Abstract
Background
Protein kinase C (PKC) is a family of serine/threonine kinases that contains more than 10 isozymes. Evidence suggests that PKC may play important roles in pain modulation, but the isozyme-specific effects of PKC on different aspects of pain modulation are not fully understood. We hypothesize that different PKC isozymes play different roles in different aspects of pain modulation.
Methods
The nociceptive behaviors of mice with deletion of PKC α, β, γ, or δ in multiple pain models were compared with their respective wild type littermates. Also, the morphine analgesia and the development of morphine tolerance in mice with deletion of PKC γ were compared with their respective wild type littermates.
Results
Thermal hyperalgesia induced by complete Freund’s adjuvant injection was significantly attenuated by the deletion of PKC β, γ or δ, but not PKC α. Deletion of PKC γ significantly attenuated neuropathic mechanical allodynia induced by spared nerve injury, whereas deletion of PKC α enhanced this allodynia. Baseline thermal and mechanical sensitivity, nociceptive behaviors induced by formalin, mechanical allodynia induced by complete Freund’s adjuvant injection, were not altered by deletion of PKC α, β, γ or δ. Finally, morphine analgesia and the development of morphine tolerance were not altered in PKC γ-deficient mice.
Conclusions
PKC plays isozyme-specific effects in pain modulation.
Introduction
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described interms of such damage.” Pain is critical for the survival of an organism because it enables the organism to detect and avoid injury. Unfortunately, sometimes pain can become a serious medical problem (e.g., cancer pain, neuropathic pain, inflammatory pain, etc.) that requires effective management.
Activation of nociceptors by noxious stimuli (e.g., heat, cold, mechanical injury, etc.) elicits the transmission of nociceptive input from the peripheral site of noxious stimuli to the central nervous system which can result in the perception of physiological pain. The transmission of nociceptive input through pain pathway is modulated by multiple mechanisms at different levels of the nervous system. For example, descending inhibitory pathways attenuate, while facilitatory pathways enhance, the perception of pain. Multiple neurotransmitters, receptor systems and intracellular signal transduction pathways participate in nociceptive transduction and modulation.
Protein kinase C (PKC) is an umbrella term for a family of serine/threonine kinases. At least 10 PKC isoforms have been identified and these have been subdivided into three groups based on calcium and diacylglycerol dependence: classical (α, βI, βII and γ; calcium and diacylglycerol-dependent), novel (δ, ε, η, and θ; calcium-independent and diacylglycerol-dependent), and atypical (ζ and ι/λ; both calcium and diacylglycerol-independent)1. These different PKC isozymes play important roles in the regulation of a variety of cellular functions (e.g., differentiation, proliferation, cell migration, apoptosis, neuron excitability and neurotransmitter release) making them attractive therapeutic targets for a host of human diseases.
Interestingly, a number of PKC isozymes have been found in the anatomical areas that regulate pain and nociception (e.g., amygdala, periaqueductal grey, dorsal root ganglia, spinal cord, etc.)2,3. As such, the role of PKC in pain and analgesia has been an area of intense research over the last two decades3. The large number of PKC isoforms and the largely overlapping substrate specificities of these isoforms in vitro make the unequivocal identification of isozymes-specific roles for PKC a major challenge. Many studies which employed pharmacological methods have shown the important roles of the PKC family in the modulation of pain3. However, the relatively poor specificity of most PKC activators and inhibitors has not only prevented the identification of the PKC isozyme-specific effects in pain, but also has caused conflicting results on the effect of PKC on pain. For example, using different PKC inhibitors, Igwe and Chronwall found that thermal hyperalgesia induced by complete Freund’s adjuvant (CFA) was mediated by protein kinase C βII4, but Yajima etc reported that thermal hyperalgesia induced by CFA did not dependent on PKC5. To gain specific insight into isozyme-specific roles for the PKCs, we used targeted gene knockout techniques in mice to investigate the specific roles of four different PKC isozymes (α, β, γ, and δ) in the modulation of pain. We hypothesized that different PKC isozymes play distinct roles in various aspects of pain modulation and analgesia. Because of the existing of conflicting reports on the effect of PKCγ on the development of morphine tolerance6,7, we also investigated the effect of PKCγ on the development of morphine tolerance using PKCγ knockout mice.
