climb onto a platform from 4 different directions. A white Styrofoam flag was attached to the platform and the platform extended 1 cm above the water level. Training consisted of five blocks of three trials with all training massed into one day. Inter-trial intervals were 20 sec and inter-block intervals were approximately 15 minutes. On each trial, the rat was placed in the water in one of four equally spaced start locations (N, S, E, and W). Subjects were allowed 60 sec to escape during each trial; if they did not escape within the allotted time, rats were gently guided to the platform. Rats remained on the platform between trials. After each trial block rats were placed in home cages under warmed air with access to added warmth from an infrared heat lamp. Platform and start locations were randomized across each trial. Rats that failed to find the platform on two out of the last three trials were removed from the study.
Three days following cue training, animals were trained on the spatial discrimination task. For spatial discrimination, the escape platform was hidden approximately 1.5 cm beneath the water level and remained in the same location relative to the distal cues in the room for the duration of spatial training. Training procedures for spatial learning were similar to the cue discrimination task, consisting of five blocks of three trials with all training massed into a single day. A single training day was employed over multiple days of training in order to increase the difficulty of acquisition and enable the examination of memory consolidation over a 24 hr period. Probe trials delivered after training and 24 hr later, were employed to assess acquisition and memory consolidation, respectively (Blalock, et al., 2003; Carter, et al., 2009; Foster, et al., 1991; Foster and Dumas, 2001; Foster and Kumar, 2007). Inter-trial intervals were 20 sec and inter-block intervals were approximately 15 minutes. On each trial, the rat was placed in the water from one of four start locations. Subjects had 60 sec to escape during each trial; if they did not escape within the allotted time, they were gently guided to the platform. Rats remained on the platform between trials and in home cages under the heat lamp after each block. Start locations were randomized across each trial. Escape latency and escape path-length was measured. Fifteen min following the end of training on block five, a free-swim probe trial was administered in order to test learning. The probe trial was followed with a refresher training block to reinforce platform location. For some rats, retention for platform location was tested 24 hr later using a second free-swim probe trial. For probe trials, the platform was removed and the animal placed in the tank for one minute. Behavioral measures acquired during the 60 sec probe trial included the time spent searching the goal and opposite quadrant, and platform crossings. A spatial discrimination index was computed according to the formula (G − O)/(G + O) where G and O represent the percent of time spent in the goal quadrant and quadrant opposite the goal, respectively (Foster, et al., 2003).
2.4 Grip Strength
Grip strength was determined using an automated grip strength meter by sensing the peak amount of force an animal applies in grasping the pull bar assembly (Columbus Instruments, Columbus, OH) as previously described (Carter, et al., 2009; Cui, et al., 2009). During the procedure, the subject was hand-held by the experimenter. For each measurement, the subject’s forelimbs were gently placed on the bar, the animal grabs the bar (a reflex response in rodents) and was then drawn along a straight line leading away from the sensor. The animal released the pull bar at some point and the maximum force attained was stored on the digital display. The peak amount of the force an animal applies in grasping the pull bar was measured. The mean force (grams) was calculated over three trials and was divided by body weight.
2.5 Gridwalk
The gridwalk apparatus consists of a wire grid bridge (1.19 × 0.30 m) suspended above (0.25 m) the ground, with an escape box at one end. The distance between the steps ranges from 2.3 – 7.6 cm. Animals are placed next to the escape box and allowed entry. After 1 min in the escape box, they are transferred to the opposite end and allowed 3 min to cross. Time to cross and foot faults are recorded. A foot fault is recorded when the animals attempts to place the foot on the grid, misses, and the foot passes the plane of the grid.
2.6 Object Memory Task (OMT)
The OMT is also both sensitive to hippocampal function and affected by aging but is less dependent on physical strength and endurance (Blalock, et al., 2003; Markowska, et al., 1998). Animals were administered a habituation session (15 min) in an empty arena (0.91 × 0.61 m). Object recognition training began 24 hr after habituation and consisted of a 15 min acquisition session during which two three-dimensional objects were placed at opposite sides of the arena, followed by a retention test sessions 24 hr after training. During the acquisition session, the cage contained two sample objects (A and B), and the time spent actively exploring each object was recorded. On the 24 hr test, one familiar object was reintroduced and the other object was replaced by a novel object. The time spent exploring each item was used to calculate a memory discrimination index (DI) as follows: DI = (N - F)/T, where N is time spent exploring the novel object, F is time spent exploring the familiar object, and T is total time spent exploring the two objects. More time spent exploring the novel object (higher DI) is considered to reflect greater memory retention for the familiar object.
2.7 Hippocampal Slice Preparation
The methods for hippocampal slice preparation and recording have been published previously (Foster and Dumas, 2001; Kumar, et al., 2007; Norris, et al., 1996). Briefly, rats were anesthetized with isoflurane (Halocarbon Laboratories, River Edge, NJ) and swiftly decapitated. The brains were rapidly removed and the hippocampi were dissected. Hippocampal slices (~ 400 µm) were cut parallel to the alvear fibers using a tissue chopper. The slices were incubated in a holding chamber (room temperature) containing standard artificial cerebrospinal fluid (ACSF) (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and glucose 10. Thirty to sixty min before recording, 2–3 slices were transferred to a standard interface recording chamber (Harvard Apparatus, Boston, MA); the chamber was continuously perfused with standard oxygenated (95% O2, 5% CO2) ACSF at a flow rate of 2 ml/min. The pH and temperature were maintained at 7.4 and 30 ± 0.5°C, respectively. Humidified air (95% O2, 5% CO2) was continuously blown over the slices.
2.8 Extracellular Recordings
Extracellular synaptic field potentials from CA3-CA1 synaptic contacts were recorded with glass micropipettes (4–6 MΩ) filled with ACSF. Stimulating electrodes (stainless steel, tip diameter ~0.13 mm) were positioned on either side (approximately 1 mm) of a recording electrode localized to the middle of stratum radiatum and single diphasic stimulus pulses were alternated between pathways such that each pathway was activated at 0.033 Hz. One stimulating electrode activated a control pathway used to insure that the effects of pattern stimulation were specific to activated synapses and not due to a change in slice health. Stimulation intensity was set to elicit a response that was 50% of maximum excitatory postsynaptic potential (EPSP) response and a stable baseline synaptic response was recorded for at least 10 min before pattern stimulation using the same stimulation intensity. Following pattern stimulation, the response was recorded for 35 min. Threshold long-term depression (LTD) was induced by low frequency stimulation (LFS, 1 Hz, 900 pulses). Theta burst stimulation (TBS, two sets of five bursts of 4 pulses at 100 Hz with 200 msec intervals between bursts and the two sets separated by a 10 sec interval) was used for induction of threshold long-term potentiation (LTP). The signals were amplified, filtered between 1 Hz and 1 kHz and stored on computer disk for off-line analysis (Data Wave Technologies, Longmont, CO). Two cursors were placed around the initial descending phase of the waveform and the maximum slope (mV/ms) of the EPSP was determined by a computer algorithm that found the maximum change across all sets of consecutively recorded points (20 kHz sampling rate) between the two cursors. For statistical analysis, the average response during the last 5 min of recording of the recording session was used to calculate the percent change relative to the averaged baseline response collected 10 min prior to pattern stimulation.
2.9 Intracellular Recordings
Microelectrodes were pulled from thin-wall 1.0 mm microfiber-filled borosilicate capillaries using a Flaming/Brown horizontal micropipette puller (Sutter Instruments, San Rafael, California). The resistance of microelectrodes when filled with 3 M potassium acetate ranged from 50 to 100 MΩ. Microelectrodes were visually positioned in the CA1 pyramidal cell layer using a dissecting microscope (SZH10, Optical Elements Corporation, Washington D.C). Intracellular sharp electrode recordings were obtained from CA1 hippocampal pyramidal neurons. The signals were amplified using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA), and recordings were sampled at 1 kHz, and stored on computer disk for off-line analysis (Data Wave Technologies, Longmont, CO). An acceptance criterion was established for cell health such that only neurons with resting membrane potential less than −60 mV measured in the absence of injected holding current, an input resistance >20 MΩ, and an action potential amplitude of 70 mV were included in the analysis. Bridge balance was obtained before the start of recording. Voltage deflections resulting from hyperpolarizing current pulses (100 ms, 0.2 nA) were used to determine input resistance and check bridge balance. For examination of the AHP, the membrane potential was maintained at ~ 64 mV with a constant current injection (0.48 ± 0.02nA, mean ± sem) to minimize the effects of voltage-dependent alterations in membrane conductance. Depolarizing current pulses (100 msec, 0.1–1.2 nA) were delivered every 20 sec through the intracellular electrode to elicit a sodium spike bursts of 4 action potentials. The AHP was measured as the difference between the holding potential during the 100 msec period immediately before the onset of the depolarizing current and the membrane potential 500 msec after the offset of the depolarizing current.
2.10 Western blot
Hippocampi were frozen in liquid nitrogen for storage at −80°C. Tissue was homogenized in RIPA buffer (Thermo Scientific, Rockford, IL) containing protease inhibitors (Thermo Scientific) and centrifuged at 20000 × g for 30 min at 4°C. The supernatant was collected and protein concentrations were determined using a Pierce BCA protein assay (Thermo Scientific). Lysates were denatured in Laemmli sample buffer with 2-mercaptoethanol (Bio-Rad, Hercules, CA) and boiled at 100°C for 5 min before SDS polyacrylamide gel electrophoresis. Aliquots (15 µg/lane for brain-derived neurotrophic factor (BDNF) and 20 µg/lane for all other proteins) and Kaleidoscope protein standards (Bio-Rad) were separated on 10~20% Tris-HCl gels (Bio-Rad) for BDNF and 4–15% Tris-HCl gels (Bio-Rad) for all other proteins. The gels were transferred to PVDF membranes (GE Healthcare, Little Chalfont, Buckinghamshire). The immunoblots were blocked in Tris buffered saline with 5% non-fat powdered milk and 0.1% tween-20 for 1 hr at room temperature followed by overnight incubation at 4°C with diluted primary antibodies, 4-hydroxy-2-nonenal (HNEJ-2) (1:100; Abcam #ab48506); malondialdehyde (MDA), (1:800; Abcam #ab27642); BDNF (1:200; Santa Cruz Biotechnology, Santa Cruz, CA#sc-546); glutathione peroxidase 1 (GPX1) (1:1000; Abcam #ab22604, Cambridge, MA); superoxide dismutase 1 (SOD1) (1:5000; Abcam #ab13498) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:10000, EnCor Biotechnology Inc #MCA-1D4, Gaivesville, FL). This was followed by anti-mouse or anti-rabbit secondary antibodies (Cell Signaling Technology, Danvers, MA, anti-mouse #7076, anti-rabbit #7074; 1:1000–4000), and an ECL Plus kit (GE Healthcare #RPN2132) for visualization. Blots were exposed to Bio Max MR film (Kodak, Rochester, NY), developed on a film processor (Konica Medical Corp, Ramsey NJ), scanned using GS-800 Calibrated Densitometer (Bio-Rad), and densitometry determined using ImajeJ software (National Institute of Health, Bethesda, MD). Densitometry measures were normalized to GAPDH. The specificity of the BDNF antibody was tested using human recombinant BDNF (Santa Cruz Biotechnology, Inc, #sc-546). For both hippocampal tissue and human recombinant BDNF a single band was observed ~14 kD indicating specificity (data not shown).
