Abstract
Background and purpose
Early brain injury is an important pathological process following subarachnoid hemorrhage (SAH). The goal of this study was to evaluate whether the Alpha7 nicotinic acetylcholine receptor (α7nAChR) agonist PNU-282987 attenuates early brain injury after SAH and whether α7nAChR stimulation is associated with down regulation of caspase activity via PI3K-Akt signaling.
Methods
The perforation model of SAH was performed and neurological score, body-weight loss and brain water content were evaluated 24 and 72 hours after surgery. Western blot and immunohistochemistry were used for quantification and localization of phosphorylated Akt (p-Akt) and Cleaved Caspase-3 (CC3). Neuronal cell death was quantified with TUNEL staining. α7nAChR antagonist Methylcaconitine and PI3K inhibitor Wortmannin were used to manipulate the proposed pathway and results were quantified with western blot.
Results
PNU-282987 improved neurological deficits both 24 and 72 hours after surgery and reduced brain water content in left hemispheres 24 hours after surgery. PNU-282987 significantly increased p-Akt levels and significantly decreased CC3 levels in ipsilateral hemispheres after SAH. Methylcaconitine and Wortmannin reversed effects of treatment. P-Akt and CC3 were co-localized to neurons in the ipsilateral basal cortex. P-Akt was mainly localized in TUNEL negative cells. PNU-282987 significantly reduced neuronal cell death in ipsilateral basal cortex.
Conclusion
α7nAChR stimulation decreased neuronal cell death, brain edema and improved neurological status in a rat perforation model of SAH. α7nAChR stimulation is associated with increasing phosphorylation of Akt and decreasing cleaved caspase-3 levels in neurons.
Keywords: Alpha7 nicotinic acetylcholine receptor, PNU-282987, Subarachnoid hemorrhage, Early Brain Injury, Apoptosis, Akt, Cleaved Caspase-3
Introduction
Subarachnoid hemorrhage (SAH), with an annual incidence of 7 per 100,000 and a case fatality rate of around 50%, is a severe condition caused in approximately 85% by rupture of intracranial aneurysm (1). Early brain injury is a major contributor to the high mortality and morbidity after SAH and apoptosis is a significant element of early brain injury (2). The PI3K-Akt pathway is an important pathway in neuronal cells, decreasing activation of pro-apoptotic caspases (3).
Alpha7 nicotinic acetylcholine receptors (α7nAChR) are ion channels expressed mainly in neurons, immune and endothelial cells where they are responsible for providing anti-apoptotic and anti-inflammatory effects (4, 5). The anti-apoptotic effect of α7nAChR activation in neuronal cells was reported to involve PI3K-Akt (5). The protective role of PI3K-Akt pathway in reducing cleaved caspase-3 (CC3) after SAH was previously described (6) however references regarding α7nAChR and SAH are still missing.
In this study we tested two hypotheses. (A) The α7nAChR agonist PNU-282987 attenuates early brain injury after SAH. (B) The α7nAChR stimulation is associated with PI3K-Akt signaling pathway which decreases pro-apoptotic caspase activation.
Material and Methods
Animals and Drugs
All experiments were approved by the IACUC of Loma Linda University. Adult male Sprague-Dawley rats (277–320g) were purchased from Harlan Laboratories (Indianapolis, Indiana). Animals were fed ad libitum. We used 134 animals in this study, assigned to following groups: Naïve (n=4), Sham (n=24), Vehicle (n=39), PNU-12 (n=36), PNU-4 (n=10), MLA (n=12) and WOR (n=9).
All drugs were purchased from Sigma-Aldrich. Drugs were dissolved and administered according to previously published data (7–10). α7nAChR agonist PNU-282987 was dissolved in saline and was injected intraperitoneally 1 hour after surgery in doses of 12mg/kg (PNU-12, MLA and WOR groups) or 4mg/kg (PNU-4 group). In the Naïve group, 12mg/kg PNU-282987 was injected intraperitoneally 15 minutes after initiation of physiological parameters monitoring. α7nAChR antagonist Methylcaconitine was dissolved in saline and injected intraperitoneally at a dose of 6mg/kg 15 minutes before administration of PNU-282987 (MLA group). PI3K inhibitor Wortmannin (Sigma-Aldrich) was dissolved in saline and injected intravenously at a dose of 15µg/kg 90 minutes before PNU-282987 administration.
Sham or Vehicle animals received equivalent volumes of saline given to animals in other groups (500µl).
