Abstract
Background and Purpose
Few studies have examined the early effects of statins on carotid artery elasticity, a potential surrogate marker of cardiovascular risk. This study examined the short-term effects of atorvastatin 80 mg daily on carotid elasticity measured by high resolution B-mode ultrasound.
Methods
The study included 40 stroke-free and statin-naïve subjects over age 45 (mean age 70±7 years; 55% men; 64% Caribbean-Hispanic). Outcome measures included carotid stiffness indices at 14 and 30 days after initiation of treatment. The systolic (SD) and diastolic (DD) diameters of the right common carotid artery were averaged from multiple B-mode imaging frames. Absolute and relative changes of Strain [(SD-DD)/DD], Stiffness (β) [ln (systolic/diastolic blood pressure)/strain] and distensibility (1/β adjusted for wall thickness) from baseline were compared by the repeated measures t-test and considered significant at α of 0.05.
Results
Baseline mean stiffness was 0.08 (95% CI: 0.06–0.10). It significantly decreased at Day 30 to 0.05 (CI: 0.04–0.06; p<0.01). Mean baseline distensibility was 15.25 [CI: 13.18–17.32], increasing significantly at Day 30 to 17.23 [CI: 14.01–20.45, p<0.05]. An improvement in distensibility of ≥ 10% from baseline was observed in 29 (73%) subjects. Changes in stiffness and distensibility were maximal among subjects with baseline low-density lipoprotein (LDL) levels <130 mg/dL.
Conclusions
Short-term treatment with high-dose atorvastatin was associated with improvement in the carotid elasticity metrics. Carotid artery elasticity measured by B-mode ultrasound is a simple non-invasive measure of arterial wall function and may be a useful surrogate endpoint in clinical trials targeting individuals at increased risk for atherosclerosis.
Keywords: statins, carotid arteries, elasticity, carotid ultrasound
Introduction
Endothelial dysfunction is among the earliest events in the cascade of atherogenesis. Much effort has focused on identifying non-invasive measures of endothelial function as well as on modifying endothelial dysfunction via pharmacologic or other means (1, 2). Identification of a simple, non-invasive method by which the immediate effects of therapy could be observed would be ideal, with potential application in both the research and clinical settings.
Carotid artery elasticity may serve as a surrogate marker for cardiovascular risk. Previous population-based studies including the Rotterdam study (3), the SMART study (4) and ARIC (5) have shown stiffness of the common carotid artery to be strongly associated with atherosclerosis and cardiovascular risk factors. In subjects with type 2 diabetes, carotid artery elasticity was related to cardiovascular risk factors as well as carotid intima-media thickness (IMT) (6). Recently, common carotid artery stiffness was shown to be an independent risk factor for ischemic stroke (7).
Arterial elasticity represents the ability of the arterial wall to contract and expand with the cardiac cycle. Increased stiffness and decreased distensibility represent impairments of the artery wall function which may be an early step in development of atherosclerosis. Previous work from our group has demonstrated good reproducibility and reliability of B-mode ultrasound measurements of common carotid artery diameters during the cardiac cycle and derived metrics of carotid elasticity (8).
Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, are well known to have beneficial effects on arterial wall properties including improvement of an ultrasound measure of flow-mediated dilatation (FMD), endothelium-dependent vasodilatation of the brachial artery (9, 10). One group found that 1 year of statin treatment improved carotid artery stiffness (11), but the early impact of statins on carotid artery wall function has not been investigated.
This study aimed to examine the effects of atorvastatin on metrics of carotid artery wall elasticity including stiffness and distensibility at 14 and 30 days after initiation of atorvastatin using high-resolution B-mode carotid ultrasound imaging. We hypothesized that carotid artery stiffness would decrease with a concomitant increase in carotid artery distensibility within 30 days of initiation of atorvastatin and that this effect would be independent of the degree of lipid-lowering effect.