Materials and Methods
Animals
All experiments were performed according to the guidelines of the National Institutes of Health and were approved by the Animal Care and Use Committee of Washington University School of Medicine, St. Louis, Missouri. Mice were housed with a 12 h/12h light/dark cycle with ad libitum access to food and water. Targeted deletions of PKC α8, β9, γ10, or δ11 were achieved by homologous recombination in processes described in detail previously. These animals were obtained from various sources, and thus have a variety of genetic backgrounds, each involving a varying number of backcrosses onto the C57BL/6 strain. PKC α, β and δ mice were generated in house by one of the authors, whereas PKC γ knockout mice were purchased from Jackson Labs, Bar Harbor, ME. All lines were maintained as het × het crosses to generate wild type and knockout mice from the same crosses. Because of the varying genetic backgrounds of these mice, each knockout was compared only to its own wild type littermates as controls. The experimenter was blind to the genotypes of mice in all behavior experiments.
Rotarod Test
An accelerating Rotarod (Ugo Basile, Comerio, Italy) was used to assess motor coordination. Latency to fall as the Rotarod accelerated from 4 to 40 rpm over 5 min was assessed. Five consecutive trials were performed, with at least 15 min between each trial.
Hot Plate Test
The surface of the hotplate (IITC Life Science, Woodland Hills, CA) was heated to a constant temperature of 52°C. Mice were placed on top of the hot plate which was enclosed with clear Plexiglas chambers (width, 10 cm; length, 10 cm; height, 15 cm). The latency to respond with hind paw licking or jumping (whichever occurred first) was measured. The mouse was immediately removed from the hot plate and returned to its home cage. Each animal was tested only once.
Water Immersion Tail-Flick Test
Mice were held gently in a towel and the distal one -third of their tail was immersed in a temperature-controlled water bath (52°C). The latency between tail immersion and the tail flick response was recorded. A cutoff of 10s was applied to prevent tissue damage.
Hargreaves Test
Paw withdrawal latencies to radiant heat stimuli were recorded using a plantar thermal stimulator (IITC Life Sciences) 12. Mice were placed into Plexiglas chambers on a glass surface that was heated to 30°C. Following a 30-min acclimation period, a radiant heat stimulus was applied to each hind paw, and the time to paw withdrawal was measured. Each hind paw was tested three times with a minimum inter-trial interval of 5min. A 20-s exposure limit was imposed to prevent tissue damage. The paw withdrawal latency of each mouse was calculated as the mean of the three trials.
von Frey Filament Test
Mechanical paw withdrawal thresholds were measured using the up-down testing paradigm 13,14. The von Frey filaments (0.02, 0.04, 0.08, 0.16, 0.32, 0.64, 1.28, and 2.56 g) were applied to the lateral part of the hind paw (in spared nerve injury (SNI) neuropathic pain model) or the middle plantar surface of the hind paw until paw withdrawal or a maximal duration of 1 s. The 0.32-g stimulus was applied first. Whenever a withdrawal response to a given filament occurred, the next smaller von Frey filament was applied. Whenever a negative response occurred, the next higher von Frey filament was applied. The test continued until a) the responses of four additional stimuli after the first change in response had been obtained, or b) the upper/lower end of the von Frey filament was reached. Threshold values were derived according to the method described by Chaplan et al13.
Formalin Test
Mice were placed into a transparent observation chamber (30 × 30 × 25 cm) for adaptation 30 min before the experiments. Mice were then gently restrained by hand and injected subcutaneously with 10 μl of 2% formalin solution in the plantar surface of the right hind paw with a 30-gauge needle. After formalin injection, mice were then placed into the original chamber and were observed for licking and flinching behavior. Time spent licking or flinching was recorded in 5-min bins, starting from the time of formalin administration through 60 min post-injection.