Physiological parameters
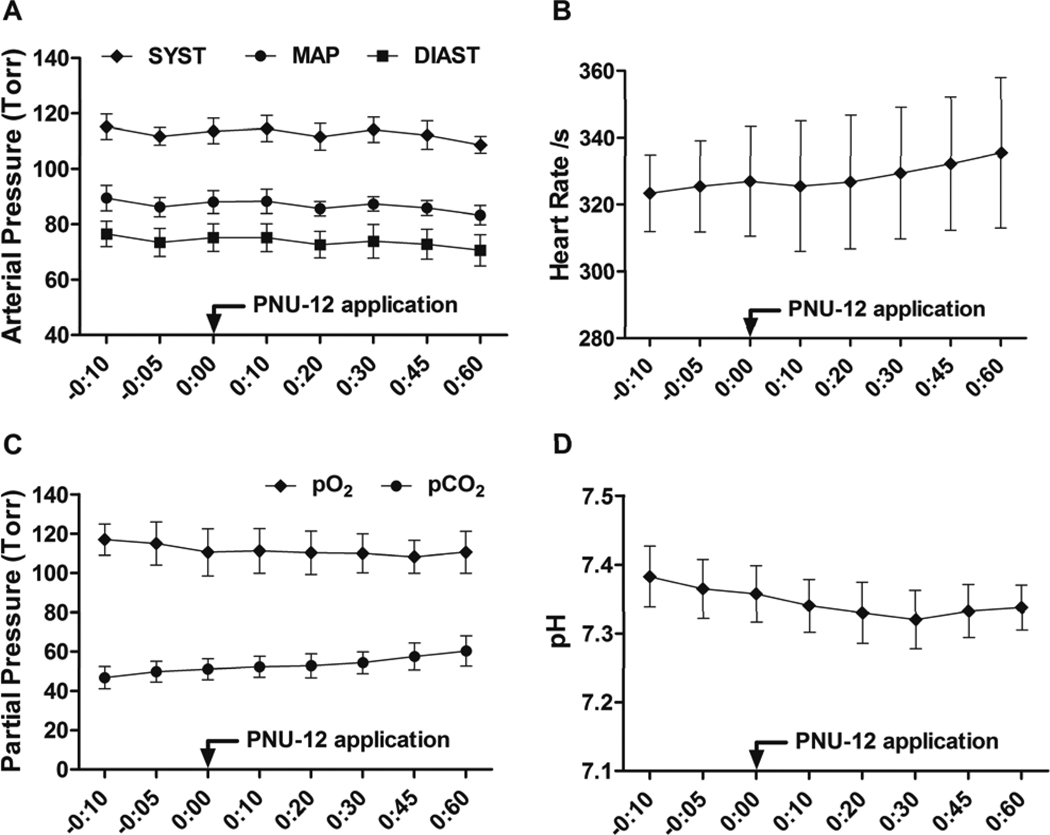
Blood pressure/heart rate measurements and analysis of blood samples were performed in naïve animals. Animals were anesthetized, intubated and kept on artificial ventilation using Isoflurane 3% in 60/40% medical gas/oxygen mixture. The right femoral artery was cannulated for invasive blood pressure/heart rate monitoring (DIGI-MED Blood Pressure Analyzer) and collection of blood samples for subsequent analysis (IL GEM 4000 Premiere automatic analyzer). The following parameters were evaluated from the blood samples: pH, pO2, pCO2, Na+, K+, Cl−, and Glucose. Monitoring was performed from 10 minutes before (3 measurements) and up to 60 minutes after (5 measurements) application of PNU-282987 (12mg/kg). Mean values of physiological parameters before PNU-282987 administration were compared to the mean values after administration. Selected data are shown in Figure 1 at each time point.
Figure 1. Effects of PNU-282987 on basic physiological parameters.
Arterial pressure (A), heart rate (B), pO2, pCO2 (C) and pH (D) from 10 minutes before to 60 minutes after application of PNU-282987 in naïve animals (N=4). Data are expressed as a mean and SEM. SYST: Systolic pressure, MAP: Mean arterial pressure, DIAST: Diastolic pressure.
Surgery
The endovascular perforation model of SAH was performed as previously described (11). Briefly, rats were anesthetized, intubated and kept on artificial ventilation during surgery with 3% Isoflurane in 60%/40% medical-air/oxygen. Body temperature was monitored by rectal probe and normo-thermia was maintained by a heating lamp. A sharpened 4-O nylon suture was introduced into the left internal carotid artery until resistance was felt (approximately 18 mm from the common carotid bifurcation). The suture was then pushed further to perforate the bifurcation of the anterior and middle cerebral arteries until resistance was overcome and withdrawn immediately after perforation. In sham operated animals the suture was inserted into the left carotid artery, however no perforation was performed. After suture removal the incision was closed, and rats were individually housed in heated cages until recovery.
Body weight and SAH grade
Body weight was measured after induction of anesthesia before intubation and before sacrifice. Results are expressed as a ratio of body weight after surgery to body weight before surgery.
SAH grade was evaluated as previously described (12). Briefly, after removing the brain, a picture of the underside of the brain was taken and pictures were divided into six parts (left and right frontal, left and right temporal, upper and lower brain stem). Each part was sub-scored according to occurrences of blood in the subarachnoid space and a total score (max 18) was calculated as the sum of all sub-scores. Animals with SAH score of 5 or less were excluded from the study for low SAH grade (10, 12).