Materials and Methods
Subjects
This study included 40 stroke-free and statin-naive subjects over age 45who were eligible for treatment according to the National Cholesterol Education Program: Adult Treatment Panel III (12). To be eligible, participants were required to have either: (a) coronary heart disease (CHD) or CHD-equivalent (peripheral artery disease, abdominal aortic aneurysm, symptomatic carotid artery disease, diabetes, or multiple risk factors conferring a 10-year risk for CHD >20%); (b) ≥ 2 risk factors for CHD and an LDL ≥ 130 mg/dL; or (c) < 2 risk factors and an LDL ≥ 160 mg/dL. NCEP ATP III risk factors for CHD include: (a) age: men ≥ 45 years; women ≥ 55 years; (b) hypertension: blood pressure ≥ 140/90 mmHg, or need for antihypertensive medication; (c) high-density lipoprotein (HDL) <40 mg/dL; (d) cigarette smoking; and (e) family history of premature CHD: CHD in a male first-degree relative <55 years or in a female first-degree relative < 65 years.
Exclusion criteria were any use of fibrates or other lipid-lowering medication; hospitalization for acute coronary syndrome within the past 6 months; hepatic or renal dysfunction; connective tissue or chronic inflammatory disease; history of malignancy; any acute illness; leukocytosis (white blood cell count >10,000/cu mm); thrombocytosis (platelet count >450,000/cu mm); anemia (hematocrit <40%); corticosteroid use; pregnancy; and breastfeeding.
The study was performed in the Department of Neurology at Columbia University, NY. It was approved by the Western Institutional Review Board. Written informed consent was obtained from all participants.
All subjects received atorvastatin 80 mg daily for 30 days. Study assessments at each of 3 visits (baseline prior to atorvastatin, Day 14, and Day 30) consisted of a carotid scan, blood pressure measurements, and fasting laboratory assays for lipids and liver enzymes. Subjects were also questioned regarding adverse effects including muscle pain, weakness, and gastrointestinal complaints.
Measurement of blood pressure
After resting for 10 minutes in the supine position, subjects’ blood pressures were obtained using a semi-automated oscillometric blood pressure recorder (Dinamap Pro100; Critikon, Inc, Tampa, FL) on the right brachial artery. Blood pressures were measured before and after the carotid ultrasound examination and then averaged.
Measurement of carotid artery elasticity
Subjects underwent carotid artery scanning at baseline before initiation of atorvastatin, on Day 14, and on Day 30 using high-resolution B-mode ultrasound on a GE LOGIQ 700 system (GE Healthcare, Milwaukee, WI). The right common carotid artery (CCA) was scanned using an 11 MHz linear-array transducer according to a standard protocol as previously reported (8). In brief, the systolic (SD) and diastolic (DD) diameters of the right CCA were obtained by measuring 10 mm of the right CCA below the origin of the carotid bulb. Offline measurement was performed using Image Pro image analysis software (Microsoft Corporation, Redmond, WA). Carotid IMT was measured in the same 10 mm segment using Image Pro according to the previously reported protocol (8, 13). Both near and far wall interfaces defining the blood-intima boundaries were maximized and clearly depicted on B-mode images. M-mode images were obtained perpendicular to the arterial walls and were adjusted for the clearest representation of the CCA walls throughout the cardiac cycle. Two wall interfaces were tracked in up to 10 consecutive cardiac cycles. SD and DD were measured from the three B/M mode registrations and averaged. Inter- and intra-observer reproducibility was high in our previous reports (8, 13).
Carotid artery elasticity metrics were calculated as follows:
; where SD was the systolic CCA diameter, and DD was the diastolic CCA diameter. Strain was expressed as a percent change, representing the amount of wall deformation compared to the unstressed state.
; where SBP and DBP were mean systolic and diastolic brachial blood pressures. Stiffness represents a stress-to-strain ratio.
Statistical analysis
Carotid elasticity parameters and blood test results are expressed as means (± SD) and inter-quartile ranges. ANOVA was used to compare elasticity parameters at baseline, Day 14, and Day 30. Absolute changes in stiffness and distensibility were compared between Day 14 (or Day 30) and baseline using the paired, repeated measures t test. Relative changes in these parameters were also calculated by dividing percent differences from baseline by baseline values, multiplied by 100. An improvement of relative change in these parameters was defined as a decrease of 10% or more in carotid stiffness and an increase of 10% or more in carotid distensibility. Improvement in stiffness and distensibility (vs. non-improvement) was compared between Day 14 (or Day 30) and baseline. Stratified analyses by the LDL levels at baseline (< 130 vs. ≥ 130 mg/dL) were performed. Differences were one-tailed and considered statistically significant at α= 0.05.
