Abstract
Background
The pathophysiology of interstitial cystitis (IC) is unknown. Deficits in urothelial cell layers and autoimmune mechanisms may play a role.
Objective
To examine whether immunization of mice with recombinant mouse uroplakin II (rmUPK2), a bladder-specific protein, would provoke an autoimmune response sufficient to create an IC phenotype.
Design, setting, and participants
RmUPK2 complementary DNA was generated, transferred into a bacterial expression vector, and the generated protein was purified. Eight-week-old SWXJ female mice were immunized with rmUPK2 protein via subcutaneous injection of 200 µg of rmUPK2 protein in 200 µl of an emulsion.
Measurements
Mice were euthanized 5 wk after immunization. Axillary and inguinal lymph node cells were tested for antigen-specific responsiveness and cytokine production, serum isotype antibody titers against rmUPK2 were determined, and gene expression of inflammatory mediators was measured in the bladder and other organs. For functional analysis, mice were placed in urodynamic chambers for 24-h micturition frequency and total voided urine measurements.
Results and limitations
Immunization with rmUPK2 resulted in T-cell infiltration of the bladder urothelium and increased rmUPK2-specific serum antibody responses in the experimental autoimmune cystitis (EAC) mice models compared with controls. The ratio of bladder to body weight was increased in EAC mice. Quantitative reverse transcriptase polymerase chain reaction analysis showed elevated gene expression of tumor necrosis factor α, interferon γ, interleukin (IL)-17A, and IL-1β in bladder urothelium but not in other organs. Evaluation of 24-h micturition habits of EAC mice showed significantly increased urinary frequency (p < 0.02) and significantly decreased urine output per void (p < 0.021) when compared with control mice.
Conclusions
Our study showed that a bladder-specific autoimmune response sufficient to induce inflammation and EAC occurs in mice following immunization with rmUPK2. EAC mice displayed significant evidence of urinary frequency and decreased urine output per void. Further phenotype characterization of EAC mice should include evidence for pain and/or afferent hypersensitivity, and evidence of urothelial cell layer damage.
Keywords: Autoimmune, Animal model, Bladder, Cystitis, T cell
1. Introduction
Interstitial cystitis (IC) is a chronic sterile inflammation of the bladder [1,2], characterized by chronic symptoms of urinary frequency and urgency accompanied by discomfort or pain in the bladder and the lower abdomen, requiring the recent addition of the term painful bladder syndrome (PBS) [1,3,4]. IC/PBS primarily affects women, with a female-to-male ratio of 5:1 [1,5]. It is estimated that as many as a million people in the United States are affected by IC [6]. The etiology of IC remains unknown. Potential pathophysiologic causes proposed include inflammatory, neurogenic, autoimmune, vascular, or lymphatic disorders; self-destruction by loss of the glycosaminoglycan layer from superficial cells; and the presence of toxic substances in the urine [7]. IC may have multiple etiologies, all of which result in a similar clinical manifestation. A possible autoimmune etiopathogenesis for IC continues to trigger interest in the scientific community with increasing reports on the association between IC and other autoimmune diseases such as lupus erythematosus, rheumatoid arthritis, ulcerative colitis, and thyroiditis [8,9], and reports of higher incidence of autoantibodies in patients with IC [9]. Whether the chronic inflammation and consequent tissue damage expose bladder tissues to further noxious stimuli and thus eventually lead to an autoimmune response warrants further investigation.
Recently, the use of experimental autoimmunity by the induction of proinflammatory type 1 T-cell responses to targeted self-antigens has contributed to the creation of useful models of autoimmune conditions including autoimmune encephalomyelitis [10], autoimmune myocarditis [11], autoimmune oophoritis [12], and experimental autoimmune cystitis (EAC) that mimic the phenotype of human IC [13]. In our prototypic model we immunized mice with a lyophilized mouse bladder homogenate for induction of bladder autoimmunity. However, this method can induce nonspecific systemic autoimmune complications because the bladder homogenates. In the current study, we aimed to generate an enhanced and more specific EAC model by targeting uroplakin II (UPK2). Uroplakins (UPK 1, 2, and 3) are a family of integral membrane proteins of urothelium [14] highly expressed in bladder tissue. In a transgenic mouse model, UPK2 promoter-driven expression of SV40 induced bladder urothelium cancer specifically [15]. Multiple studies have demonstrated the stability of UPK2 expression in human bladder cancer cell lines [16]; therefore, the durable expression of UPK2 is a good target for the creation of EAC. We postulated that immunization with UPK2 would cause bladder-specific inflammation without any collateral or systemic autoimmune damage. Here we show that immunization of mice with rmUPK2 results in an autoimmune phenotype confined to the bladder that mimics many of the clinical and histopathologic features of human IC.