Neurological Score
Neurological scores were evaluated before sacrifice in a blinded fashion using a Garcia scoring system (13). The following were evaluated: Spontaneous activity, symmetrical movements of limbs, forelimbs outstretching, climbing a wall of a wire cage, axillary touch response and vibrissae touch response. Minimal score was 0 (worst) and maximal 3 (the best) for each sub-test and total score was calculated as a sum of all sub-tests.
Brain Water Content
Brains were removed 24 or 72 hours after surgery and separated into left hemisphere, right hemisphere, cerebellum and brain stem. Each part was weighed immediately after removal (wet weight) and after drying in 100 °C for 72 hours (dry weight). The percentage of water content was calculated as [(wet weight- dry weight)/wet weight]*100%.
Western Blot
Animals were euthanized 24 hours after surgery and ipsilateral hemispheres were processed for Western Blot as previously described (11). Equal amounts (50ug) of total protein were separated in 12% SDS-PAGE and transferred onto nitrocellulose membranes. As primary antibodies we used anti-Phospho-Akt (Ser 473; 1:1000; Cell Signaling, #9271), anti-Cleaved Caspase-3 (1:1000; Cell Signaling, #9661) and anti-β-Actin (1:1000; Santa Cruz Biotechnology, SC-1616) and appropriate secondary antibodies (Santa Cruz Biotechnology) were used in dilution 1:1000. Bands were visualized using the enhanced chemiluminescence reagent kit (Amersham ECL plus kit, GE Healthcare UK Limited) and quantification was performed by optical density methods using ImageJ software (NIH). Results are expressed as a relative density to β-Actin subsequently normalized to the mean value of the Sham group.
Immunohistochemistry and TUNEL staining
Animals were euthanized 24 hours after surgery and brains were processed as previously described (14). Double-fluorescence labeling for NeuN (1:100, Millipore, MAB377) and CC3 (1:1000; Cell Signaling,#9661) or NeuN and p-Akt (Ser473; 1:25; Cell Signaling, #9271) was performed as described previously (14). TUNEL staining (In situ Cell Death Detection Kit, Roche Inc., Germany) either with NeuN and p-Akt for neuronal localization or with NeuN alone for cell counting was performed as previously described (3). For negative controls the same staining without primary antibody was performed. The stained sections were processed using a fluorescent microscope and Magna Fire SP system (Olympus). The TUNEL positive cells were counted in the ipsilateral basal cortex in 3 fields per animal at × 400 magnification and data was expressed as the number of TUNEL positive neurons/mm2 (15).
Statistical analysis
Data are expressed as a mean and standard error of the mean. Mortality data was analyzed by Fischer exact test. SAH grading data in the 72 hours study were evaluated by Unpaired t-test. Physiological parameters were evaluated by Paired t-test. All other data were analyzed by One-way ANOVA followed by Tukey post test. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 5.02 for Windows.
Results
We performed 134 surgeries, 29 animals died because of severe SAH and 11 animals were excluded because of low SAH grade: 5 animals in Vehicle group, 3 in PNU-12 group, 1 in PNU-4 group and 2 in MLA group. Mortality rate: Sham 0% (0 of 24 animals), naïve + PNU-12 0% (0 of 4 animals), Vehicle 29% (10 of 34 animals), PNU-12 27% (9 of 33 animals), PNU-4 33% (3 of 9 animals), MLA 40% (4 of 10 animals) and WOR 33% (3 of 9 animals). Mortality occurred within 6 hours after surgery and there were no statistically significant difference between Vehicle and PNU-12 group (p=NS, Fisher exact test).
Effect of PNU-282987 administration on physiological parameters
Blood pressure/heart rate monitoring and blood sample analysis (pH, pO2, pCO2, Na+, K+, Cl−, Glucose) was performed in naïve animals (n=4) from 10 minutes before and up to 60 minutes following application of PNU-282987 (12mg/kg).
There were no significant differences in any measured parameters between mean value before application and mean value after PNU application (p=NS, Table 1). Selected data (Blood pressure, heart rate, pH, pO2, pCO2) are also shown in Figure 1 at each time point.
Table 1.
Physiological parameters before and after application of PNU-282987 in naïve animals (n=4). Data are expressed as mean and SEM. MAP: Mean arterial pressure, SYST: Systolic pressure, DIAST: Diastolic pressure, HR: Heart rate.
| MAP (mmHg) |
SYST (mmHg) |
DIAST (mmHg) |
HR (/s) |
pO2 (mmHg) |
pCO2 (mmHg) |
pH | Na+ (mmol/l) |
K+ (mmol/l) |
Cl− (mmol/l) |
Glu (mg/dl) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Before PNU |
87.9 (±2.90) |
113.5 (±3.87) |
75.1 (±3.19) |
325.3 (±13.76) |
114.2 (±10.11) |
49.17 (±5.17) |
7.36 (±0.042) |
132.8 (±0.854) |
4.89 (±0.038) |
103.4 (±0.84) |
185.3 (±12.53) |
|
After PNU |
86.1 (±2.91) |
112.2 (±3.70) |
73.0 (±5.07) |
329.9 (±20.26) |
110.1 (±10.03) |
55.40 (±6.237) |
7.33 (±0.038) |
134.3 (±0.919) |
5.03 (±0.043) |
102.6 (±0.56) |
183.7 (±10.47) |
Outcome Study
Neurological score, body weight, and brain water content 24 hours after surgery
For the 24 hours outcome study we used Sham, Vehicle, PNU-12 and PNU-4 groups (n=6 in each group).