Chronic Inflammatory Pain Model
Intraplantar injection of CFA (10 μl, 1.0 mg/ml; Sigma-Aldrich) was used as a chronic inflammatory pain model. Following CFA injection, thermal and mechanical sensitivity were measured using the von Frey filament and Hargreaves tests as described above.
SNI Neuropathic Pain Model
All animals underwent the SNI operation as described previously 15,16. Briefly, under anesthesia with pentobarbital (50 mg/kg, intraperitoneal), the left sciatic nerve and its three terminal branches (sural, common peroneal, and tibial) were exposed at the mid-thigh. The left tibial and common peroneal nerves were tightly ligated with 8-0 silk and transected distal to the ligation, and 1 mm of the distal nerve stump was removed. Care was taken to prevent damage to the sural nerve. Muscle layers were closed using 6-0 suture, and the skin incision was closed with wound staples.
Morphine Analgesia
To measure the analgesic effect of morphine, two cumulative morphine-dosing regimens were used: In naïve mice the doses of morphine were 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, and 10.0 mg/kg; in morphine-tolerated mice the doses of morphine were 3, 6, 10, 15, 30, 60, 100, and 300 mg/kg). Morphine was administered intraperitoneally in a volume of 200μl which was diluted in normal saline. The interval between drug injections was 30 min. Tail-flick latencies were tested 30 min after each injection of a drug dose.
Morphine analgesia was calculated using the percent maximum possible effect using the following equation: percent maximum possible effect = ([Measured tail-flick latency] − [baseline tail-flick latency]) × 100/(10 -[baseline tail-flick latency]), wherein 10 is the number of seconds used as the cut-off time for tail-flick latency measurement.
Induction of Morphine Tolerance
Two paradigms were used to induce morphine tolerance. 1) One 75mg slow-release morphine pellet (provided by National Institute on Drug Abuse) was implanted subcutaneously; 2) one 25 mg release-controlled morphine pellet was implanted subcutaneously followed by another 25 mg morphine pellet implanted subcutaneously 4 days later. All implantations were done under anesthesia with pentobarbital.
Statistical Analysis
Results are expressed as mean ± S.E.M. Statistical analysis was performed using STATISTICA software (version 6, StatSoft, Inc., Tulsa, OK). Comparisons of two means were made by unpaired two-tailed t test. Comparisons of time sequence data between groups were made by repeated measures two-way ANOVA followed by Tukey’s post-hoc test. Differences were considered statistically significant when p ≤ 0.05.
Results
Deletion of PKC α, β, γ, or δ does not change the gross motor function of mice
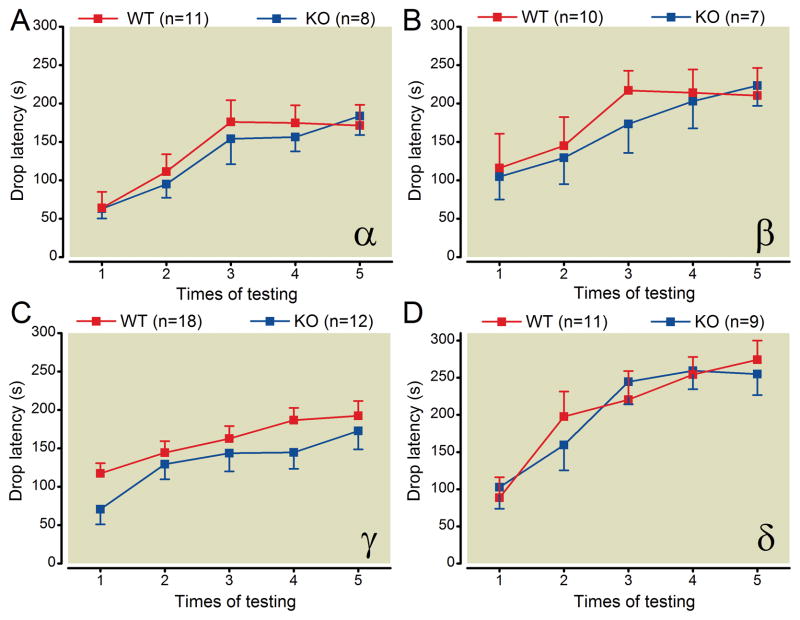
The accelerating rotarod test was employed to measure the gross motor functions of mice. Compared with their respective wild type littermates, PKC α, β, γ or δ knockout mice exhibited no statistically significant difference in the latency to drop from the rotarod (fig. 1). In addition, all knockout mice demonstrated trial-to-trial improvement in performance on the rotarod that was indistinguishable from their respective wild type littermates, consistent with a lack of impairment in simple motor learning. These results suggest that deletion of PKC α, β, γ, or δ does not change gross motor function or impair simple motor learning in mice.