2.11 Statistical Analysis
In general, multiple analyses of variance (ANOVAs) were used to establish main effects and interactions. Repeated measures ANOVAs on escape latency or distance, across blocks of trials, were employed to examine effects of training on the water maze swim task (Blalock, et al., 2003; Carter, et al., 2009; Foster, et al., 1991; Foster and Dumas, 2001; Foster and Kumar, 2007; Foster, et al., 2003). Follow-up ANOVAs and/or Fisher’s PLSD post hoc comparisons with p < 0.05 were employed to determine specific differences. Student t tests were used to determine whether quadrant search behavior was different than that expected by chance and whether patterned stimulation resulted in a change in synaptic strength different from baseline. A Mann-Whitney U test was employed for gridwalk crossing times. For electrophysiological studies, the average of five to ten AHPs was calculated for each cell. An ANOVA was used to determine main effects. Post hoc analyses were conducted (Stat view, SAS Institute Inc., Cary, NC) using Fisher’s PLSD tests, with significance set at p < 0.05.
3. Results
Our previous work indicates that, despite differences in the distance traveled, the two exercise paradigms have similar effects on behavior and markers of brain aging (Cui, et al., 2009). This was confirmed in the current study such that a significant (p < 0.001) difference was observed in average distance traveled over the 10–12 weeks prior to behavioral testing. The distance traveled was increased in conditioned animals which worked for food according to a set criterion (4216 ± 199 m) relative to calorically restricted animals which substituted wheel running for foraging activity (1623 ± 212 m). No difference was observed between the two exercise groups for weight or behavioral measures on the water maze, grip strength, gridwalk, or object recognition task, and no correlation was observed between distance traveled and behavioral measures. Therefore, the two exercise groups were combined for behavioral comparisons with young, A-SED, and A-ENR rats. An examination of body weight across all groups on the day prior to behavioral testing indicated a group effect [F(3,116) = 27.2, p < 0.0001] and post hoc tests indicate that A-EX and young rats had reduced body weight relative to A-SED and A-ENR (Fig 1).
Figure 1.
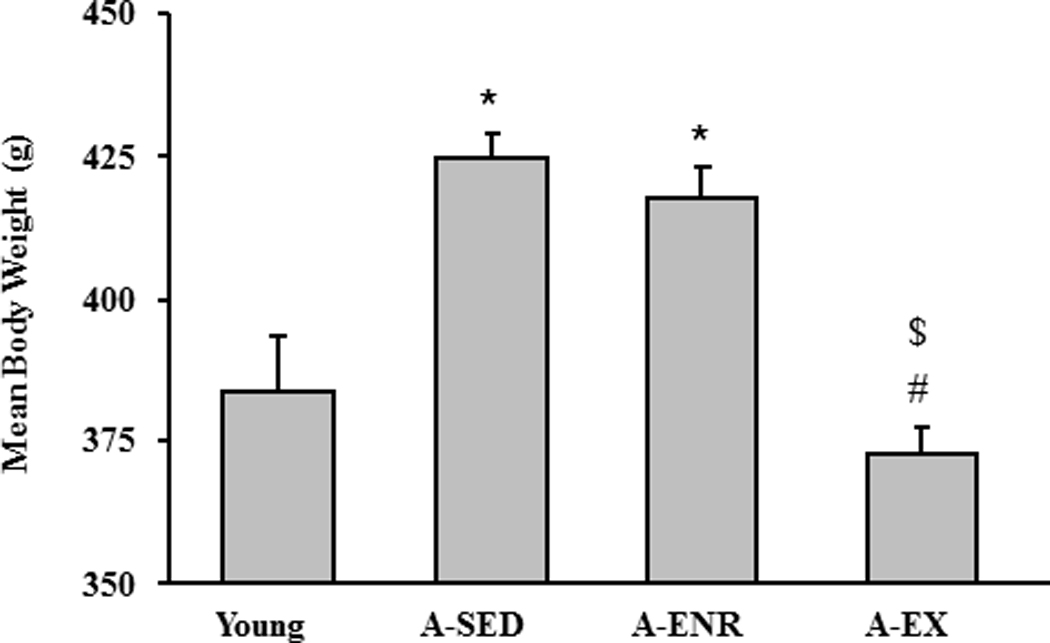
Bars represent the mean body weight (+SEM) for young, A-SED, A-ENR, and A-EX animals. Asterisk indicates significant difference from young, pound sign indicates significant difference from A-SED, and dollar sign indicates a significant difference from A-ENR in this and subsequent figures.
3.1 Cue discrimination learning of aged animals is facilitated by exercise
For testing on the water maze, the initial groups included young (n = 12), A-SED (n = 39), A-ENR (n = 22), and A-EX (n = 21). Eight A-SED animals (20%), three A-EX (14%) and three A-ENR animals (13%) did not reach the platform within 60 sec for two out of the last three trials of cue discrimination training. These animals were classified as having sensory-motor deficits or impaired procedural learning which would have detrimental effects on the spatial version of the task (Blalock, et al., 2003; Foster and Kumar, 2007). Therefore, these animals were removed from further analysis (Table 1). For the remaining animals, a repeated measures ANOVA on escape latency during cue training (Fig 2A) indicated a significant main effect of training across blocks [F(4, 304) = 20.5, p < 0.0001], a tendency for a group effect (p = 0.053), and an interaction of training and group [F(3, 304) = 2.5, p < 0.005]. Post hoc ANOVAs within each group indicated that young [F(4,44) = 8.83, p < 0.0001], A-SED [F(4,120) = 4.67, p < 0.005], and A-EX [F(4,68) = 14.7, p < 0.0001] exhibited a significant change in latency during training. A comparison of the mean escape latency for the last three blocks indicated a group effect [F(3, 76) = 3.2, p < 0.05] and post hoc analysis indicated that latency was increased in A-SED rats compared to young and A-EX rats (Fig 2B). Interestingly, no difference was observed between young and A-EX rats.
Figure 2.
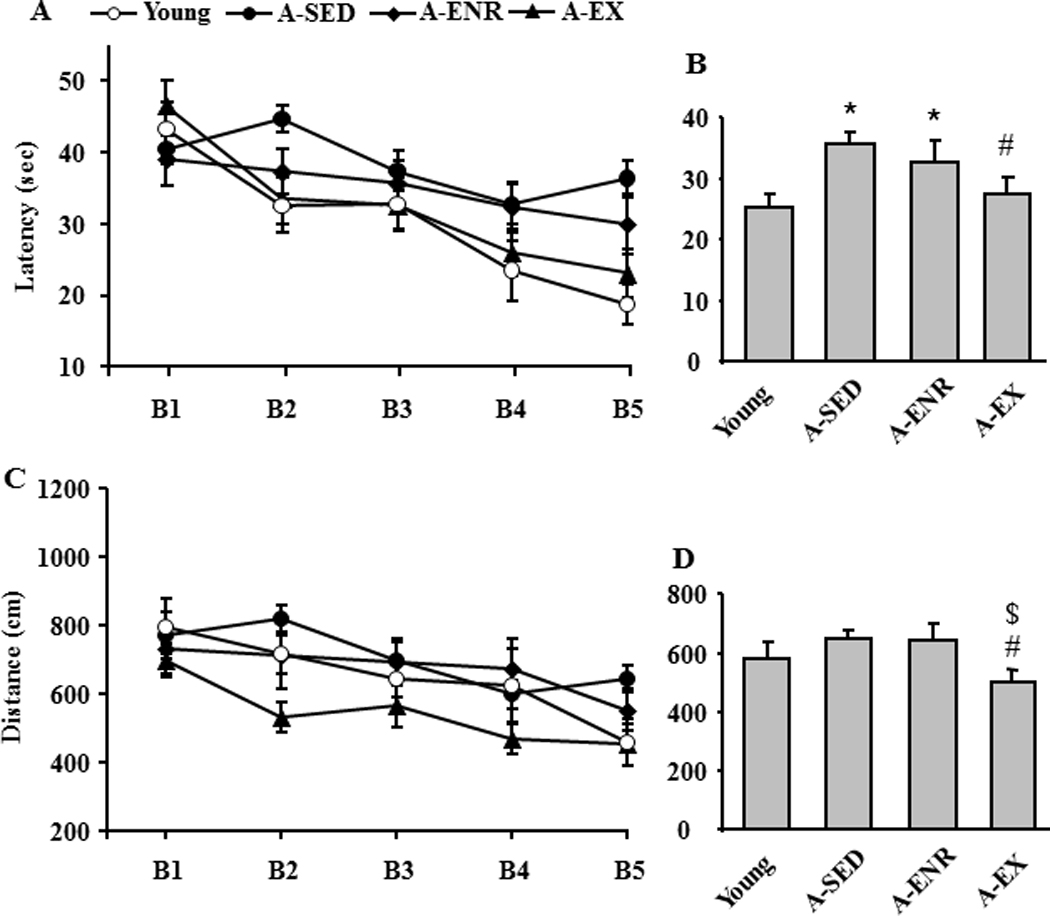
Environmental enrichment and exercise improve cue discrimination performance. A) Mean latency on each of the five training blocks (B1–B5) during cue discrimination training for the four groups: young (open circle), A-SED (filled circle), A-ENR (filled diamond) and A-EX (filled triangle). B) Comparison of the mean escape latency averaged over the last three blocks of training. C) Mean distance traveled during each block for each group. D) Comparison of the mean escape distance averaged over the last three blocks.