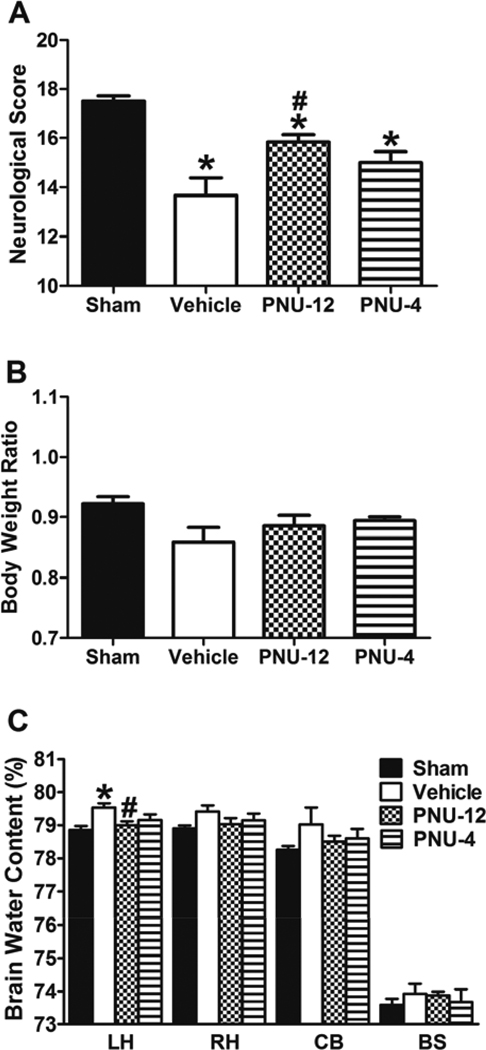
There was no significant difference in SAH grade between Vehicle (12.33 ± 0.71), PNU-12 (11.83 ± 1.01) and PNU-4 (11.67 ± 1.09) groups (p=NS). Neurological score (Figure 2A) was significantly higher in Sham group (17.50 ± 0.22) compared to Vehicle (13.67 ± 0.71), PNU-12 (15.83 ± 0.31) and PNU-4 (15.00 ± 0.45) groups 24 hours after surgery (p<0.05). Compared to Vehicle, neurological score was significantly higher in PNU-12 group (p<0.05), however there was no significant difference between Vehicle and PNU-4 group (p=NS). Body weight ratio 24 hours after surgery (Figure 2B) was not significantly different between groups (p=NS). Brain water content (Figure 2C) was significantly higher in the left hemisphere of Vehicle animals (79.54 ± 0.12) compared to Sham (78.86 ± 0.11) and PNU-12 (79.00 ± 0.12) groups (p<0.05). There was no significant difference in brain water content in left hemisphere between PNU-4 group (79.16 ± 0.17) and Vehicle group (p=NS). There were no significant difference between groups in brain water content in right hemisphere, cerebellum and brain stem (p=NS).
Figure 2. Effects of PNU-282987 at 24 hours after surgery.
Neurological score (A), body weight ratio (B) and brain water content (C) 24 hours after surgery. Data are expressed as a mean and SEM. * p<0.05 compared to Sham, # p<0.05 compared to vehicle. LH: Left hemisphere, RH: Right hemisphere, CB: Cerebellum, BS: Brain stem.
Neurological score, body weight, and brain water content 72 hours after surgery
At 72 hours we evaluated the same parameters as in the 24 hours outcome study in Sham, Vehicle and PNU-12 groups (n=6 in each group). We did not use 4mg/kg dose of PNU-282987, because this dosage did not show significant effects after 24 hours.