2. Materials and methods
2.1. Generation and purification of recombinant mouse uroplakin 2
Total RNA was extracted from mouse bladder with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The complementary DNA (cDNA) was synthesized with random hexamers (Applied Biosystems, Foster City, CA, USA) using M-MLV reverse transcriptase (Promega, Madison, WI, USA). Mouse UPK2 was subcloned into the pET 30b vector (EMD Chemicals, Gibbstown, NJ, USA) using NcoI and HindIII (NEB, Ipswich, MA, USA) restriction enzymes. The cloned vector was transformed into BL21 (DE3) and expressed at 20°C overnight (approximately 22 h) after the addition of 1 mM isopropyl β-D-1-thiogalactopyranoside. The expressed protein was purified using Ni-column affinity purification under denaturing conditions followed by reverse-phase high-performance liquid chromatography (HPLC) purification to remove any residual endotoxin.
2.2. Mice and immunization
SWXJ (H-2q,s) female mice (n = 90) were generated by mating SJL/J (H-2s) males with SWR/J (H-2q) females at Jackson Laboratory (Bar Harbor, ME, USA). At 6–8 wk of age, mice were injected subcutaneously in the abdominal flank with 200 µl of an emulsion of equal volumes of water and complete Freund’s adjuvant (CFA) with (EAC mice) or without (control mice) 200 µg of recombinant mouse uroplakin 2 (rmUPK2) protein and 400 µg of Mycobacteria tuberculosis H37RA (Difco, Detroit, MI, USA) as previously described [12]. This maximum concentration of rmUPK2 (200 µg) was chosen based on previous studies [12,17]. Mice were euthanized by asphyxiation with carbon dioxide (CO2) followed by cervical dislocation 5 wk after immunization. All protocols were preapproved by the institutional animal care and use committee of Case Western Reserve University in compliance with the Public Health Service policy on humane care and use of laboratory animals.
2.3. Cell culture and proliferation assay
As described previously [11,12,17,18], to determine immunogenicity, inguinal and axillary lymph node cells (LNCs) were removed from 10 mice 10 d after immunization with rmUPK2 and cultured as a single-cell suspension in 96-well flat-bottom microtiter Falcon plates (BD Biosciences, San Jose, CA, USA) at 3 × 105 cells per well in Dulbecco modified Eagle medium (DMEM) (Mediatech CellGro, Herndon, VA, USA) with 10% fetal bovine serum (HyClone), 5% HEPES buffer, 2% L-glutamine, and 1% penicillin/streptomycin (Invitrogen Life Technologies) added. Then rmUPK2 and recombinant mouse anti-Müllerian hormone (AMH) (as a control) were added in serial 10-fold dilutions to triplicate wells with positive control wells containing 2 µg/ml antimouse CD3 (BD Biosciences). In some experiments, CD4+ and CD8+ T cells were purified from 10-d primed LNC by positive selection using anti-CD4- and anti-CD8-coated magnetic beads and double passage through a MACS LS column using a MidiMACS cell separator (Miltenyi, Auburn, CA, USA). The purified cells were cultured with 5 × 105 gamma-irradiated (2000 rads) syngeneic splenocyte feeders. All cell cultures were incubated at 37°C in humidified air containing 5% CO2. After 96 h, wells were pulsed with [methyl-3H] thymidine (l µCi per well; specific activity 6.7 Ci/mmol; New England Nuclear, Boston, MA, USA) and harvested 16 h later by aspiration onto glass fiber filters.
2.4. Histologic analysis
Bladders were removed and fixed in 10% neutral formalin overnight. Paraffin-embedded tissue was cut into sections 5 mm thick and then stained with hematoxylin and eosin. Gross histologic observations were performed using light microscopy.
2.5. Cytokine enzyme-linked immunosorbent assays
Cytokine concentrations were determined by enzyme-linked immunosorbent assay (ELISA) measurement as described previously [12]. Briefly, 48-h supernatants of 10-d primed LNC were cultured in supplemented DMEM at 5 × 106 cells per well in 24-well flat-bottom Falcon plates (BD Biosciences) in the presence of 20 µg/ml rmUPK2 and rmAMH (as a control). Absorbance was measured at 405 nm using a model 550 ELISA microplate reader (Bio-Rad, Hercules, CA, USA).
2.6. Immunocytochemistry
Immunostaining was performed as described [12]. Briefly, unmasked and blocked formalin-fixed paraffin-embedded tissues at 6 µm were treated with a 1:50 dilution of rat antimouse CD3 (Novacastra, Newcastle Upon Tyne, UK) followed by a 1:100 dilution of mouse-adsorbed biotinylated goat antirat immunoglobulin (Ig) G (BD Biosciences). Slides were developed conventionally using streptavidin-horseradish peroxidase complex (Vector Laboratories, Burlingame, CA, USA) and examined by light microscopy.