Similar results were observed for escape path length with main effects of training [F(4, 304) = 9.9, p < 0.0001] and group [F(3, 76) = 3.5, p < 0.05] in the absence of any interactions (Fig 2C). Post hoc ANOVAs indicated that young [F(4,44) = 4.27, p < 0.01], A-SED [F(4,120) = 4.57, p < 0.005], and A-EX [F(4,68) = 5.42, p < 0.001] exhibited a decrease in escape path length over the course of training. A comparison of the mean escape path length for the last three blocks indicated a tendency for a group effect (p = 0.069) and post hoc comparisons indicated that distance was reduced in A-EX rats compared to A-SED and A-ENR (Fig 2D). Together, the results indicate that exercise can improve cue discrimination performance.
3.2 Spatial discrimination learning is impaired by exercise training
A repeated measures ANOVA on escape latency during spatial discrimination testing indicated significant main effects of training [F(5, 380) = 16.4, p < 0.0001] and group [F(3, 76) = 9.4, p < 0.001] and an interaction between group and training F(15, 380) = 1.7, p < 0.05] (Fig 3A). Post hoc ANOVAs within each group indicated that young [F(5,55) = 9.64, p < 0.0001], A-SED [F(5,150) = 6.78, p < 0.0001], and A-ENR [F(5,90) = 3.53, p < 0.01] exhibited a decrease in escape latency over the course of training. An ANOVA on the mean latency for the last three blocks indicated a group effect [F(3, 76) = 11.0, p < 0.0001] and post hoc analysis indicated that young rats exhibited reduced latency relative to the other three groups (Fig 3B). Furthermore, A-ENR animals exhibited a reduced latency relative to the A-EX group.
Figure 3.
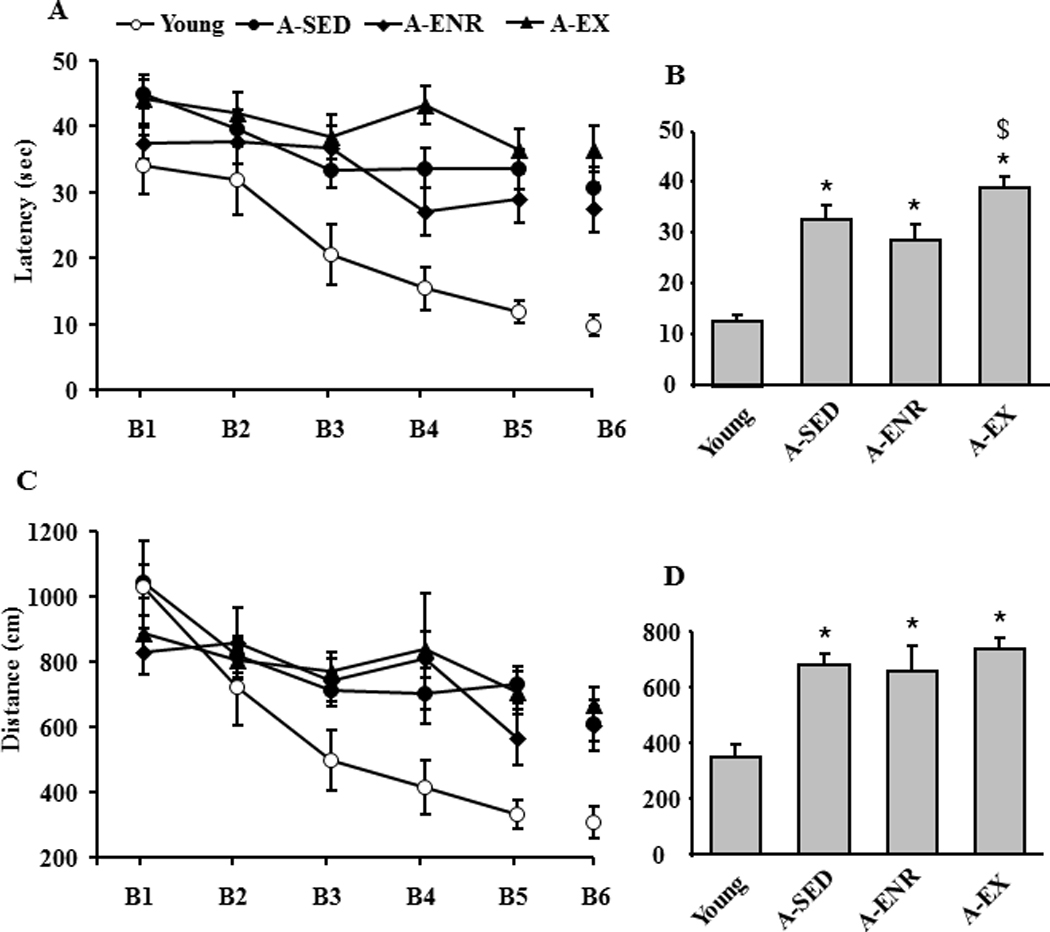
Performance on spatial discrimination training. A) Mean latency for the six training blocks (B1–B6) of the spatial discrimination task for the four groups: young (open circle), A-SED (filled circle), A-ENR (filled diamond) and A-EX (filled triangle). The gap between blocks 5 and 6 indicate the time in which the probe trial to test for acquisition was delivered. B) Comparison of the mean escape latency averaged over the last three blocks of training. C) Mean distance traveled during each block for each group. D) Comparison of the mean escape distance averaged over the last three blocks.
A repeated measures ANOVA for escape path length for the spatial task indicated significant main effects of training [F(5, 380) = 17.9, p < 0.0001] and group [F(3, 76) = 3.4, p < 0.05] with a group by training interaction [F(15, 380) = 2.1, p < 0.05] (Fig 3C). Post hoc ANOVAs indicated that young [F(5,55) = 14.74, p < 0.0001] and A-SED [F(5,150) = 11.33, p < 0.0001] groups exhibited a decrease in escape path length over the course of training. An ANOVA on the mean escape path length for the last three blocks indicated a group effect [F(3, 76) = 6.1, p < 0.001] and post hoc analysis indicated that young exhibited reduced path length relative to the other three age groups (Fig 3D).
An examination of data from the probe trial delivered between training blocks 5 and 6 indicated poor acquisition of a spatial discrimination search strategy by the A-EX group (Fig 4). An ANOVA on platform crossings indicated a significant group effect [F(3, 76) = 8.5, p < 0.0001] and post hoc comparisons indicated that young rats exhibited more crossings relative to the other three groups (Fig 4A). However, platform crossing is influenced by age and does not serve as a good measure of memory function across age-groups (Foster and Dumas, 2001). Therefore, the percent dwell time in the goal quadrant and opposite quadrant (Fig 4B) were used to calculate a spatial discrimination search index. An ANOVA on the discrimination index indicated an effect of group [F(3, 76) = 6.0, p < 0.001] and post hoc tests indicated that the discrimination index was reduced in A-EX compared to the other three groups (Fig 4C). A comparison of the discrimination index relative to that expected by chance (i.e. 0) indicated that all groups, except A-EX, focused their search on the goal quadrant. The results indicate that, as a group, the A-EX animals did not acquire a spatial search strategy. While A-SED rats exhibited an index score above chance, the index score was decreased relative to young controls suggesting that some animals in this group failed to acquire a spatial search strategy.
Figure 4.
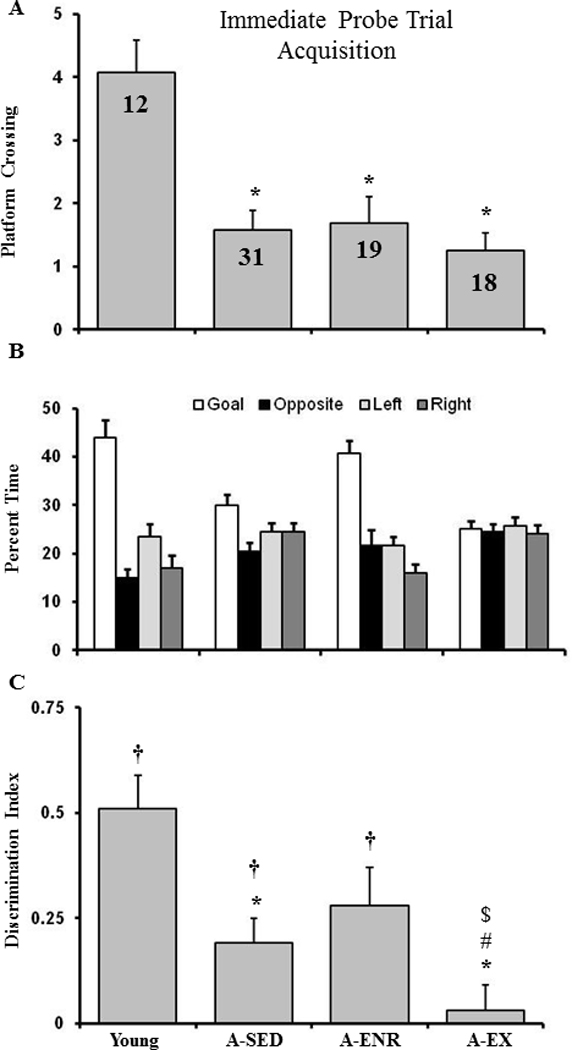
Impaired acquisition of a spatial discrimination associated with exercise in aged rats. The figure shows (A) the platform crossings, (B) percent dwell times (mean + SEM) for the goal quadrant (open bars), opposite quadrant (filled bars), quadrant to the left of the goal (light gray), quadrant to the right of the goal(dark gray), and (C) the discrimination index calculated from the dwell times for the acquisition probe trial conducted between blocks 5 and 6 of the spatial discrimination training for the four groups. The † sign indicates that the discrimination index was significantly (p < 0.05) above chance (i.e. 0). Note that only A-EX animals did not exhibit a discrimination index above chance.