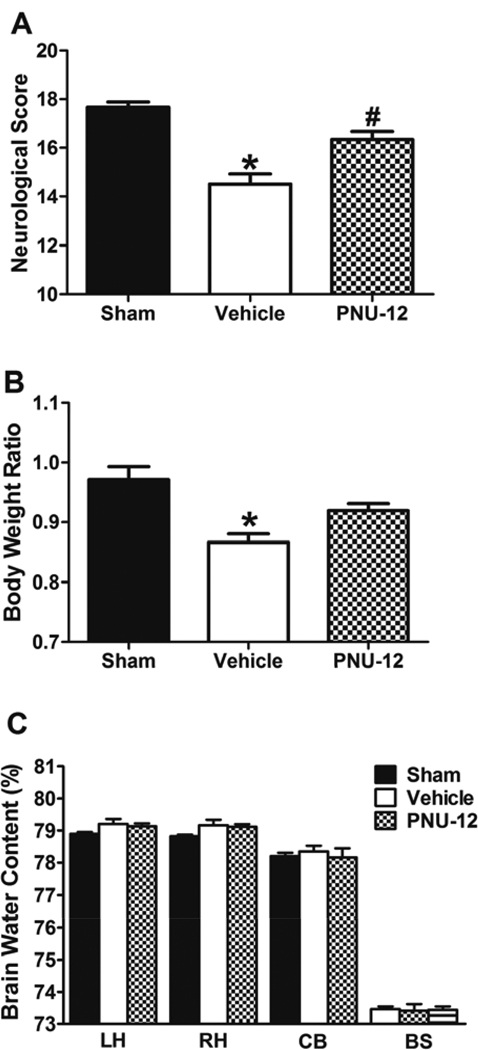
There was no significant difference in SAH grade between Vehicle (10.33 ± 0.92) and PNU-12 (11.50 ± 0.99) groups (p=NS). Neurological score (Figure 3A) was significantly lower in Vehicle group (14.50 ± 0.43) compared to Sham (17.67 ± 0.21) and PNU-12 (16.33 ± 0.33) groups (p<0.05). There was a significant difference in neurological score between Sham and PNU-12 groups (p=NS). Body weight ratio (Figure 3B) was significantly lower in Vehicle (0.87± 0.014) compared to Sham (0.97± 0.022) group (p<0.05). There were no significant differences in body weight ratio of PNU-12 (0.92 ± 0.012) compared to Sham or Vehicle groups (p=NS). Brain water content (Figure 3C) was not significantly different between groups in any other part of the brain 72 hours after surgery (p=NS).
Figure 3. Effects of PNU-282987 at 72 hours after surgery.
Neurological score (A), body weight ratio (B) and brain water content (C) 72 hours after surgery. Data are expressed as a mean and SEM. * p<0.05 compared to Sham, # p<0.05 compared to vehicle. LH: Left hemisphere, RH: Right hemisphere, CB: Cerebellum, BS: Brain stem.
Mechanism Study
Quantification of phosphorylated-Akt and cleaved caspase-3 at 24 hours after surgery
Western Blot analysis was used for quantification of p-Akt and CC3 levels in ipsilateral hemispheres 24 hours after surgery in Sham, Vehicle, PNU-12, MLA, and WOR animals (n=6 in each group).
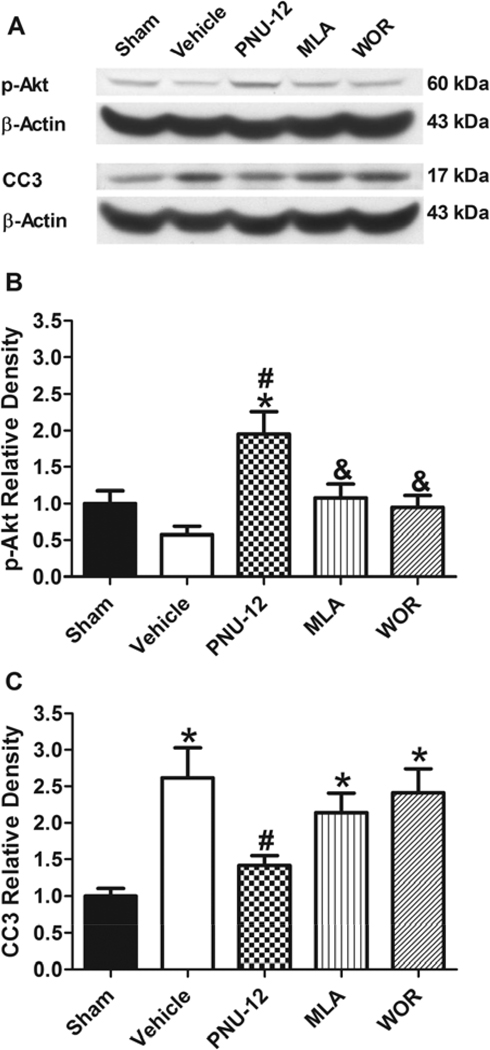
There was no significant difference in SAH grade between Vehicle (12.67± 0.56), PNU-12 (12.00 ± 0.73), MLA (11.33 ± 0.61) and WOR (11.17 ± 0.48) groups (p=NS). The levels of p-Akt (Figure 4A+4B) in the ipsilateral hemisphere was significantly higher in the PNU-12 group (1.95 ±0.31) compared to both Vehicle (0.58 ± 0.11) and Sham (1.00 ± 0.17) groups (p<0.05), while there was no significant difference between Sham and Vehicle groups (p=NS). P-Akt level was significantly lower in both MLA (1.08± ± 0.19) and WOR (0.95 ± 0.16) groups compared to the PNU-12 group (p<0.05). CC3 levels (Figure 4A+4C) were significantly higher in the Vehicle (2.62 ± 0.31) compared to Sham (1.00 ± 0.10) and PNU-12 (1.41 ± 0.14) groups (p<0.05), while there was no significant difference between Sham and PNU-12 groups (p=NS). CC3 levels in both MLA (2.16 ± 0.26) and WOR (2.41 ± 0.33) groups were significantly higher compared to Sham (p<0.05), however there was no significant difference between these and the PNU-12 group (p=NS).