Results
The mean subject age was 70 ± 7 years, and 55% were men; 64% were Caribbean-Hispanic, 24% were African-American, and 12% were Caucasian.
There was a significant treatment effect of atorvastatin on reduction of both total and LDL cholesterol levels compared to baseline (p<0.01). LDL decreased from a mean baseline level of 143.98±38.44mg/dL to 70.08±25.40mg/dL at Day 30. Total carotid IMT did not change from baseline (baseline IMT 0.81±0.09 mm; 30 Day IMT 0.79±0.08 mm; p=0.275).
Atorvastatin significantly affected metrics of carotid artery elasticity. Compared to baseline [0.08, 95% confidence interval, CI: 0.06–0.10], carotid artery stiffness decreased at Day 14 [0.07, CI: 0.05–0.08] and significantly decreased at Day 30 [0.05, CI: 0.04–0.06, p<0.01] as shown in Figure 1. Carotid artery distensibility increased with atorvastatin treatment as illustrated in Figure 2. Compared to baseline [15.25, CI: 13.18–17.32], distensibility was increased at Day 14 [16.09, CI 13.70–18.48] and was significantly increased at Day 30 [17.23, CI: 14.01–20.45, p<0.05].
Figure 1.
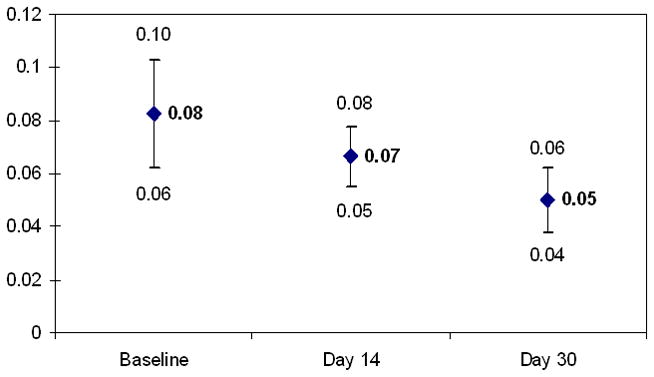
Mean carotid artery stiffness and 95% confidence intervals at Baseline, Day 14, and Day 30 of atorvastatin treatment (Baseline vs. Day 30, t test, p<0.01)
Figure 2.

Mean carotid artery distensibility and 95% confidence intervals at Baseline, Day 14, and Day 30 of atorvastatin treatment (Baseline vs. Day 30, t test, p<0.05)
The overall 30 Day decline in LDL cholesterol from baseline was not significantly correlated with the change in elasticity (p=0.539). However, changes in carotid artery stiffness and distensibility were significant among subjects with baseline LDL levels<130 in comparison to those with baseline LDL ≥130. As shown in Figure 3, more subjects (25%) experienced an improvement of 10% or more in carotid artery stiffness from baseline if the baseline LDL was <130 compared to those with baseline LDL ≥130 (13%). Carotid artery stiffness was not significantly different between the two groups at baseline [0.09 ±0.04 in the group with baseline LDL<130; and 0.05 ± 0.02 in the group with LD ≥ 130, p=0.333] (data not shown).
Figure 3.

Changes in carotid stiffness (improvement of 10% or more from baseline) by the LDL levels
Similarly, changes in carotid artery distensibility differed by baseline LDL level as shown in Figure 4. More subjects experienced improvement of 10% or more in carotid artery distensibility after atorvastatin treatment if their baseline LDL was <130 (22%) compared to those with baseline LDL ≥130 (13%). Carotid artery distensibility was not significantly different between the two groups at baseline; 11.65±6.93 in the group with baseline LDL < 130, and 21.14 ±12.80 in the group with LDL ≥130 (p=0.166) (data not shown).
Figure 4.

Changes in carotid distensibility (improvement of 10% or more from baseline) by the LDL levels
Discussion
In the current study, we observed a significant improvement in carotid artery elasticity (reduced stiffness and increased distensibility) 30 days following initiation of treatment with atorvastatin. This effect was more pronounced among those subjects with baseline LDL levels below 130 mg/dL and regardless of the degree of reduction in LDL and total cholesterol levels.