3.3 Retention of spatial discrimination is improved by enrichment
Previous work indicates that aging is associated with an increase incidence of impaired recollection and more rapid forgetting in humans (Davis, et al., 2003) and rodents (Bizon, et al., 2009; Countryman and Gold, 2007; Foster, 1999; Foster and Kumar, 2007). In examining retention deficits, it is important to control for differences in the degree of learning. Cut-off criteria can be employed to identify animals that successfully acquired a spatial search strategy (Foster and Kumar, 2007; Tombaugh, et al., 2005). In order to remove animals that failed to acquire a spatial search strategy, the performance of young animals was used to set a cut-off criterion one standard deviation below the mean for the young group (i.e. a discrimination index of 0.23). Those animals that did not meet the criterion were considered to have failed to acquire the spatial discrimination. The criterion resulted in the loss of one rat from the young group (8%) thirteen rats from the A-SED group (42%), seven animals from A-ENR (37%), and fourteen from A-EX (78%). The remaining animals that were classified as acquiring a spatial search strategy (A-ENR = 12; A-EX = 4; A-SED = 18; young = 11) were tested for retention using a probe trial 24 hr after acquisition training. An ANOVA for platform crossings indicated a significant group effect [F(3, 41) = 5.28, p < 0.005] and post hoc comparisons indicated that young rats exhibited more crossings relative to the other three groups (Fig 5A). An ANOVA on the discrimination index indicated no effect of group. However, a comparison of the discrimination index relative to that expected by chance (i.e. 0) indicated that only young and A-ENR rats exhibited retention, focusing their search on the goal quadrant (Fig 5C).
Figure 5.
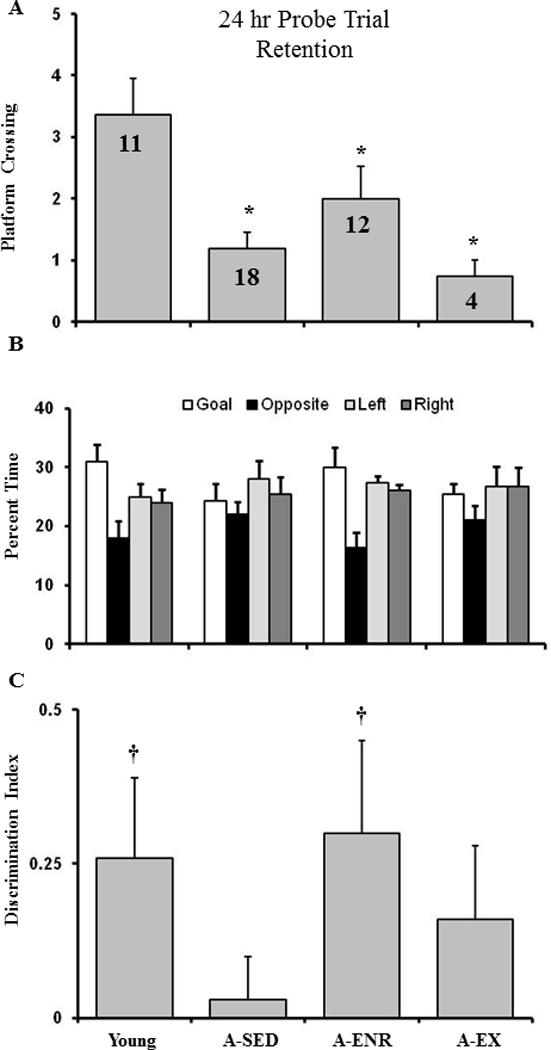
Exposure to environmental enrichment is associated with improved memory consolidation. The figure shows (A) and platform crossings, (B) percent dwell times (mean + SEM) for the goal quadrant (open bars), opposite quadrant (filled bars), quadrant to the left of the goal (light gray), quadrant to the right of the goal(dark gray), and (C) discrimination index for the retention probe trial conducted 24 hr after spatial discrimination training. The † sign indicates that the discrimination index is significantly (p < 0.05) difference from chance (i.e. 0). Note, that only young and A-ENR rats exhibit a spatial search strategy focused on the goal quadrant.
In order to insure that exercise improved motor function, young (n = 6 previously tested on the water maze), A-SED (n = 39, 29 previously tested on the water maze and 10 naive), A-ENR (n = 16 previously tested on the water maze) and A-EX (n = 31, 18 previously tested on the water maze, in addition to naïve caloric restriction wheel running (n = 6) and naïve conditioned wheel running (n = 7)) rats were tested for grip strength. The results indicate a tendency for a difference across groups, and post hoc tests indicated that grip strength was increased in A-EX relative to A-SED (Fig 6A). A subset of the A-EX (n = 21) and A-SED (n = 12) animals were further tested on a gridwalk crossing task, which has been shown to be sensitive to exercise (Chang, et al., 2009; Ding, et al., 2002). An ANOVA on the number of foot faults indicated a tendency (p = 0.051) for fewer foot faults (Fig 6B) and a Mann-Whitney U (U = 69.5, p < 0.05) indicated reduced median time to cross the grid walk bridge in the A-EX rats (Fig 6C).
Figure 6.
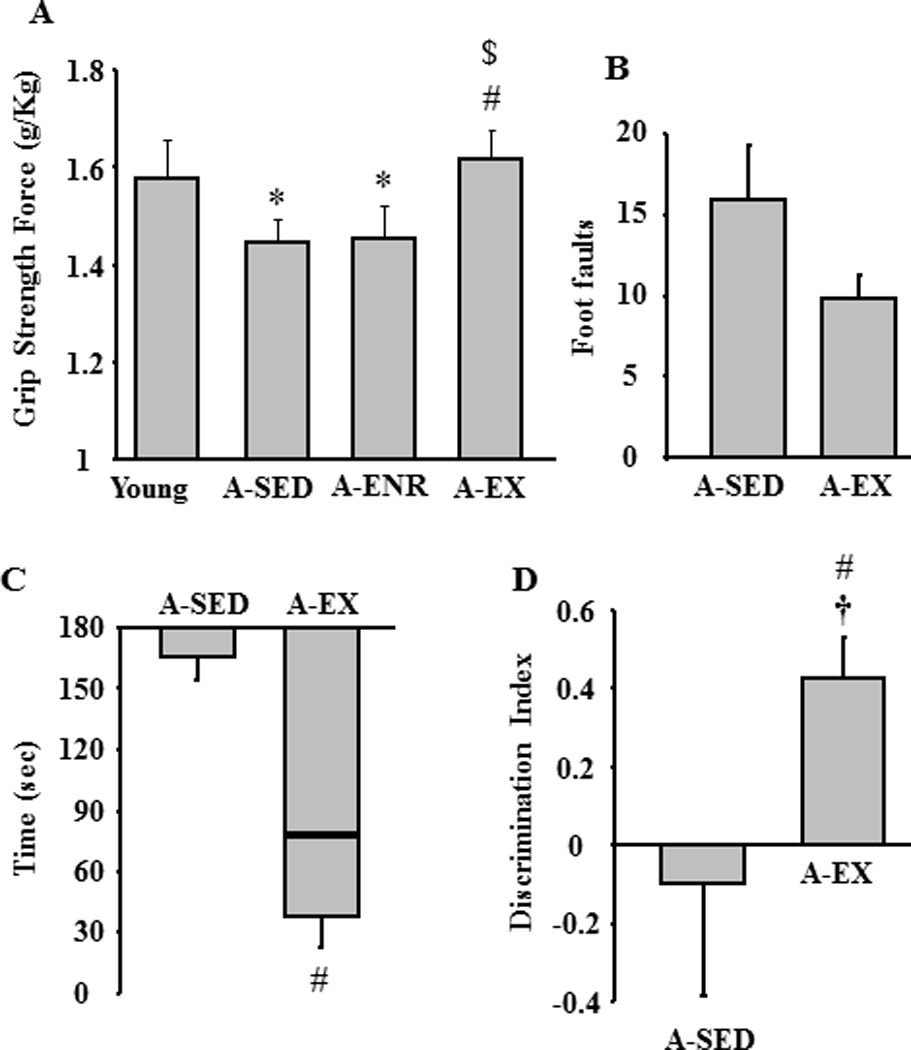
Influence of exercise on grip strength, grid walk, and object recognition. A) Exercise increased the grip strength of aged rats. Compared to A-SED, exercise enhanced performance on the grid walk task observed as a decrease in B) the mean number of foot faults and C) median latency to cross the grid walk bridge. The box in C represents the inter-quartile range and line within the box represents the median score for each age group. The bar extending away from the box denote the minimum value. D) Mean discrimination index for object recognition examined 24 hr after initial exposure showing superior memory for A-EX relative to A-SED rats.
The results indicate that, exercise improved motor function including sensory-motor learning on the water maze. In contrast, exercise was associated with impaired performance on the spatial discrimination learning task. One possibility is that the exercise treatment biased the animals to select egocentric or non-spatial response strategies. Previous studies indicate that exercise can increase object recognition memory in young animals (Garcia-Capdevila, et al., 2009; Griffin, et al., 2009; O'Callaghan, et al., 2007) and performance on this task declines with advanced age (Blalock, et al., 2003; Dellu, et al., 1992; Ennaceur and Meliani, 1992; Hauser, et al., 2009). To determine whether exercise would influence performance on this non-spatial memory task, we examined object recognition in A-SED (n = 6) and A-EX (n = 14) rats. The results indicate better performance [F(1, 18) = 4.5, p < 0.05] by A-EX rats and comparison of the discrimination index for object recognition relative to chance indicated that only the A-EX rats exhibited memory over a 24 hr period (Fig 6D).
3.4 Effects of differential experience on senescent physiology
In some cases, the differential housing conditions were maintained for at least 2–4 weeks following behavioral characterization in order to reduce the effects of behavioral testing on subsequent biological measures. Previous work indicates that caloric restriction can reverse the LTP impairment observed in aged animals (Eckles-Smith, et al., 2000; Hori, et al., 1992). However, a number of studies suggest that the effects of calorie restriction may be due in part to the increase in motor activity (Carter, et al., 2009; Duffy, et al., 1989; Duffy, et al., 1997; Duffy, et al., 1997; Goodrick, et al., 1983; Goodrick, et al., 1983; Weed, et al., 1997; Yu, et al., 1985). In order to better isolate the effect of exercise from caloric restriction on physiological parameters, only animals conditioned to run on the wheel were included in the A-EX group. The maximum EPSP slope was determined for the CA3-CA1 synaptic response. An ANOVA on the maximum response indicated a tendency for a difference across groups (p = 0.09) and post hoc tests indicated that the response was reduced in A-EX relative to young (Fig 7). Following determination of the maximum response, the synaptic response was set at 50% of maximum for examination of synaptic plasticity. Figure 8A shows the time course for the synaptic responses following LFS in slices from young (n = 12/8, slices/animals), A-SED rats (n = 10/10, slices/animals), A-EX (n = 8/7, slices/animals) and A-ENR (n = 12/8, slices/animals). When comparisons were performed on the response relative to baseline, only A-SED rats exhibited significant (p < 0.05) LTD (Fig 8B).