Figure 4. Effect of PNU-282987 on p-Akt and CC3 at 24 hours after surgery.
Representative Western blots of p-Akt, CC3 and β-Actin at 24 hours after surgery (A), Quantitative analysis of p-Akt (B) and CC3 (C). Data are expressed as a mean and SEM. * p<0.05 compared to Sham, # p<0.05 compared to vehicle, & p<0.05 compared to treatment.
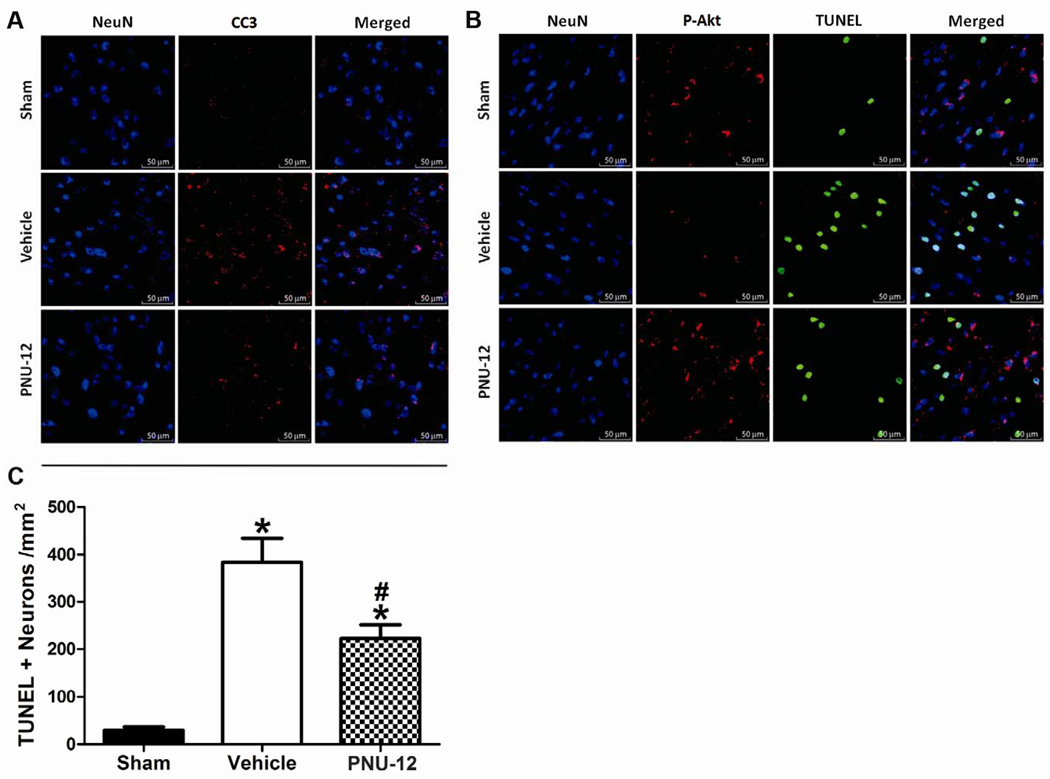
Neuronal co-localization of phosphorylated-Akt and cleaved caspase-3 immunoreactivity and neuronal cell death quantification
Localization of previously described changes was performed by immunohistochemistry, in Sham, Vehicle, PNU-12 groups (n=2 in each group). Neuronal cell death was quantified by TUNEL cell counting in the above groups (n=6 in each group). Ipsilateral basal cortex was the area of interest in all cases.
CC3 immunoreactivity (Figure 5A) was enhanced in the Vehicle group compared to both PNU-12 and Sham groups with good co-localization to neurons. P-Akt immunoreactivity (Figure 5B) in the PNU-12 group tended to be higher than the Sham group and even higher when compared to the Vehicle group. P-Akt signal was mainly co-localized to neurons and TUNEL positive cells did not generally co-localize with p-Akt positive cells. Number of TUNEL positive neurons in the ipsilateral basal cortex was significantly lower in Sham (29.67 ± 7.2) compared to both Vehicle (383.79 ± 50.6) and PNU-12 (222.51 ± 29.6) groups. There was statistically a significant difference in TUNEL positive neuron counts between Vehicle and PNU-12 groups (p<0.05, Figure 5C).
Figure 5. Neuronal localization of p-Akt, CC3, TUNEL staining and neuronal cell death quantification at 24 hours after surgery.