2.7. Antibody isotyping
Isotype-specific antibody titers to rmUPK2 and rmAMH (as a control) were determined in serum samples from mice immunized with rmUPK2, according to the manufacturer’s instructions using the mouse MonoAB ID/SP ELISA kit (Zymed, Invitrogen, Carlsbad, CA, USA).
2.8. Micturition habits by urinary frequency-volume assessment
The 24-h micturition and drinking habits of mice were measured as described previously [19].
2.9. Real-time reverse transcriptase polymerase chain reaction
Total RNA was extracted from different tissues, converted to cDNA, and analyzed for gene expression by real-time reverse transcriptase polymerase chain reaction (RT-PCR) using the following primer pairs: interferon (IFN) γ, sense, TGATGGCCTGATTGTCTTTCAA, antisense, GGATATCTGGAGGAACTGGCAA; tumor necrosis factor (TNF) α, sense, CAAAGGGAGAGTGGTCAGGT, antisense, ATTGCACCTCAGGGAAGAGT; interleukin (IL) 1β, sense, GAGTGTGGATCCCAAGCAAT, antisense, AGACAGGCTTGTGCTCTGCT; IL-17A, sense, TCCACCGCAATGAAGAC, antisense, CTTTCCCTCCGCATTGAC; β-actin, sense, GGTCATCACTATTGGCAACG, antisense, ACGGATGTCAACGTCACACT.
2.10. Statistical analysis
The unpaired student t test was used to analyze differences in micturition frequency, mean urine output-to-micturition ratio, and ratio of bladder weight to body weight between rmUPK2 and control immunized mice. For RT-PCR experiments, statistical analysis was performed using a one-way analysis of variance with the Tukey post hoc test for comparisons between groups. The mean and standard error of three mice per group and standard deviations were calculated.
3. Results
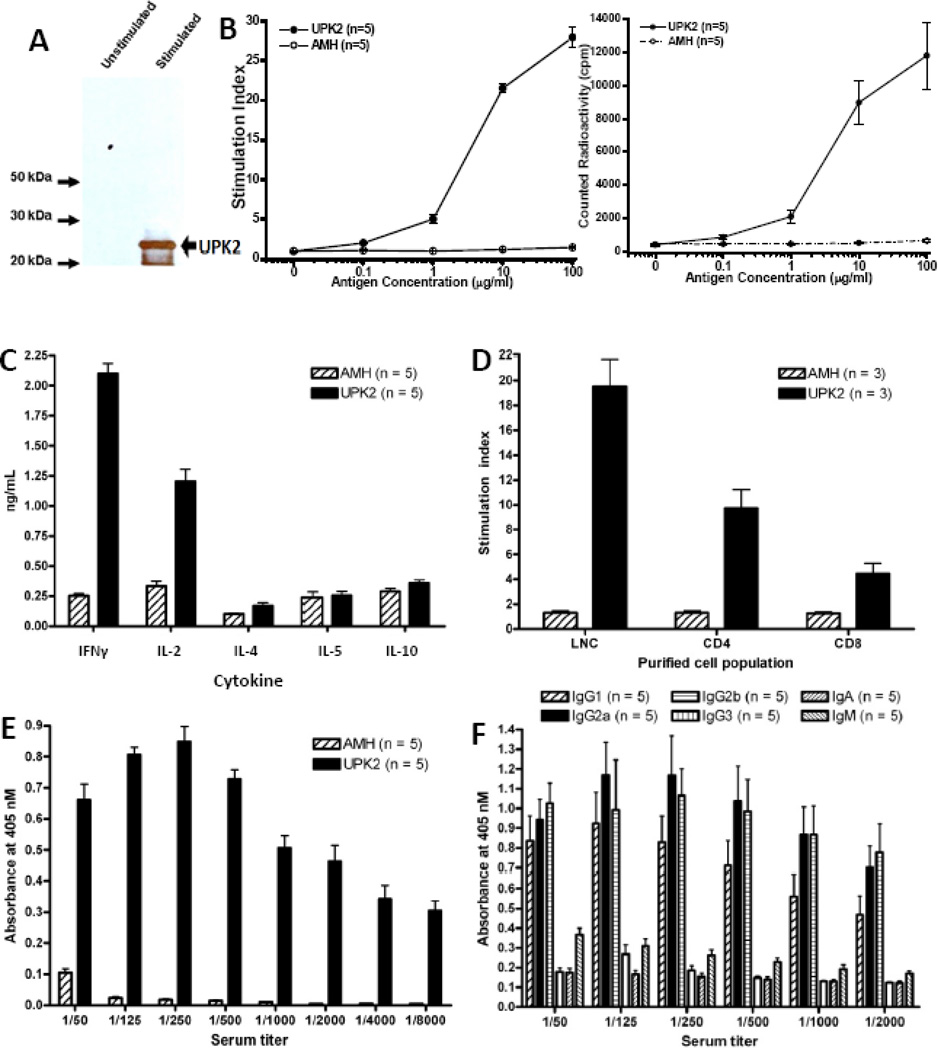
RmUPK2 was produced from a bacterial expression plasmid and purified using a 6X-His-tag mediated Ni-NTA affinity followed by reverse phase HPLC (Fig. 1a).