Fig. 1.
Deletion of PKC isoforms α, β, γ, or δ does not alter gross motor behavior in the accelerating Rotarod Test. Cohorts of the various knockout mice were compared to their wild-type littermates on the same day, with the experimenter blind to genotype of the animals.
Deletion of PKC α, β, γ, or δ does not change the baseline sensitivity of mice to thermal stimuli
Three different tests were used to determine if deletion of an individual PKC isozyme alters sensitivity to noxious heat: hot-plate test, tail-flick test and hargreaves test.
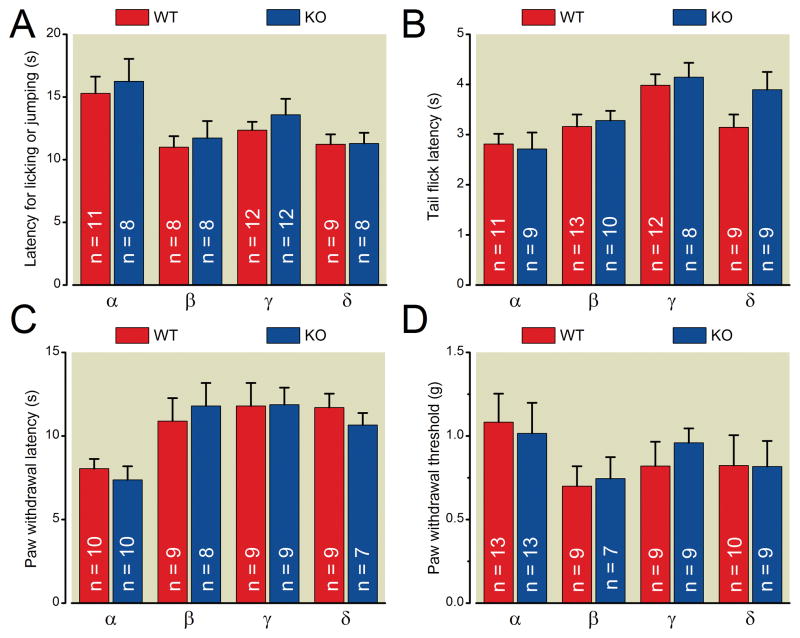
In the hot-plate test, sensitivity to noxious heat applied to the paws was evaluated by measuring the latency paw licking or jumping after mice were placed on the surface of a 52°C hot plate. The response latencies for PKC α, β, γ, or δ knockout mice were not statistically significantly different from their respective wild-type littermate mice (fig. 2 A).
Fig. 2.
PKC α, β, γ or δ knockout mice show no statistically significant difference in their performance on the hot-plate test (A), tail-flick test (B), Hargreaves Test (C) or von Frey Filament Test (D) compared with wildtype littermate control mice (group main effects). Details of each test are provided in “materials and methods.” For all experiments, the experimenter blind to genotype of the animals.
The tail-flick test was used to evaluate sensitivity to noxious heat applied to the tail. The latency to respond with a tail flick, following immersion of the tail in a hot water bath (52°C), was measured. The tail-flick latencies of PKC α, β, γ, or δ knockout mice of were not statistically significantly different from their respective wild-type littermate mice (fig. 2 B).