Representative immunofluorescence of NeuN and CC3 in the ipsilateral basal cortex (A), NeuN and p-Akt combined with TUNEL staining (B) and neuronal cell death quantification (C) in the ipsilateral basal cortex at 24 hours after surgery. Data are expressed as mean and SEM. * p<0.05 compared to Sham, # p<0.05 compared to vehicle.
Discussion
In this study we tested two hypotheses. (A) The α7nAChR agonist PNU-282987 attenuates early brain injury after SAH. (B) The α7nAChR stimulation is associated with PI3K-Akt signaling pathway which decreases pro-apoptotic caspase activation. In the descriptive part (A) we focused on brain water content, neurological score and body weight loss; while in the mechanistic part (B) we focused on the involvement of p-Akt mediated decrease in caspase activation and neuronal cell death after α7nAChR stimulation.
SAH grade, at each time point, was comparable between groups. This precaution was taken to allow comparison of measured parameters. All cases of mortality suffered severe SAH. The mortality rate was not improved by treatment with PNU-282987 compared to Vehicle possibly due SAH grade.
Basic physiological parameters were not affected by the application of PNU-282987 in our study. We performed monitoring for 60 minutes after PNU-282987, because this procedure is invasive and a longer exposure of to anesthesia could affect physiological parameters. Physiological parameters during our experiment were comparable with previous work from our lab (10, 14). PNU-282987 was previously reported to inhibit human ERG potassium channels however this adverse effect is associated with the structure of this drug, not with specific α7nAChR stimulation. It should be noted that a newer α7nAChR agonist with reduced hERG has been developed (16).
Neurological dysfunction is an important clinical and experimental outcome present after SAH. Neurological deficits follow the peak of brain edema after SAH (17). The Garcia test is widely used in animal models of SAH (10–12). In our experiment PNU-282987 improved neurological deficit at 24 hours in a dose dependent manner and significantly improved neurological function 72 hours after surgery.
Body weight can be considered as a nonspecific marker, providing information about the animal’s overall condition. In our experiment body weight was not significantly different between groups 24 hours after surgery. Body weight at 72 hours was significantly lower in Vehicle compared to Sham and PNU-282987 groups showed a tendency to decrease body weight loss. Given these results, we believe that body weight is influenced more by surgery than by SAH at 24 hours, and this effect is not present at 72 hours.
Brain edema is an important element of early brain injury, the etiology of which is cytotoxic and vasogenic (2). Brain edema peaks 24 after surgery in rat perforation model of SAH (17). Previous studies show increased brain water content after SAH in the left hemisphere only (17) as well as in all parts of brain (14). In our study PNU-282987 reduced brain water content in left hemispheres 24 hours after surgery in a dose dependent manner, while no significant differences in brain water content were found 72 hours after surgery.
The PI3K-Akt signaling pathway plays important roles in preventing apoptosis (18). Akt is a member of serine/threonine kinase family which is activated directly by PI3K-mediated phosphorylation. Phosphorylated Akt activates several anti-apoptotic mechanisms; among them is the regulation of BCL-2 family proteins which control pro-apoptotic caspase activation (3, 18). The anti-apoptotic role of PI3K-Akt pathway in neurons after SAH was previously reported (6) and the neuroprotective effects of α7nAChR stimulation via PI3K-Akt pathway was also reported (5). In our study we evaluated p-Akt and CC3 levels 24 hours after surgery in ipsilateral hemispheres and we used Methylcaconitine and Wortmannin to manipulate this anti-apoptotic pathway. Methylcaconitine is a potent competitive antagonist of α7nAChR (8), while Wortmannin is an irreversible inhibitor of PI3K, which is widely used to inhibit downstream Akt phophorylation (10). We did not evaluate total levels of Akt because expression of Akt was reported not to be changed after SAH (6) or after α7nAChR stimulation/inhibition (19,20). In our study p-Akt levels were increased in ipsilateral hemishperes after PNU-282987 treatment compared to Vehicle and both Methylcaconitine and Wortmannin reversed the effect of treatment. CC3 was increased in ipsilateral hemispheres in the Vehicle group compared to the PNU-282987 treated group and both Methylcaconitine and Wortmannin showed tendencies to reverse the effect of PNU-282987 treatment.
α7nAChR is reported in endothelial cells and immune cells (4) as well as neurons (5). Our focus centered around neuronal apoptosis. Therefore we used immunohistochemistry to localize our findings and found that both p-Akt and CC3 were co-localized to neurons in ipsilateral basal cortex. Additionally we used TUNEL staining, a marker for cells undergoing DNA fragmentation in the final phase of apoptotic cell death (21).
Apoptosis is an important component of early brain injury after SAH which occurs both in neurons and endothelial cells (22). In our study we used TUNEL staining for quantification of neuronal cell death in the ipsilateral basal cortex (22) and PNU-282987 significantly reduced neuronal cell death in this area 24 hours after SAH.