Fig. 1.
Characterization of the immune response to recombinant mouse uroplakin II (rmUPK2). (a) RmUPK2 was generated as an N-terminal 6X-His tagged fusion protein. An anti-His western blot performed after purification detected rmUPK2 as a prominent band with a secondary lower molecular weight band resulting from truncated transcription of the N-terminal His tagged protein. (b–d) Lymph node cells (LNCs) removed 10 d after immunization of SWXJ female mice (n = 10) with rmUPK2 were cultured as described to determine immunogenicity. (b) Antigen-specific recall proliferative responses were measured using scintillation spectrometry in cultured LNCs. Responses in LNCs were observed to 20 µg/ml rmUPK2 but not to 20 µg/ml recombinant mouse anti-Müllerian hormone (AMH) generated similarly using Escherichia coli transfection. Results expressed are the mean of triplicate or quadruplicate cultures pulsed with [3H]-thymidine graphed based on the stimulation index (left) or radioactivity in counts per minute (right) plus or minus the standard error. (c) Enzyme-linked immunosorbent assay (ELISA) analysis of cytokines from the supernatant of cultured LNCs demonstrated a proinflammatory type 1 phenotype with high production of inteferon-γ and interleukin (IL) 2 and low production of IL-4, IL-5, and IL-10 in response to rmUPK2. (d) Magnetic bead separation of cultured LNCs demonstrated recall proliferative responses from both CD4+ and CD8+ T cells purified (>90%) by (e) direct ELISA analysis of sera taken 5 wk after immunization with rmUPK2 or rmAMH. Results showed high titer-specific responses to rmUPK2 but not rmAMH even at titers of 1:8000. (f) Direct ELISA analysis of serum samples taken 5 wk after immunization with rmUPK2 demonstrated that the antibody response to rmUPK2 was predominantly a type 1 response involving high production of immunoglobulin (Ig) G2a and low production of IgG1 (n = 5). All values represent the mean plus or minus the standard deviation.
LNCs taken 10 d after immunization with rmUPK2 showed specific recall proliferative responses to rmUPK2 but were unresponsive to rmAMH, a control protein generated from Escherichia coli in a manner similar to rmUPK2 (Fig. 1b). LNCs responding to rmUPK2 showed a proinflammatory type 1 cytokine response characterized by enhanced expression of IFN-γ and IL-2 and low production of IL-4, IL-5, and IL-10 (Fig. 1c). Specific responses to rmUPK2 were elicited from both purified (>90%) CD4+ and CD8+ T cells (Fig. 1d). High-titer serum antibody responses were observed 5 wk after immunization with rmUPK2 (Fig. 1e) and consisted of a predominant type 1 response with elevated production of IgG2a and low production of IgG1 (Fig. 1f).