These findings are consistent with previous clinical studies demonstrating the beneficial effects of statins on endothelial function using brachial artery FMD (14–16). The present study extends these observations to the improvement of arterial function in the carotid artery, another vascular bed commonly affected by early atherosclerosis, and demonstrates that changes may occur as early as 30 days after treatment initiation.
Several possible mechanisms may explain the observed results, further highlighting the pleiotropic effects of statins (17). First, atorvastatin is known to influence the bioavailability of nitric oxide via effects on endothelial nitric oxide synthase (eNOS). Simvastatin and lovastatin have been shown to induce transcriptional activation of the nitric oxide [NO] synthase gene in human endothelial cells in vitro (18). Atorvastatin may also improve endothelial function by this mechanism (16,19,20). Second, atorvastatin reduces oxidized LDL which thus decreases degradation of NO produced by the endothelium and increases vasodilatation reserve, by which it may improve carotid distensibility. Statins reduce the copper-catalyzed LDL oxidation by increasing the proportion of protein within the LDL particle (21) and decreasing superoxide anion formation by macrophages (22), decreasing the oxidative aldehyde production derived from LDL oxidation. Furthermore, statins can downregulate Angiotensin II receptor in the endothelium (23) with subsequent relative indifference to the potent vasoconstrictor Angiotensin II and also decrease the enhanced release of free radicals, which might account for the vasodilator effect on the arterial wall. Finally, statins affect smooth muscle cell (SMC) maintenance, migration, and proliferation, making the SMC resistant to apoptosis (24). These effects are known to be independent of the effect on LDL reduction.
The anti-inflammatory properties of atorvastatin may also contribute to the observed effects on carotid artery elasticity. Decreased inflammatory activity in both peripheral blood mononuclear cells and in carotid atherosclerotic plaque was observed among patients treated with 80 mg of atorvastatin daily for 1 month (25). A recent randomized trial found that 4 days of atorvastatin treatment may prevent the potentially deleterious effects of inflammation on endothelial function measured by brachial FMD (26).
The beneficial effects on endothelial function appear unique to statins and are not seen, for example, with other cholesterol-lowering agents such as ezetimibe. In one study, endothelial function was studied by measuring forearm blood flow responses to acetylcholine and sodium nitroprusside using venous occlusion plethysmography in patients with stable coronary artery disease (27). Both atorvastatin in dose escalation from 10 mg to 40 mg and atorvastatin initiation with 40 mg improved endothelial function after treatment for 4 weeks, but ezetimibe had no effect despite a comparable reduction in LDL levels.
Several explanations may exist for the pronounced effect of atorvastatin on carotid elasticity among subjects with lower baseline LDL levels observed in our study. The short-term duration of treatment could produce improvement of arterial wall function among those with lower LDL levels but not among those with greater LDL because they may have had a lower degree of pro-inflammatory state since elevated LDL levels are well-known to decrease the bioavailability of endothelium-derived NO and to downregulate endothelial eNOS (28). Also, the absolute LDL cholesterol levels measured after treatment were lower among those who started with lower LDL levels, although a similar relative LDL reduction was achieved for all. Second, those with higher pre-treatment LDL levels might have already had more structural atherosclerotic wall changes and therefore the effect of atorvastatin on the arterial wall function was less effective. In our measure of carotid distensibility however, we have accounted for the carotid artery wall thickness (IMT), a measure of the structural atherosclerotic changes. Regardless, the same observation remained indicating that the alterations in the arterial wall architecture (e.g. changes in elastin/collagen ratio) may be more important for arterial wall function than arterial wall thickness per se (29). Finally this observation may have been found by chance due to a small number of patients in the stratified analyses.
Future studies are needed to establish the dose-response effect of atorvastatin on arterial elasticity. A previous study found that 10 mg and 20 mg of atorvastatin had similar effects on endothelium-dependent vasodilatation and carotid artery distensibility in patients with ischemic heart disease and hyperlipidemia (30). However, in a study of patients heterozygous for familial hypercholesterolemia (31), 80 mg of atorvastatin led to a significant improvement in endothelial function as measured by FMD whereas 20 mg had no significant effect. Meanwhile, the FMD results were independent of the reduction in LDL. While previous data would suggest a class-effect rather than a drug effect, future studies are needed to determine if our observations are specific to atorvastatin.