Figure 7.
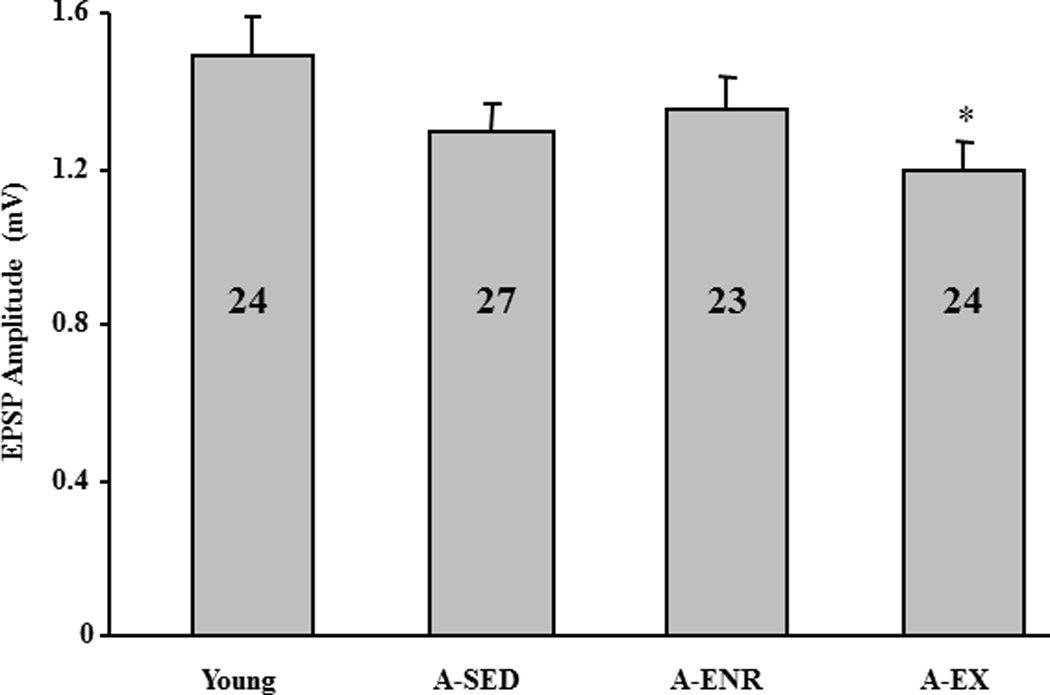
Maximum EPSP slope across the four groups. The asterisk indicates a significance difference relative to young. The numbers in each bar represent the number of slices recorded to calculate the mean.
Figure 8.
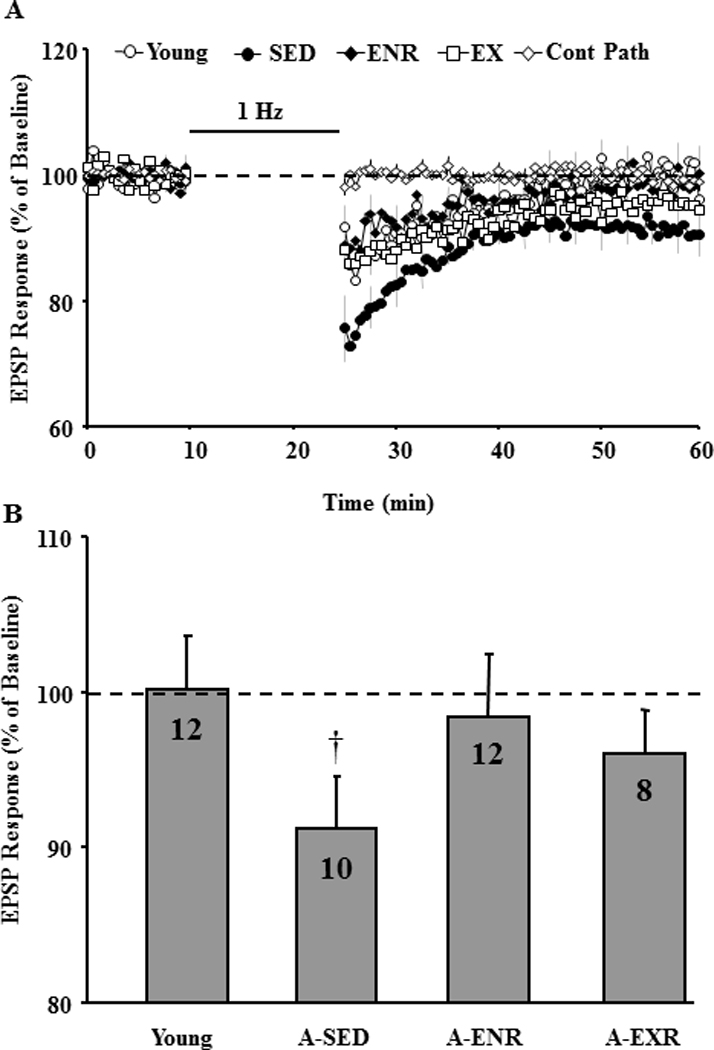
Effect of environmental enrichment or exercise on LTD. A) Time course of the field EPSP measurements obtained from hippocampal slices 10 min before and 35 min after 1 Hz stimulation (900 pulses, 1 Hz, horizontal bar) for the slices obtained from young (open circle), A-SED (filled circle), A-ENR (filled diamond) and A-EX (filled triangle) animals. A non-tetanized control path (Cont Path, open diamond) was used to make sure that the changes in EPSP responses were specific to pattern stimulation and not due to change in slice health. Each individual response was computed as a percent of the mean baseline response (dashed line) collected during the 10 min just prior to pattern stimulation. LTD was measured as the slope of field potentials normalized to baseline and is plotted against time. B) Bar diagram showing the average magnitude of LTD during the last 5 min of recording. The number in each bar represents the number of slices used to calculate the mean. † represents significance difference from baseline (dotted line). Note only A-SED animals exhibited LTD.
An ANOVA for TBS-induced LTP revealed a significant treatment effect [F(3, 52) = 6.57, p < 0.001] and post hoc comparisons indicated that the level of LTP was decreased in young (n = 12/4, slices/animals), A-SED (n = 17/10, slices/animals), and A-ENR (n = 11/6, slices/animals) relative to A-EX (n = 16/10, slices/animals) rats (Fig 9). In addition, LTP in young tended (p = 0.06) to be greater than A-SED. A comparison of the response 35 min following TBS relative to baseline indicated that LTP was observed for young, A-ENR, and A-EX, but not in A-SED animals.
Figure 9.
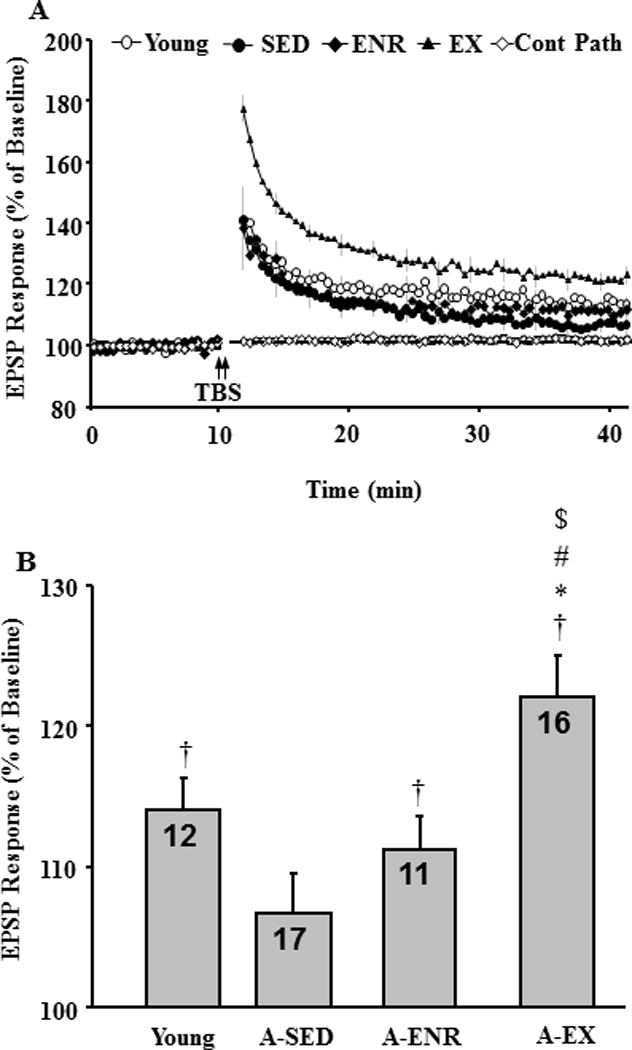
Exposure to environmental enrichment or exercise facilitates induction of LTP in aged animals. A) Time course of the field EPSP measurements obtained from hippocampal slices 10 min before and 35 min after TBS (arrow) for the slices obtained from young (open circle), A-SED (filled circle), A-ENR (filled diamond) and A-EX (filled triangle) animals. A non-tetanized control path (cont path, open diamond) was used to make sure that the changes in EPSP responses were specific to pattern stimulation and not due to change in slice health. Each individual response was computed as a percent of the mean baseline response (dashed line) collected during the 10 min just prior to pattern stimulation. LTP was measured as the slope of field potentials normalized to baseline and is plotted against time. B) Bar diagram showing the average magnitude of LTP during the last 5 min of recording. The number in each bar represents the number of slices used to calculate the mean. † represents significance difference from baseline, * represents significance difference from young, # represents significance difference from A-SED, $ represents significance difference from A-ENR.
A total of 117 intracellularly recorded cells (young 40/12, cells/animals; A-SED 39/14, cells/animals; A-ENR 21/11, cells/animals; A-EX 17/5, cells/animals) were acceptable according to our cell health criteria. Passive membrane properties: input resistance, resting membrane potential, and spike amplitude were not different between the four groups (Table 2). AHPs were elicited by a burst of 4 action potentials and the amplitude was measured as the difference between the holding potential during the 100 msec period immediately before the onset of the depolarizing current and the membrane potential 500 msec after the offset of the depolarizing current. For each cell, the average of five to thirty AHPs was calculated and used for statistical analysis. An ANOVA on the AHP amplitude indicated a significant group effect [F(3, 113) = 6.8, p < 0.0005] and post hoc comparisons confirmed a reduced AHP in young and A-ENR compared to A-SED (Fig 10). Furthermore, the AHP amplitude for the A-ENR group was reduced relative to A-EX and there was a tendency (p = 0.06) for the AHP to be reduced in young relative to A-EX.