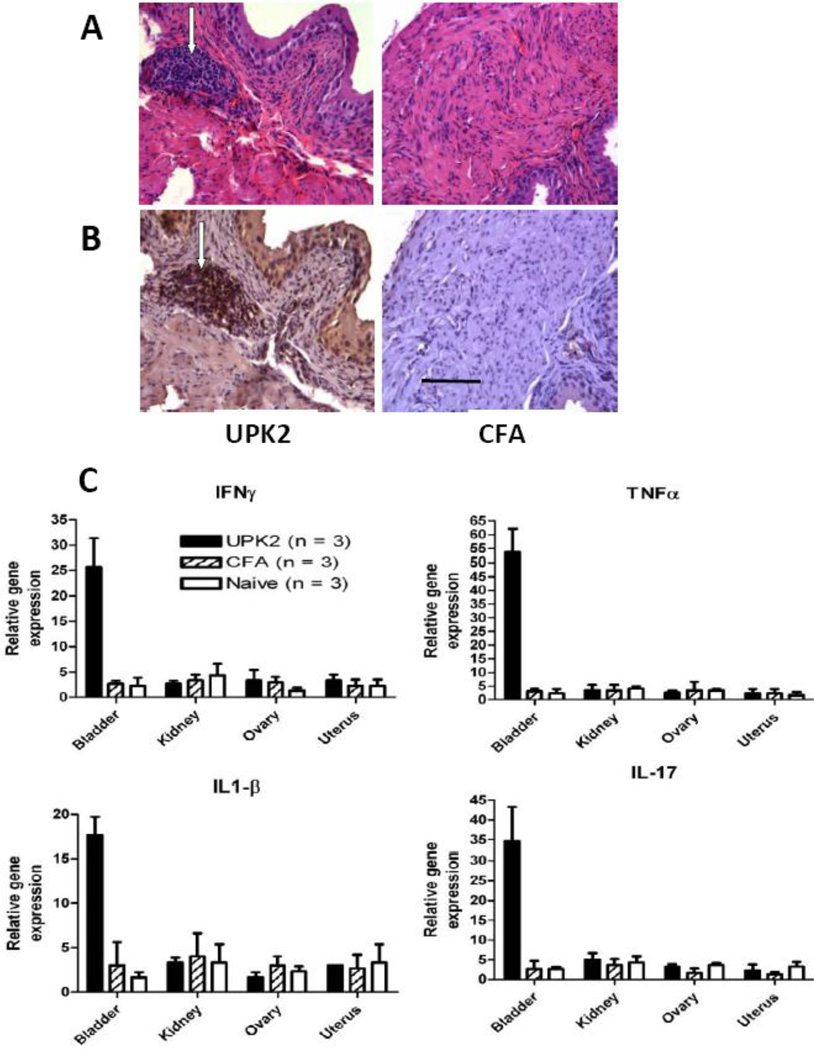
Histologic analysis of bladder tissue 5 wk after immunization with rmUPK2 showed extensive perivascular leukocytic infiltration (Fig. 2a, left) that was not evident in any bladder sections taken from control mice immunized with CFA alone (Fig. 2a, right). Immunocytochemical analysis showed that the bladder-infiltrating cells were predominantly CD3+ T cells (Fig. 2b, left) and that such T-cell infiltration was not evident in bladders from CFA-immunized control mice (Fig. 2b, right). RT-PCR analysis showed significantly (p < 0.001 for all) elevated gene expression levels of the inflammatory cytokines TNF-α, IL-17A, IFN-γ, and IL-1β in bladder tissue of mice immunized with rmUPK2 compared with bladders either from age- and sex-matched naive mice or from control mice immunized with CFA alone (Fig. 2c). Elevated gene expression levels of TNF-α, IL-17A, IFN-γ, and IL-1β were not observed (p > 0.05) in the ovary, kidney, or uterus of rmUPK2 immunized mice or from naive and CFA-immunized control mice (Fig. 2c).
Fig. 2.
Immunocytochemical and molecular analysis of bladder tissue from recombinant mouse uroplakin II (rmUPK2). (a) Example of hematoxylin and eosin–stained bladder sections taken 5 wk after immunization of a SWXJ female mouse with rmUPK2 showed extensive perivascular leukocytic infiltration (arrows in left panel) not evident in sections taken from a complete Freund adjuvant (CFA) immunized control mouse (right panel). (b) Example of immunostaining with CD3 antibody showed a predominance of T cells in the bladder infiltrates of a SWXJ female mouse immunized with rmUPK2 (arrows in left panel) not evident in anti-CD3 stained sections from a control mouse immunized with CFA alone (right panel). Solid bar = 50 µm for all tissue sections. (c) Quantitative reverse transcription polymerase chain reaction analysis showed significantly elevated gene expression levels of tumor necrosis factor α, interleukin (IL) 17A, interferon-γ, and IL-1β in the bladder but not in the ovary, kidney, or uterus of mice immunized with rmUPK2 compared with tissues taken from age- and sex-matched naive mice or control mice injected with CFA alone (* p < 0.001 for all). Relative gene expression was calculated as the ratio of individual gene expression to β-actin in each tissue type using the comparative threshold cycle method [17]. All values represent the mean plus or minus the standard deviation.