The Hargreave’s test was used to evaluate sensitivity to noxious heat applied to the hind paws. The withdrawal latency to a radiant heat stimulus applied to the hindpaw was measured. The paw withdrawal latencies of PKC α, β, γ, or δ knockout mice were not significantly different from their respective wild-type littermate mice (fig. 2 C).
Together, these data indicate that deletion of individual PKC isozymes α, β, γ, or δ does not change the baseline sensitivity of mice to noxious heat stimuli.
Deletion of PKC α, β, γ, or δ does not change the baseline sensitivity of mice to mechanical stimuli
The mechanical sensitivity of mice was tested with von Frey filaments. The paw withdrawal thresholds to mechanical stimuli were calculated using the up-down method 13,14. The paw withdrawal thresholds of PKC α, β, γ, or δ knockout mice were not statistically significantly different from their respective wild-type littermate mice (fig. 2 D). These results indicate that deletion of an individual PKC isozyme α, β, γ, or δ does not change the baseline sensitivity of mice to mechanical stimuli.
Deletion of PKC α, β, γ, or δ has no effect on the nociceptive responses induced by plantar injection of formalin
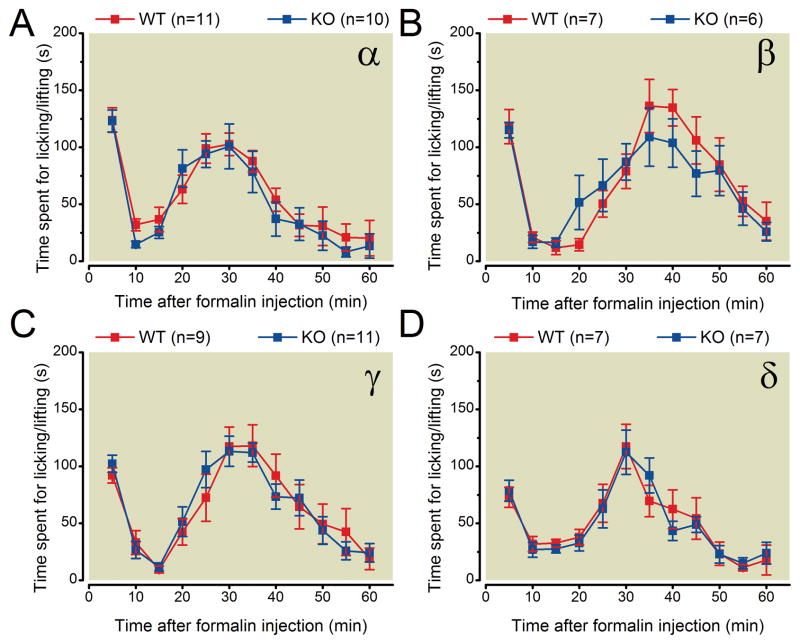
In the formalin test, we recorded the time that mice spent licking or flicking their hindpaws after intraplantar injection of formalin solution. The time spent licking or flicking after formalin injection was not statistically significantly different in PKC α, β, γ, or δ knockout mice compared with their respective wild-type littermate mice (fig. 3). These results indicate that deletion of individual PKC α, β, γ, or δ isozymes has no effect on the nociceptive response induced by formalin.
Fig. 3.
PKC α, β, γ, or δ knockout mice show no statistically significant differences in formalin-induced pain behavior compared with wildtype littermate control mice.