Table 2.
Biophysical properties of CA1 neurons recorded from aged male F 344 rats.
| IR (MΩ) | RMP | Spike Amplitude | |
|---|---|---|---|
| Young (40/12) | 39.2 ± 2.40 | −61.7 ± 0.79 | 84.2 ± 0.78 |
| A-SED (39/14) | 37.1 ± 1.55 | −61.1 ± 0.85 | 84.9 ± 0.83 |
| A-ENR (21/11) | 41.8 ± 4.64 | −62.9 ± 1.15 | 83.1 ± 0.81 |
| A-EX (17/5) | 33.5 ± 1.71 | −62.6 ± 0.82 | 87.1 ± 1.21 |
Values are ± SEM. RMP, Resting membrane potential, IR, Input resistance, A-SED, aged-sedentary; A-ENR, aged enriched; A-EX, aged exercise. RMP and spike amplitude values are in mV. Numbers in parentheses indicate number of cells and animals respectively for each condition.
Figure 10.
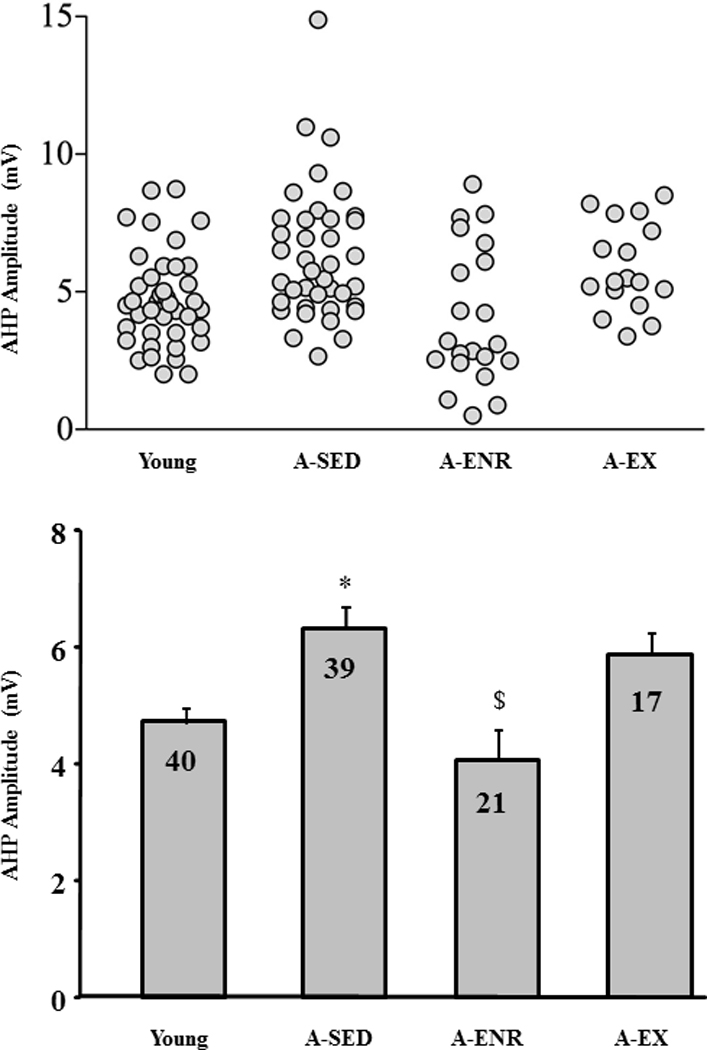
Exposure to environmental enrichment or exercise reduced the aged associated enhanced amplitude of AHP. A) Figure illustrates distribution of AHP amplitude of individual cells for slices obtained from young (n = 40 cells), A-SED (n = 39 cells), A-ENR (n = 21 cells), and A-EX (n = 28 cells) animals. B) Bar diagram shows the mean amplitude of AHP for the different groups. The number in each bar represents the number of cells used to calculate the mean. * represents significant difference from young, # represents significant difference from A-SED, and $ represents significant difference from A-ENR animals.
To determine whether our exercise procedure reduced oxidative stress in the hippocampus, we measured lipid oxidation using western blots (Fig 11). The analysis indicated no difference (A-SED: n = 10; A-EX: n = 10) in HNE levels detected between 30 kDa and 70 kDa. There was a slight decrease in MDA levels for the A-EX group when measures included all bands. The decrease was largely due to a decrease in two bands between 90 kDa and 120 kDa (p < 0.05). Furthermore, no group differences were observed for expression of BDNF, GPX1, or SOD1.
Figure 11.
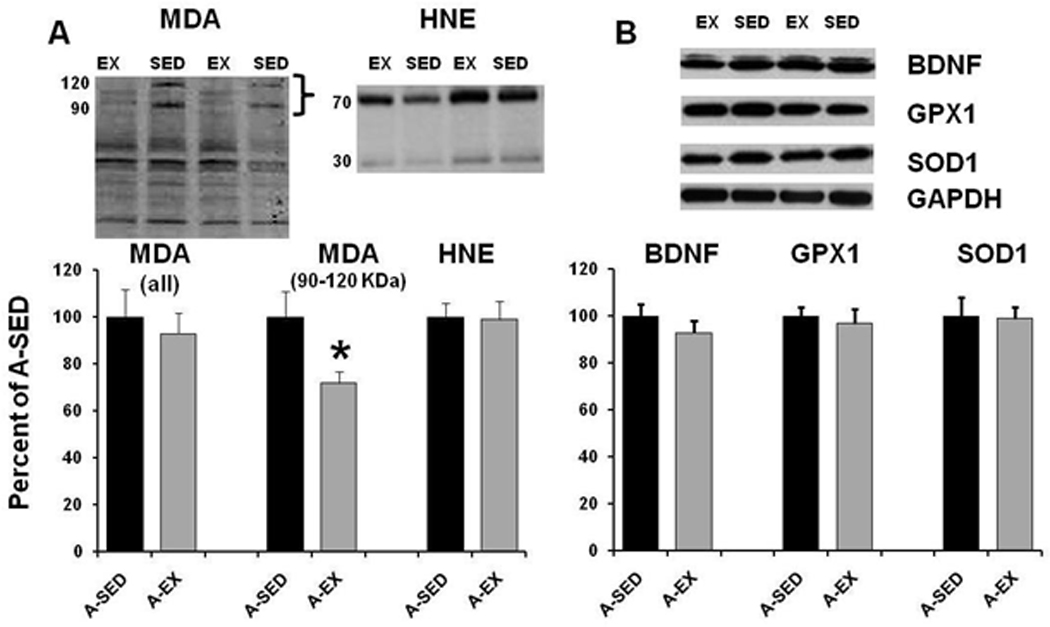
Protein analysis of lipid oxidation. Western blots for A-SED (filled bars) and A-EX (gray bars) showing the mean expression of A) MDA and HNE or B) BDNF, GPX1, and SOD1. Densitometry readings for each protein of interest were standardized by the corresponding reading for GAPDH. Inserts provide example blots. All measures were normalized to the A-SED group. MDA measured between 90 – 120 KDa (bracket on the blot insert) was decreased in the A-EX group. Asterisk indicates a significant (p < 0.05) difference.
4. Discussion
The current study set out to compare the effects of environmental enrichment and exercise on age-related cognitive decline and senescent physiology. The results on environmental enrichment are consistent with a number of studies, which demonstrate that environmental enrichment improves cognition in rats (Lores-Arnaiz, et al., 2006; Soffie, et al., 1999; Winocur, 1998) and acquisition of the spatial version of the water maze in rats and mice (Fernandez, et al., 2004; Frick and Fernandez, 2003; O'Callaghan, et al., 2009). We now add that environmental enrichment increased memory consolidation in aged rats. Thus, while young, A-SED, and A-ENR rats acquired a spatial search strategy (Fig 4C), only young and A-ENR rats exhibited retention over the 24 hr period (Fig 5C). Aged animals can rapidly acquire spatial information; however, impairment is observed as the retention delay is increased (Bizon, et al., 2009; Driscoll, et al., 2006; Foster, 1999; Foster, et al., 1991; Foster and Kumar, 2007). Furthermore, impaired memory consolidation is highly sensitive to aging, emerging in middle-age (Bizon, et al., 2009; Blalock, et al., 2003; Driscoll, et al., 2006; Foster, et al., 2003). For studies that examine training across multiple days, aged rats initially exhibit learning within each day of training and memory consolidation deficits are observed as impaired performance for the first trial on the following day(Foster, 1999). This “saw tooth” pattern of behavior disappears with continued training as incremental learning permits age animals to establish a long term spatial memory (Foster and Kumar, 2007; Rapp, et al., 1987; van Groen, et al., 2002).
One question concerns which aspects of environmental enrichment contribute to improved cognitive function. The environmental enrichment procedures provide social interactions, cognitive stimulation, exposure to novelty, and increased motor activity. Previous research indicates a major role for cognitive stimulation (Cracchiolo, et al., 2007; Harburger, et al., 2007; Marques, et al., 2009; Pietropaolo, et al., 2006) and novelty (Pardon, et al., 2009; Veyrac, et al., 2009) in improving spatial learning and memory, with a relatively minor influence of social interactions (Diniz, et al., 2010; Schrijver, et al., 2002; Schrijver, et al., 2004; Winocur, 1998). Motor activity interacts with the other variable such that improved memory is observed when motor activity occurs in an environment that is separate from the home cage or requires extensive handling (Harburger, et al., 2007; Lambert, et al., 2005; O'Callaghan, et al., 2009). In the current study, exercise occurred in the home cage and was associated with impaired spatial water maze performance. On the surface, the results would support work indicating little or no benefit from exercise on cognition in aging rats (Asl, et al., 2008; Barnes, et al., 1991; Hansalik, et al., 2006). Alternatively, it is possible that the exercise condition predisposed the animals to focus on an egocentric or response strategy rather than a spatial strategy (DeCoteau and Kesner, 2000; McDonald and White, 1993; Potegal, 1972). The A-EX group exhibited superior performance on the cue discrimination task, suggesting enhance reliance on systems other than the hippocampus McDonald and White, 1994; Packard and McGaugh, 1992). In older humans, exercise may promote more general attentional processes with limited influence specific to spatial processing (Colcombe and Kramer, 2003). The object recognition task requires attentional processing (Levin, et al., 2011) and minimizes the need for spatial orientation. Furthermore, performance on this task declines with advanced age (Blalock, et al., 2003; Dellu, et al., 1992; Ennaceur and Meliani, 1992; Hauser, et al., 2009). We observed that exercise increased object recognition memory in older rats, a result similar to that observed in young animals (Garcia-Capdevila, et al., 2009; Griffin, et al., 2009; O'Callaghan, et al., 2007), suggesting that exercise can improve memory in older animals. Together, the results suggest that the exercise conditions altered the response selection, such that when presented with the option of using a spatial or a response strategy in the water maze, animals with a history of 10–12 weeks of exercise biased their behavior to employ an egocentric response strategy.