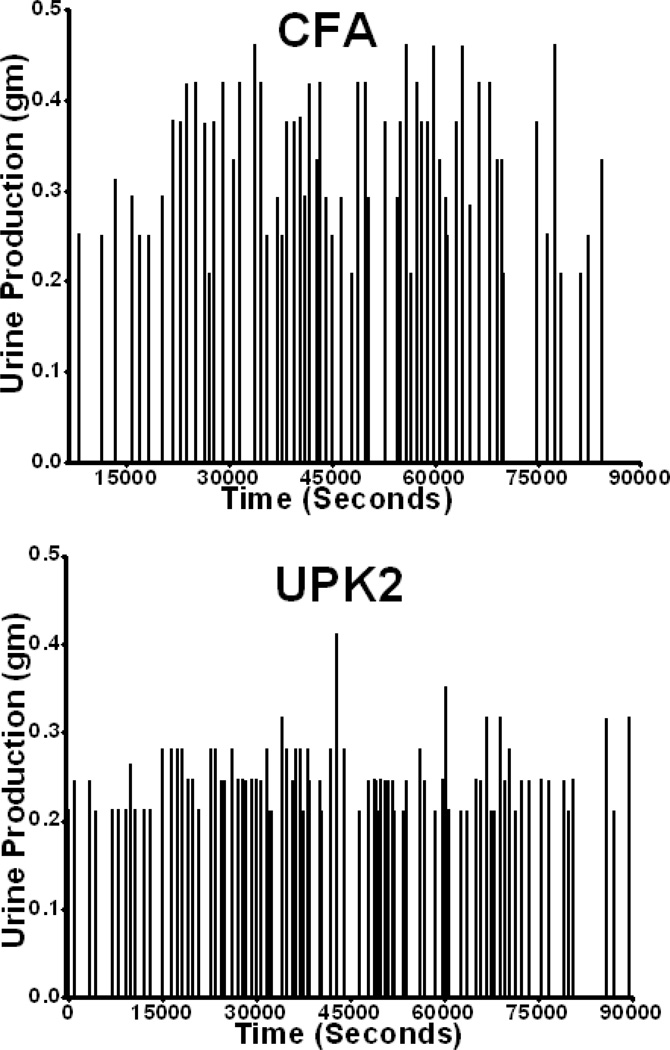
Functional analysis demonstrated that 5 wk after immunization with rmUPK2, mice had significantly increased urinary frequencies (75.70 ± 3.701 vs 64.52 ± 2.88; p = 0.02; Fig. 3a and 4) and significantly decreased mean urine outputs per void (0.31 ± 0.009 vs 0.34 ± 0.01; p = 0.02; Fig. 3b and 4). In addition, rmUPK2-immunized mice displayed significantly increased ratios of bladder weight (in milligrams) to body weight (in grams), a measure of organ inflammation and damage (0.63 ± 0.01 vs 0.56 ± 0.01; p = 0.003; Fig. 3c).
Fig. 3.
Bladder dysfunction in mice immunized with recombinant mouse uroplakin II (rmUPK2). (a) The 24-h micturition frequencies were significantly higher (*p = 0.02) 5 wk after immunization of SWXJ female mice with rmUPK2 compared with control mice immunized with complete Freund adjuvant (CFA) alone. (b) Inversely, urine output/micturition was significantly lower (*p = 0.02) in mice immunized with rmUPK2 compared with control mice injected with CFA alone. (c) Bladder weight (milligrams) to body weight (g) ratios were significantly higher (p = 0.003) in mice immunized with rmUPK2 compared with control mice injected with CFA alone. All values represent the mean plus or minus the standard deviation.
Fig. 4.
Representative micturition frequency and output traces from mice immunized with complete Freund adjuvant (CFA; upper panel) and recombinant mouse uroplakin II (rmUPK2; lower panel). Each vertical line represents a micturition event, and the height of each vertical line indicates the micturition output measured in grams.
4. Discussion
The pathophysiology of IC/PBS remains elusive. The most recent wave of initiatives by research-funding organizations such as the National Institutes of Health of the United States have led to an emphasis on combining translational and clinical approaches for improving our understanding of this mysterious disease and thus highlighting the importance of translational models (www.mappnetwork.org). At least 16 animal models have been investigated in the past 20 yr that mimic all or part of the IC/PBS phenotype [20]. Creation of most of these animals relies on some form of bladder insult such as inflammation induced by intravesical administration of an irritant or immune stimulant, systematic and environmentally induced inflammation (eg, glycerol, mustard oil, acetic acid, cyclophosphamide [CYP]), or autoimmunity [21–23]. The CYP-induced cystitis model is well characterized and used extensively to study chronic cystitis [24]. However, this model exhibits dissimilarities in its pathogenesis with that of IC/PBS. In 2004, a naturally occurring model of IC/PBS in domestic cats was characterized [25] although not widely implemented by other investigators.
In our initial report [23], we used female mice bladder homogenate to immunize female mice and evaluated bladder function 4 mo later. Immunized mice displayed bladder autoimmunity and significant evidence of increased frequencies of urination and decreased intercontraction intervals and urine output per void [23]. These observed abnormalities were comparable with those occurring in the human phenotype and encouraged us to develop a more targeted autoimmune attack directed against a bladder-specific antigen.