In this study we focused on PI3K-Akt signaling which plays a significant role in apoptosis, a major contributor to early brain injury after SAH. However there are also other mechanisms of α7nAChR stimulation which could have a beneficial effect after SAH. α7nAChR is a part of the cholinergic anti -inflammatory pathway (23) which attenuates the inflammatory response by decreasing both endothelial cell and macrophage activation (4). Inflammation is reported to be involved after SAH, both in early brain injury (24) and vasospasm (25). The cholinergic anti-inflammatory pathway is mostly associated with the parasympathetic nervous system, but α7nAChR stimulation is also reported to induce nitric oxide dependent dilation of the basilar artery associated with the sympathetic nervous system (26). Taken together the anti-apoptotic effect of α7nAChR stimulation in combination with the anti- inflammatory and ant-vasospatic effects make this a promising approach for treatment after SAH, however further studies are required to elucidate those beneficial mechanisms.
Conclusion
α7nAChR stimulation decreased neuronal cell death, brain edema and improved neurological status in a rat perforation model of SAH. α7nAChR stimulation is associated with increasing phosphorylation of Akt and decreasing cleaved caspase-3 levels in neurons. These results indicate that α7nAChR agonists are promising treatments after SAH.
Acknowledgments
Sources of Funding
This study is partially supported by NIH NS053407 to JHZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
none.
References
- 1.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Qu Y, Tang J, Chen D, Fu X, Mao M, et al. PI3K/Akt signaling pathway is required for neuroprotection of thalidomide on hypoxic-ischemic cortical neurons in vitro. Brain Res. 2010;1357:157–165. doi: 10.1016/j.brainres.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, et al. Tracey. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A. Acetylcholinesterase inhibitors used in treatment of Alzheimer's disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology. 2006;51:474–486. doi: 10.1016/j.neuropharm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Cheng G, Chunlei W, Pei W, Zhen L, Xiangzhen L. Simvastatin activates Akt/glycogen synthase kinase-3beta signal and inhibits caspase-3 activation after experimental subarachnoid hemorrhage. Vascul Pharmacol. 2010;52:77–83. doi: 10.1016/j.vph.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Waring JF, Abel S, Li J, Bitner RS, Nikkel AL, Blomme EA, et al. Analysis of gene expression profiles in rat hippocampus following treatment with nicotine and an alpha7 nAChR selective agonist. Neurosci Res. 2008;60:266–274. doi: 10.1016/j.neures.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP. A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods. 1995;61:113–118. doi: 10.1016/0165-0270(95)00032-p. [DOI] [PubMed] [Google Scholar]
- 9.Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 10.Sugawara T, Ayer R, Jadhav V, Chen W, Tsubokawa T, Zhang JH. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. 2008;86:3635–3643. doi: 10.1002/jnr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42:477–483. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- 16.Walker DP, Wishka DG, Piotrowski DW, Jia S, Reitz SC, Yates KM, et al. Design, synthesis, structure-activity relationship, and in vivo activity of azabicyclic aryl amides as alpha7 nicotinic acetylcholine receptor agonists. Bioorg Med Chem. 2006;14:8219–8248. doi: 10.1016/j.bmc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Thal SC, Sporer S, Klopotowski M, Thal SE, Woitzik J, Schmid-Elsaesser R, et al. Brain edema formation and neurological impairment after subarachnoid hemorrhage in rats. Laboratory investigation. J Neurosurg. 2009;111:988–994. doi: 10.3171/2009.3.JNS08412. [DOI] [PubMed] [Google Scholar]
- 18.Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, et al. alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 20.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, et al. In situ apoptotic cell labeling by the TUNEL method:improvement and evaluation on cell preparations. J Histochem Cytochem. 1996:959–968. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- 23.Marrero MB, Bencherif M, Lippiello PM, Lucas R. Application of alpha7 nicotinic acetylcholine receptor agonists in inflammatory diseases: an overview. Pharm Res. 2011;28:413–416. doi: 10.1007/s11095-010-0283-7. [DOI] [PubMed] [Google Scholar]
- 24.Prunell GF, Svendgaard NA, Alkass K, Mathiesen T. Inflammation in the brain after experimental subarachnoid hemorrhage. Neurosurgery. 2005;56:1082–1092. [PubMed] [Google Scholar]
- 25.Bavbek M, Polin R, Kwan AL, Arthur AS, Kassell NF, Lee KS. Monoclonal antibodies against ICAM-1 and CD18 attenuate cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Stroke. 1998;29:1930–1935. doi: 10.1161/01.str.29.9.1930. [DOI] [PubMed] [Google Scholar]
- 26.Si ML, Lee TJ. Alpha7-nicotinic acetylcholine receptors on cerebral perivascular sympathetic nerves mediate choline-induced nitrergic neurogenic vasodilation. Circ Res. 2002;91:62–69. doi: 10.1161/01.res.0000024417.79275.23. [DOI] [PubMed] [Google Scholar]