The results on physiology confirm an age-related increase in the susceptibility to induction of LTD (Foster and Kumar, 2007; Foy, et al., 2008; Hsu, et al., 2002; Norris, et al., 1996), impaired LTP induction (Coultrap, et al., 2008; Deupree, et al., 1993; Eckles-Smith, et al., 2000; Moore, et al., 1993; O'Callaghan, et al., 2009; Shankar, et al., 1998), and an increase in the AHP(Bodhinathan, et al., 2010; Disterhoft, et al., 1996; Gant and Thibault, 2009; Kumar and Foster, 2007; Kumar and Foster, 2002; Landfield and Pitler, 1984; Tombaugh, et al., 2005). Consistent with a number of studies, mainly in young animals, environmental enrichment decreased LTD and improved LTP (Artola, et al., 2006; Duffy, et al., 2001; O'Callaghan, et al., 2009; Yang, et al., 2006; Yang, et al., 2007). Similarly, we confirmed that exposure to novelty decreases the AHP in aged rats (Kumar and Foster, 2007). The fact that the AHP was larger in A-SED and A-EX, groups that performed poorly on the water maze, is consistent with the idea that learning reduces the AHP amplitude (Oh, et al., 2010). Previous work indicates that exercise in young animals does not enhance LTP in region CA1 (Lange-Asschenfeldt, et al., 2007; Tartar, et al., 2006; van Praag, et al., 1999). In contrast, we observed that the influence of exercise on synaptic plasticity was qualitatively similar to environmental enrichment, reducing the susceptibility to induction of LTD and enhancing LTP. The difference may be due to baseline differences in the threshold or susceptibility to LTP induction, such that exercise improved LTP induction only in aged animals that normally exhibit impaired LTP.
The rejuvenation of senescent physiology by environmental enrichment and exercise has important implications for our understanding of brain aging. The improvement in synaptic plasticity is likely due to factors that are common across the two conditions including increased hippocampal activity associated with locomotion (Bland, 1986; Buzsaki, 2002; Czurko, et al., 1999; Foster, et al., 1989). The idea that neural activity modifies Ca2+-dependent processes is consistent with gene microarry studies (Molteni, et al., 2002; Stranahan, et al., 2008; Tong, et al., 2001). This work indicates that exercise increases the expression of genes linked to neural activity and synaptic plasticity and reduces the expression of genes linked to oxidative stress. In some cases, the increase in neural activity due to exercise results in increased expression of BDNF (Berchtold, et al., 2005; Griffin, et al., 2009; Molteni, et al., 2002; O'Callaghan, et al., 2009) and protection against oxidative stress (Nakajima, et al., 2010; Rodrigues, et al., 2010; Stranahan, et al., 2010; Vaynman, et al., 2006). We did not observe an increase in BDNF expression, which is consistent with several other studies (Cechetti, et al., 2008; Lou, et al., 2008; Marais, et al., 2009; Vaynman, et al., 2004). Taken together, the results indicate that the ability to observe an increase in BDNF is related to the specific region of the hippocampus examined and a number of ancillary factors associated with the exercise treatment including the age of the animal, intensity, and duration of the exercise.
Exercise can shift the redox state of the brain (Radak, et al., 2007). It might be expected that a shift in redox state would influence oxidative damage. We observed a decrease in lipid peroxidation, which was limited to certain bands on the western blot. The results are consistent with other studies that observed minimal changes in lipid peroxidation in the hippocampus following exercise training (Acikgoz, et al., 2006; Cechetti, et al., 2008; Devi and Kiran, 2004; Jolitha, et al., 2006). Thus, it is possible that a shift in redox state has large effects on the activity of signaling cascades that are important for hippocampal function (Bodhinathan, et al., 2010; Bodhinathan, et al., 2010) with limited effects on oxidative damage.
In summary, the data indicate that both exercise and environmental enrichment ameliorated the physiological markers of hippocampal aging including synaptic plasticity (Fig 8&Fig 9). In contrast, the results indicate that only environmental enrichment improved hippocampal-dependent spatial discrimination learning (Fig 4C), ameliorated the memory consolidation deficit observed in older animals (Fig 5C), and improved cell excitability (Fig 10). Taken together with other studies comparing the effects of exercise and experience on age-related cognitive decline, our results suggest that neural activity associated with locomotion, exploration, and novelty may rejuvenate hippocampal neural plasticity processes; however, the history of experience may predispose the animal to employ a limited behavioral repertoire. Thus, exercise appears to provide a means to promote healthy brain aging; nevertheless, in the absence of concomitant cognitive training, the benefits may not be fully realized. Consequently, physical activity and the associated activation of hippocampal circuits may be useful as an adjunct to various cognitive training programs.
Acknowledgement
This work was supported by National Institutes of Aging Grant AG14979, AG037984, AG036800 and the Evelyn F. McKnight Brain Research Foundation. Thanks to Prasanna Durairaj, Jose Herrera, and Vijay Parekh for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors of this manuscript have no actual or potential conflicts of interest. Procedures involving animals have been reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
References
- Acikgoz O, Aksu I, Topcu A, Kayatekin BM. Acute exhaustive exercise does not alter lipid peroxidation levels and antioxidant enzyme activities in rat hippocampus, prefrontal cortex and striatum. Neurosci Lett. 2006;406(1–2):148–151. doi: 10.1016/j.neulet.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Artola A, von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23(1):261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Asl NA, Sheikhzade F, Torchi M, Roshangar L, Khamnei S. Long-term regular exercise promotes memory and learning in young but not in older rats. Pathophysiology. 2008;15(1):9–12. doi: 10.1016/j.pathophys.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Forster MJ, Fleshner M, Ahanotu EN, Laudenslager ML, Mazzeo RS, Maier SF, Lal H. Exercise does not modify spatial memory, brain autoimmunity, or antibody response in aged F-344 rats. Neurobiol Aging. 1991;12(1):47–53. doi: 10.1016/0197-4580(91)90038-l. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30(4):646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23(9):3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26(1):1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Intracellular Redox State Alters NMDA Receptor Response during Aging through Ca2+/Calmodulin-Dependent Protein Kinase II. J Neurosci. 2010;30(5):1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Redox sensitive calcium stores underlie enhanced after hyperpolarization of aged neurons: role for ryanodine receptor mediated calcium signaling. J Neurophysiol. 2010;104(5):2586–2593. doi: 10.1152/jn.00577.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. The journals of gerontology. 2009;64(8):850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetti F, Fochesatto C, Scopel D, Nardin P, Goncalves CA, Netto CA, Siqueira IR. Effect of a neuroprotective exercise protocol on oxidative state and BDNF levels in the rat hippocampus. Brain Res. 2008:1188182–1188188. doi: 10.1016/j.brainres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Chang HC, Yang YR, Wang SG, Wang RY. Effects of treadmill training on motor performance and extracellular glutamate level in striatum in rats with or without transient middle cerebral artery occlusion. Behav Brain Res. 2009;205(2):450–455. doi: 10.1016/j.bbr.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Bickford PC, Browning MD. Blueberry-enriched diet ameliorates age-related declines in NMDA receptor-dependent LTP. Age (Dordr) 2008;30(4):263–272. doi: 10.1007/s11357-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman RA, Gold PE. Rapid forgetting of social transmission of food preferences in aged rats: relationship to hippocampal CREB activation. Learn Mem. 2007;14(5):350–358. doi: 10.1101/lm.524907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracchiolo JR, Mori T, Nazian SJ, Tan J, Potter H, Arendash GW. Enhanced cognitive activity--over and above social or physical activity--is required to protect Alzheimer's mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem. 2007;88(3):277–294. doi: 10.1016/j.nlm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Hofer T, Rani A, Leeuwenburgh C, Foster TC. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiol Aging. 2009;30(6):903–909. doi: 10.1016/j.neurobiolaging.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czurko A, Hirase H, Csicsvari J, Buzsaki G. Sustained activation of hippocampal pyramidal cells by 'space clamping' in a running wheel. Eur J Neurosci. 1999;11(1):344–352. doi: 10.1046/j.1460-9568.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- Davis HP, Small SA, Stern Y, Mayeux R, Feldstein SN, Keller FR. Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003;39(4–5):1063–1091. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP. A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav Neurosci. 2000;114(6):1096–1108. doi: 10.1037//0735-7044.114.6.1096. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992;588(1):132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14(3):249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging. 2004;25(4):501–508. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li J, Lai Q, Azam S, Rafols JA, Diaz FG. Functional improvement after motor training is correlated with synaptic plasticity in rat thalamus. Neurol Res. 2002;24(8):829–836. doi: 10.1179/016164102101200816. [DOI] [PubMed] [Google Scholar]
- Diniz DG, Foro CA, Rego CM, Gloria DA, de Oliveira FR, Paes JM, de Sousa AA, Tokuhashi TP, Trindade LS, Turiel MC, Vasconcelos EG, Torres JB, Cunnigham C, Perry VH, Vasconcelos PF, Diniz CW. Environmental impoverishment and aging alter object recognition, spatial learning, and dentate gyrus astrocytes. Eur J Neurosci. 2010;32(3):509–519. doi: 10.1111/j.1460-9568.2010.07296.x. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59(5–6):413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139(4):1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Feuers RJ, Leakey JA, Nakamura K, Turturro A, Hart RW. Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev. 1989;48(2):117–133. doi: 10.1016/0047-6374(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Feuers RJ, Pipkin JL, Turturro A, Hart RW. Age and temperature related changes in behavioral and physiological performance in the Peromyscusleucopus mouse. Mech Ageing Dev. 1997;95(1–2):43–61. doi: 10.1016/s0047-6374(96)01834-9. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Leakey JE, Pipkin JL, Turturro A, Hart RW. The physiologic, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ Res. 1997;73(1–2):242–248. doi: 10.1006/enrs.1997.3714. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8(1):26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3(1):29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res. 2000;78(1–2):154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51(1):83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- Fernandez CI, Collazo J, Bauza Y, Castellanos MR, Lopez O. Environmental enrichment-behavior-oxidative stress interactions in the aged rat: issues for a therapeutic approach in human aging. Ann N Y Acad Sci. 2004:101953–101957. doi: 10.1196/annals.1297.012. [DOI] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev. 1999;30(3):236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Barnes CA, Rao G, McNaughton BL. Increase in perforant path quantal size in aged F-344 rats. Neurobiol Aging. 1991;12(5):441–448. doi: 10.1016/0197-4580(91)90071-q. [DOI] [PubMed] [Google Scholar]