In the current study, we used a focused immune response against the bladder by targeting the bladder-specific protein UPK2 and analyzed bladder function to assess the role of autoimmunity in the induction of IC and IC-related phenotypes. Phenotypical characterization of our current model showed that 5 wk after immunization with rmUPK2, SWXJ female mice had significantly increased urinary frequencies and decreased urine outputs per void (urgency) mimicking two of three of the major symptoms of IC/PBS. In addition, rmUPK2-immunized mice showed significantly increased ratios of bladder weight to body weight, a simple but reliable measure of organ inflammation. These results indicate that immunization of SWXJ mice with rmUPK2 induces an organ-specific autoimmune disorder characterized by inflammation confined to the bladder that consistently leads to bladder dysfunction similar to that observed in UPK2-null mice [26]. Sublethal whole-body irradiation was used previously to ablate the immune system; however, this method was also shown to induce cystitis [27–29]. Therefore, its usefulness in isolating the role of immune-mediated changes in this model is limited.
Due to general challenges in demonstration and quantification of visceral pain in animal models, we have not presented evidence of pelvic pain in this phenotype characterization. Despite the availability of a number of pain assessment tools for murine models [21], assessment of visceral pain and localizing the source or demarcating the related referred area of pain is a challenging task. However, future studies using von Frey filaments to assess the severity of response to mechanical stimulation (tactile allodynia) could be performed to compare values before immunization and progression over time. Further analysis using a device recently developed by our group, bladder sensory perception threshold [30], integrated with the Neurometer diagnostic neurostimulator (Neurotron, Baltimore, MD, USA), could be used to measure afferent sensory function as a phenotyping measure of pelvic pain in rmUPK2-immunized mice with EAC [18]. Overall, we have demonstrated the organ-specific autoimmune nature of rmUPK2-immunized mice with EAC and suggest this is a potentially useful model for the future investigation of pathogenesis and therapeutic intervention of IC/PBS.
5. Conclusions
Our findings show that a clinically powerful autoimmune response occurs in mice immunized with rmUPK2. This autoimmune response is specific to bladder tissue, and the resultant EAC is characterized by significant evidence of urinary frequency and decreased urine output per void. Further characterization of EAC mice as a model for IC/PBS should include evidence for pain and/or afferent hypersensitivity, and assessment of urothelium damage.
Take-home message.
This study describes a new mouse model of interstitial cystitis (IC) with a bladder-specific autoimmune response that may be a potentially useful model for the investigation of the pathogenesis of and therapeutic interventions for IC.
Acknowledgment statement
The authors acknowledge Kerry O. Grimberg for her medical editorial assistance.
Funding/Support and role of the sponsor: This work was supported by National Institutes of Health grant R03HD-061825 (Firouz Daneshgari) and R01CA-14035 (Vincent K. Tuohy). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Firouz Daneshgari and Vincent K. Tuohy had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Altuntas, Tuohy, Daneshgari.
Acquisition of data: Altuntas, Gulen, Goksoy.
Analysis and interpretation of data: Altuntas, Sakalar, Tuohy, Daneshgari.
Drafting of the manuscript: Altuntas, Sakalar, Daneshgari, Tuohy.
Critical revision of the manuscript for important intellectual content: Altuntas, Tuohy, Daneshgari.
Statistical analysis: Altuntas.
Obtaining funding: Tuohy, Daneshgari.
Administrative, technical, or material support: Goksoy, Gulen, Kavran, Li, Qin.
Supervision: Qin, Li, Tuohy, Daneshgari.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Bogart LM, Berry SH, Clemens JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. J Urol. 2007;177:450–456. doi: 10.1016/j.juro.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Forrest JB, Vo Q. Observations on the presentation, diagnosis, and treatment of interstitial cystitis in men. Urology. 2001;57:26–29. doi: 10.1016/s0090-4295(01)01121-9. [DOI] [PubMed] [Google Scholar]
- 3.Hanno PM. Re-imagining interstitial cystitis. Urol Clin North Am. 2008;35:91–99. doi: 10.1016/j.ucl.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Toft BR, Nordling J. Recent developments of intravesical therapy of painful bladder syndrome/interstitial cystitis: a review. Curr Opin Urol. 2006;16:268–272. doi: 10.1097/01.mou.0000232048.81965.16. [DOI] [PubMed] [Google Scholar]
- 5.Clemens JQ, Meenan RT, O’Keefe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174:576–580. doi: 10.1097/01.ju.0000165170.43617.be. [DOI] [PubMed] [Google Scholar]