Limitations of the present study include the small sample size and the population demographics, which included predominantly elderly Caribbean-Hispanics. No control group was available for comparison. Results were not adjusted for changes in blood pressure or for anti-hypertensive medication use which could also influence carotid artery elasticity and/or carotid IMT. Withdrawal of atorvastatin with additional measurements to test the reversibility of the effect would also be informative. While previous work has shown B-mode ultrasound measures of carotid elasticity to be reliable (8), small errors in measurement of the artery diameter can lead to variations in the derived metrics. This however would bias the observed associations towards null.
Future research should focus on standardizing protocols and techniques for measuring carotid artery elasticity. Currently there is no recommended gold standard measure of arterial elasticity for clinical research. More knowledge is needed in the area of reliability and predictive validity prior to widespread use of this technique in clinical practice. Clearly, advantages of measuring carotid artery elasticity by ultrasound lie in its simplicity, user-friendliness, accessibility, and its ability to readily detect changes within weeks. Traditionally, months to years have been required to see the pharmacologic effects on structural atherosclerotic lesions such as carotid plaque or IMT imaged by 2-dimensional ultrasound. In addition to carotid artery elasticity however, novel ultrasound techniques such as 3-dimensional measurement of plaque volume may also provide a useful tool for assessing the short-term effects of new therapies on atherosclerosis (32).
In conclusion, the current study clearly demonstrated an improvement in carotid elasticity with short-term administration of high-dose atorvastatin. Whether improvement in carotid elasticity translates into better cardiovascular outcomes is yet to be proven. Measurement of carotid elasticity holds promise as another potential surrogate endpoint in clinical trials targeting individuals at increased risk for atherosclerosis.
Acknowledgments
Sources of Funding
We want to thank Pfizer, Inc. for the support of this study (an investigator-initiated study granted to Dr. Rundek). Pfizer, Inc. supplied the atorvastatin medication for the subjects enrolled in this study. Pfizer, Inc. had no involvement in the design or conduct of this study, or in the analyses, results or preparation of this manuscript.
Footnotes
Conflict of interest: None.
References
- 1.Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: A meta-analysis of randomized controlled trials. Atherosclerosis. 2011;216:7–16. doi: 10.1016/j.atherosclerosis.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 2.Walsh SR, Tang T, Sadat U, Dutka DP, Gaunt ME. Cardioprotection by remote ischaemic preconditioning. Br J Anaesth. 2007;99:611–616. doi: 10.1093/bja/aem273. [DOI] [PubMed] [Google Scholar]
- 3.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: The Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 4.Simons PCG, Algra A, Bots ML, Grobbee DE, van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: Indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of Arterial Disease) Circulation. 1999;100:951–957. doi: 10.1161/01.cir.100.9.951. [DOI] [PubMed] [Google Scholar]
- 5.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 6.Okimoto H, Ishigaki Y, Koiwa Y, Hinokio Y, Ogihara T, Suzuki S, et al. A novel method for evaluating human carotid artery elasticity: Possible detection of early stage atherosclerosis in subjects with type 2 diabetes. Atherosclerosis. 2008;196:391–397. doi: 10.1016/j.atherosclerosis.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Tsivgoulis G, Vemmos K, Papamichael C, Spengos K, Daffertshofer M, Cimboneriu A, et al. Common carotid arterial stiffness and the risk of ischemic stroke. Eur J Neurol. 2006;13:475–481. doi: 10.1111/j.1468-1331.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 8.Godia EC, Madhok R, Pittman J, Trocio S, Ramas R, Cabral D, et al. Carotid artery distensibility: A reliability study. J Ultrasound Med. 2007;26:1157–1165. doi: 10.7863/jum.2007.26.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercuro G, Zoncu S, Saiu F, Sarais C, Rosano GM. Effect of atorvastatin on endothelium-dependent vasodilation in postmenopausal women with average serum cholesterol levels. Am J Cardiol. 2002;90:747–750. doi: 10.1016/s0002-9149(02)02602-4. [DOI] [PubMed] [Google Scholar]