- Foster TC, Castro CA, McNaughton BL. Spatial selectivity of rat hippocampal neurons: dependence on preparedness for movement. Science. 1989;244(4912):1580–1582. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- Foster TC, Dumas TC. Mechanism for increased hippocampal synaptic strength following differential experience. J Neurophysiol. 2001;85(4):1377–1383. doi: 10.1152/jn.2001.85.4.1377. [DOI] [PubMed] [Google Scholar]
- Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: relation to long-term potentiation. Brain Res. 1996;736(1–2):243–250. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87(4):522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24(6):839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Foy JG, Thompson RF. 17 beta-estradiol modifies stress-induced and age-related changes in hippocampal synaptic plasticity. Behav Neurosci. 2008;122(2):301–309. doi: 10.1037/0735-7044.122.2.301. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24(4):615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Gagne J, Gelinas S, Martinoli MG, Foster TC, Ohayon M, Thompson RF, Baudry M, Massicotte G. AMPA receptor properties in adult rat hippocampus following environmental enrichment. Brain Res. 1998;799(1):16–25. doi: 10.1016/s0006-8993(98)00451-x. [DOI] [PubMed] [Google Scholar]
- Gant JC, Thibault O. Action potential throughput in aged rat hippocampal neurons: regulation by selective forms of hyperpolarization. Neurobiol Aging. 2009;30(12):2053–2064. doi: 10.1016/j.neurobiolaging.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Capdevila S, Portell-Cortes I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behav Brain Res. 2009;202(2):162–170. doi: 10.1016/j.bbr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J Gerontol. 1983;38(1):36–45. doi: 10.1093/geronj/38.1.36. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth, activity, and lifespan in rats allowed voluntary exercise. Exp Aging Res. 1983;9(3):203–209. doi: 10.1080/03610738308258453. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19(10):973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73(5):356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansalik M, Skalicky M, Viidik A. Impairment of water maze behaviour with ageing is counteracted by maze learning earlier in life but not by physical exercise, food restriction or housing conditions. Exp Gerontol. 2006;41(2):169–174. doi: 10.1016/j.exger.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Lambert TJ, Frick KM. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behav Brain Res. 2007;185(1):43–48. doi: 10.1016/j.bbr.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Nzerem CK, Frick KM. Single enrichment variables differentially reduce age-related memory decline in female mice. Behav Neurosci. 2007;121(4):679–688. doi: 10.1037/0735-7044.121.4.679. [DOI] [PubMed] [Google Scholar]
- Hauser E, Tolentino JC, Pirogovsky E, Weston E, Gilbert PE. The effects of aging on memory for sequentially presented objects in rats. Behav Neurosci. 2009;123(6):1339–1345. doi: 10.1037/a0017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59(3):826–831. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- Hori N, Hirotsu I, Davis PJ, Carpenter DO. Long-term potentiation is lost in aged rats but preserved by calorie restriction. Neuroreport. 1992;3(12):1085–1088. doi: 10.1097/00001756-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Liang YC, Wu HM, Chen YL, Lo SW, Ho WC. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus. 2002;12(6):787–802. doi: 10.1002/hipo.10032. [DOI] [PubMed] [Google Scholar]
- Jolitha AB, Subramanyam MV, Asha Devi S. Modification by vitamin E and exercise of oxidative stress in regions of aging rat brain: studies on superoxide dismutase isoenzymes and protein oxidation status. Exp Gerontol. 2006;41(8):753–763. doi: 10.1016/j.exger.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster T. Environmental enrichment decreases the afterhyperpolarization in senescent rats. Brain Res. 2007;1130(1):103–107. doi: 10.1016/j.brainres.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. 17 beta-Estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88(2):621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thinschmidt JS, Foster TC, King MA. Aging Effects on the Limits and Stability of Long-Term Synaptic Potentiation and Depression in Rat Hippocampal Area CA1. J Neurophysiol. 2007;98(2):594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol Learn Mem. 2005;83(3):206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226(4678):1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Lohmann P, Riepe MW. Spatial performance in a complex maze is associated with persistent long-term potentiation enhancement in mouse hippocampal slices at early training stages. Neuroscience. 2007;147(2):318–324. doi: 10.1016/j.neuroscience.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Leal-Galicia P, Castaneda-Bueno M, Quiroz-Baez R, Arias C. Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem. 2008;90(3):511–518. doi: 10.1016/j.nlm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bushnell PJ, Rezvani AH. Attention-modulating effects of cognitive enhancers. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lores-Arnaiz S, Bustamante J, Arismendi M, Vilas S, Paglia N, Basso N, Capani F, Coirini H, Costa JJ, Arnaiz MR. Extensive enriched environments protect old rats from the aging dependent impairment of spatial cognition, synaptic plasticity and nitric oxide production. Behav Brain Res. 2006;169(2):294–302. doi: 10.1016/j.bbr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Lou SJ, Liu JY, Chang H, Chen PJ. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008:121048–121055. doi: 10.1016/j.brainres.2008.02.080. [DOI] [PubMed] [Google Scholar]
- Marais L, Stein DJ, Daniels WM. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab Brain Dis. 2009;24(4):587–597. doi: 10.1007/s11011-009-9157-2. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87(3):559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Marques JM, Alonso I, Santos C, Silveira I, Olsson IA. The spatial learning phenotype of heterozygous leaner mice is robust to systematic variation of the housing environment. Comparative medicine. 2009;59(2):129–138. [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61(3):260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107(1):3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Mohammed AH, Henriksson BG, Soderstrom S, Ebendal T, Olsson T, Seckl JR. Environmental influences on the central nervous system and their implications for the aging rat. Behav Brain Res. 1993;57(2):183–191. doi: 10.1016/0166-4328(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16(6):1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Moore CI, Browning MD, Rose GM. Hippocampal plasticity induced by primed burst, but not long-term potentiation, stimulation is impaired in area CA1 of aged Fischer 344 rats. Hippocampus. 1993;3(1):57–66. doi: 10.1002/hipo.450030106. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55(1):78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Ohsawa I, Ohta S, Ohno M, Mikami T. Regular voluntary exercise cures stress-induced impairment of cognitive function and cell proliferation accompanied by increases in cerebral IGF-1 and GST activity in mice. Behav Brain Res. 2010;211(2):178–184. doi: 10.1016/j.bbr.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71(6):401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long- term potentiation reversal during aging. J Neurosci. 1996;16(17):5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19(10):1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176(2):362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Oh MM, Oliveira FA, Disterhoft JF. Learning and aging related changes in intrinsic neuronal excitability. Front Aging Neurosci. 2010;2:1–10. doi: 10.3389/neuro.24.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992;106(3):439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Sarmad S, Rattray I, Bates TE, Scullion GA, Marsden CA, Barrett DA, Lowe J, Kendall DA. Repeated novel cage exposure-induced improvement of early Alzheimer's-like cognitive and amyloid changes in TASTPM mice is unrelated to changes in brain endocannabinoids levels. Neurobiol Aging. 2009;30(7):1099–1113. doi: 10.1016/j.neurobiolaging.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behav Neurosci. 2006;120(4):787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- Potegal M. The caudate nucleus egocentric localization system. Acta Neurobiol Exp (Wars) 1972;32(2):479–494. [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. Effects of exercise on brain function: role of free radicals. Appl Physiol Nutr Metab. 2007;32(5):942–946. doi: 10.1139/H07-081. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101(1):3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Dutra MF, Ilha J, Biasibetti R, Quincozes-Santos A, Leite MC, Marcuzzo S, Achaval M, Goncalves CA. Treadmill training restores spatial cognitive deficits and neurochemical alterations in the hippocampus of rats submitted to an intracerebroventricular administration of streptozotocin. J Neural Transm. 2010;117(11):1295–1305. doi: 10.1007/s00702-010-0501-9. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73(1):209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Pallier PN, Brown VJ, Wurbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res. 2004;152(2):307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79(1):334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- Soffie M, Hahn K, Terao E, Eclancher F. Behavioural and glial changes in old rats following environmental enrichment. Behav Brain Res. 1999;101(1):37–49. doi: 10.1016/s0166-4328(98)00139-9. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010;31(11):1937–1949. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65(3):191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23(10):2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25(10):2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8(6):1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Old rats remember old tricks; memories of the water maze persist for 12 months. Behav Brain Res. 2002;136(1):247–255. doi: 10.1016/s0166-4328(02)00137-7. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen JG, Chin APMJ, Hopman-Rock M, van Mechelen W. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin J Sport Med. 2008;18(6):486–500. doi: 10.1097/JSM.0b013e3181845f0b. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76(3):356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070(1):124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Sacquet J, Nguyen V, Marien M, Jourdan F, Didier A. Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuro psychopharmacology. 2009;34(3):786–795. doi: 10.1038/npp.2008.191. [DOI] [PubMed] [Google Scholar]
- Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62(1):97–103. doi: 10.1016/s0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
- Winocur G. Environmental influences on cognitive decline in aged rats. Neurobiol Aging. 1998;19(6):589–597. doi: 10.1016/s0197-4580(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Novelty exploration elicits a reversal of acute stress-induced modulation of hippocampal synaptic plasticity in the rat. J Physiol. 2006;577(Pt 2):601–615. doi: 10.1113/jphysiol.2006.120386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem. 2007;87(2):257–263. doi: 10.1016/j.nlm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40(6):657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]