- 6.Rudick CN, Schaeffer AJ, Klumpp DJ. Pharmacologic attenuation of pelvic pain in a murine model of interstitial cystitis. BMC Urol. 2009;9:16. doi: 10.1186/1471-2490-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sand PK. Proposed pathogenesis of painful bladder syndrome/interstitial cystitis. J Reprod Med. 2006;51:234–240. [PubMed] [Google Scholar]
- 8.Van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat Clin Pract Urol. 2007;4:484–491. doi: 10.1038/ncpuro0874. [DOI] [PubMed] [Google Scholar]
- 9.Ochs RL. Autoantibodies and interstitial cystitis. Clin Lab Med. 1997;17:571–579. [PubMed] [Google Scholar]
- 10.Jaini R, Hannaman D, Johnson JM, et al. Gene-based intramuscular interferon-beta therapy for experimental autoimmune encephalomyelitis. Mol Ther. 2006;14:416–422. doi: 10.1016/j.ymthe.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Jane-wit D, Yu M, Edling AE, et al. A novel class II-binding motif selects peptides that mediate organ-specific autoimmune disease in SWXJ, SJL/J, and SWR/J mice. J Immunol. 2002;169:6507–6514. doi: 10.4049/jimmunol.169.11.6507. [DOI] [PubMed] [Google Scholar]
- 12.Altuntas CZ, Johnson JM, Tuohy VK. Autoimmune targeted disruption of the pituitary-ovarian axis causes premature ovarian failure. J Immunol. 2006;177:1988–1996. doi: 10.4049/jimmunol.177.3.1988. [DOI] [PubMed] [Google Scholar]
- 13.Lin YH, Liu G, Kavran M, et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. BJU Int. 2008;102:1724–1730. doi: 10.1111/j.1464-410X.2008.07891.x. [DOI] [PubMed] [Google Scholar]
- 14.Kong XT, Deng FM, Hu P, et al. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 2004;167:1195–1204. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saban MR, Towner R, Smith N, et al. Lymphatic vessel density and function in experimental bladder cancer. BMC Cancer. 2007;7:219. doi: 10.1186/1471-2407-7-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Kakehi Y, Zeng Y, et al. Uroplakin II as a promising marker for molecular diagnosis of nodal metastases from bladder cancer: comparison with cytokeratin 20. J Urol. 2005;174:2138–2142. doi: 10.1097/01.ju.0000181214.32390.75. discussion 2142–3. [DOI] [PubMed] [Google Scholar]
- 17.Jaini R, Kesaraju P, Johnson JM, et al. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat Med. 2010;16:799–803. doi: 10.1038/nm.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jane-wit D, Altuntas CZ, Monti J, et al. Sex-defined T-cell responses to cardiac self determine differential outcomes of murine dilated cardiomyopathy. Am J Pathol. 2008;172:11–21. doi: 10.2353/ajpath.2008.070324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin YH, Liu G, Kavran M, et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. BJU Int. 2008;102:1724–1730. doi: 10.1111/j.1464-410X.2008.07891.x. [DOI] [PubMed] [Google Scholar]
- 20.Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J Urol. 2002;167:694–702. doi: 10.1016/S0022-5347(01)69129-8. [DOI] [PubMed] [Google Scholar]
- 21.Rudick CN, Schaeffer AJ, Thumbikat P. Experimental autoimmune prostatitis induces chronic pelvic pain. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1268–R1275. doi: 10.1152/ajpregu.00836.2007. [DOI] [PubMed] [Google Scholar]
- 22.Altuntas CZ, Daneshgari F, Liu G, et al. Bladder dysfunction in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;203:58–63. doi: 10.1016/j.jneuroim.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YH, Liu G, Kavran M, et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model of interstitial cystitis. Br J Urol. 2008;102:1724–1730. doi: 10.1111/j.1464-410X.2008.07891.x. [DOI] [PubMed] [Google Scholar]
- 24.Vera PL, Iczkowski KA, Wang X, Meyer-Siegler KL. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003898. e3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. 2004;172:1242–1248. doi: 10.1097/01.ju.0000137953.49304.6c. [DOI] [PubMed] [Google Scholar]
- 26.Aboushwareb T, Zho G, Turner C. Urodynamic characterization of mice lacking uroplakin II or III. FASEB J. 2007;21:921–931. [Google Scholar]
- 27.Vale JA, Bowsher WG, Liu K, et al. Post-irradiation bladder dysfunction: development of a rat model. Urol Res. 1993;21:383–388. doi: 10.1007/BF00300073. [DOI] [PubMed] [Google Scholar]
- 28.Stewart FA, Lundbeck F, Oussoren Y, Luts A. Acute and late radiation damage in mouse bladder: a comparison of urination frequency and cystometry. Int J Radiat Oncol Biol Phys. 1991;21:1211–1219. doi: 10.1016/0360-3016(91)90278-c. [DOI] [PubMed] [Google Scholar]
- 29.Knowles JF. Radiation-induced hydronephrosis in the rat: a new experimental model. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;48:737–744. doi: 10.1080/09553008514551831. [DOI] [PubMed] [Google Scholar]
- 30.Abouassaly R, Liu G, Yamada Y, Ukimura O, Daneshgari F. Efficacy of a novel device for assessment of autonomic sensory function in the rat bladder. J Urol. 2008;179:1167–1172. doi: 10.1016/j.juro.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]