- 10.John S, Schneider MP, Delles C, Jacobi J, Schmieder RE. Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am Heart J. 2005;149:473. doi: 10.1016/j.ahj.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. Impact of statin therapy on left ventricular function and carotid arterial stiffness in patients with hypercholesterolemia. Circ J. 2008;72:538–544. doi: 10.1253/circj.72.538. [DOI] [PubMed] [Google Scholar]
- 12.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, et al. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke. 2002;33:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 15.Akalin A, Temiz G, Akcar N, Sensoy B. Short term effects of atorvastatin on endothelial functions and oxidized LDL levels in patients with type 2 diabetes. Endocr J. 2008;55:861–866. doi: 10.1507/endocrj.k07e-121. [DOI] [PubMed] [Google Scholar]
- 16.Marchesi S, Lupattelli G, Siepi D, Schillaci G, Vaudo G, Roscini AR, et al. Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J Cardiovasc Pharmacol. 2000;36:617–621. doi: 10.1097/00005344-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends in Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laufs U, Fata VL, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 19.Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- 20.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 21.Kleinveld HA, Demacker PNM, De Haan AFJ, Stalenhoff AFH. Decreased in vitro oxidizability of low-density lipoprotein in hypercholesterolemic patients treated with 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors. Eur J Clin Invest. 1993;23:289 –295. doi: 10.1111/j.1365-2362.1993.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 22.Giroux LM, Davignon J, Naruszewicz M. Simvastatin inhibits the oxidation of low-density lipoproteins by activated human monocyte-derived macrophages. Biochim Biophys Acta. 1993;1165:335 –338. doi: 10.1016/0005-2760(93)90145-y. [DOI] [PubMed] [Google Scholar]
- 23.Ichiki T, Takeda K, Tokunou T, Iino N, Egashira K, Shimokawa H, et al. Downregulation of angiotensin II type 1 receptor by hydrophobic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:1896–1901. doi: 10.1161/hq1201.099430. [DOI] [PubMed] [Google Scholar]
- 24.Raiteri M, Arnaboldi L, McGeady P, Gelb MH, Verri D, Tagliabue C, et al. Pharmacological control of the mevalonate pathway: Effect on arterial smooth muscle cell proliferation. J Pharmacol Exp Ther. 1997;281:1144–1153. [PubMed] [Google Scholar]
- 25.Martín-Ventura JL, Blanco-Colio LM, Gómez-Hernández A, Muñoz-García B, Vega M, Serrano J, et al. Intensive treatment with atorvastatin reduces inflammation in mononuclear cells and human atherosclerotic lesions in one month. Stroke. 2005;36:1796–1800. doi: 10.1161/01.STR.0000174289.34110.b0. [DOI] [PubMed] [Google Scholar]
- 26.Vlachopoulos C, Aznauridis K, Dagre A, Vasiliadou C, Masoura C, Stefanadi E, et al. Protective effect of atorvastatin on acute systemic inflammation-induced endothelial dysfunction in hypercholesterolaemic subjects. Eur Heat J. 2007;10:1093. doi: 10.1093/eurheartj/ehm247. [DOI] [PubMed] [Google Scholar]
- 27.Fichtlscherer S, Schmidt-Lucke C, Bojunga S, Rössig L, Heeschen C, Dimmeler S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: Clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J. 2006;27:1182–1190. doi: 10.1093/eurheartj/ehi881. [DOI] [PubMed] [Google Scholar]
- 28.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 29.Gaballa MA, Jacob CT, Raya TE, Liu J, Simon B, Goldman S. Large artery remodeling during aging: biaxial passive and active stiffness. Hypertension. 1998;32:437–443. doi: 10.1161/01.hyp.32.3.437. [DOI] [PubMed] [Google Scholar]
- 30.Susekov AV, Rozhkova TA, Tripoten’ MI, Pogorelova OA, Kulev BD, Balakhonova TV, et al. Randomized FARVATER study. Part 2. Effect of atorvastatin on endothelial function, distensibility and stiffness of vascular wall. Kardiologiia. 2007;47:25–30. [PubMed] [Google Scholar]
- 31.Brown SL, Raal FJ, Panz VR, Stevens BA, Veller MG. High-dose atorvastatin therapy is required for significant improvement of endothelial function in heterozygous familial hypercholesterolaemic patients. Cardiovasc J S Afr. 2004;15:70–75. [PubMed] [Google Scholar]
- 32.Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D Ultrasound measurement of change in carotid plaque volume: A tool for rapid evaluation of new therapies. Stroke. 2005;35:1904–1909. doi: 10.1161/01.STR.0000178543.19433.20. [DOI] [PubMed] [Google Scholar]