Deletion of PKC α, β, γ, or δ has no effect on mechanical allodynia induced by CFA plantar injection
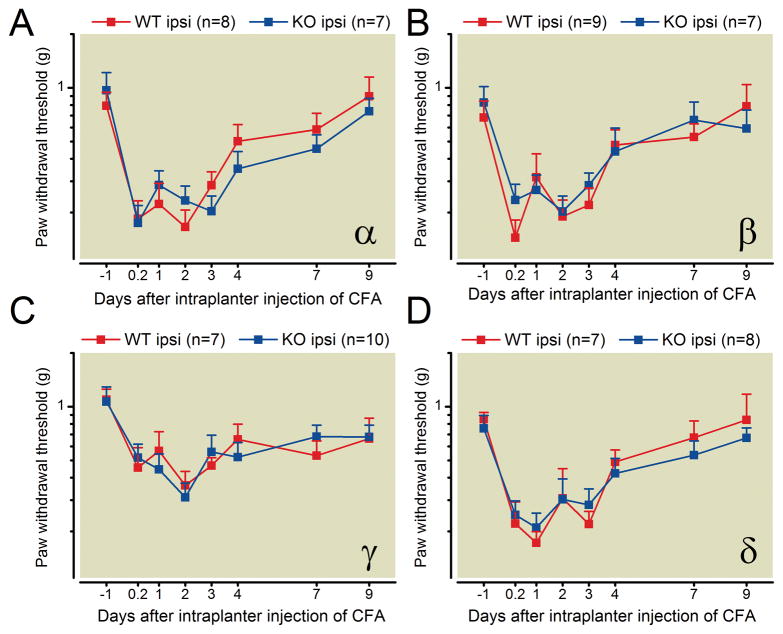
Paw withdrawal thresholds to application of a series of calibrated von Frey filaments were measured in the CFA-induced persistent inflammatory pain model. After intraplantar injection of CFA, paw withdrawal thresholds for all groups of mice were statistically significantly decreased in the injected hind paw compared to baseline, demonstrating the induction of mechanical hypersensitivity (allodynia). No statistically significant changes in the extent of CFA-induced mechanical hypersensitivity were seen in PKC α, β, γ, and δ knockout mice relative to their respective wild-type littermate mice (fig. 4). These results indicate that deletion of individual PKC α, β, γ, or δ isozymes has no effect on the mechanical allodynia induced by intraplantar injection of CFA.
Fig. 4.
PKC α, β, γ, and δ knockout mice have no statistically significant differences in CFA-induced mechanical allodynia compared with wildtype littermate control mice. For all experiments, the experimenter blind to genotype of the animals.
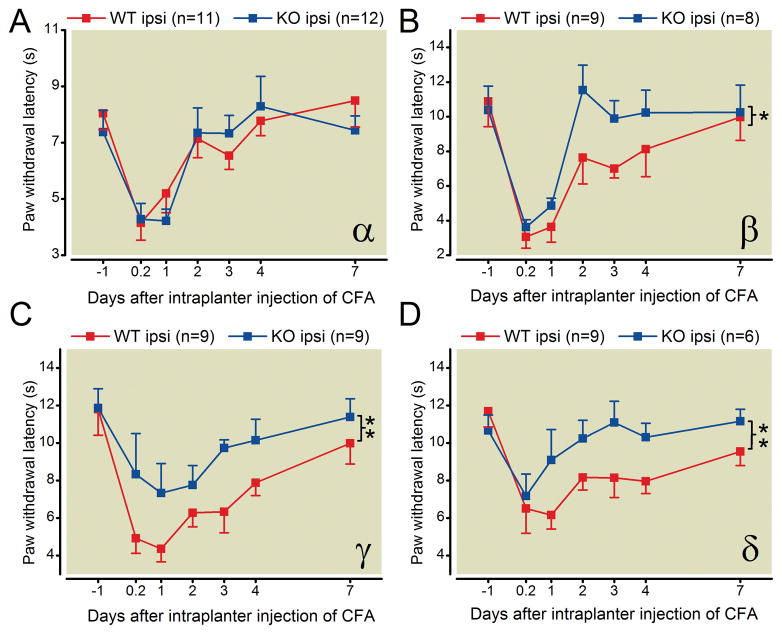
Deletion of PKC β, γ or δ, but not PKCα, attenuates the thermal hyperalgesia induced by CFA plantar injection
Paw withdrawal latencies to radiant heat stimuli were measured in the CFA-induced chronic inflammatory pain model. After intraplantar injection of CFA, paw withdrawal latencies of wild type mice were statistically significantly decreased compared with their respective baseline, indicating the mice developed thermal hyperalgesia. CFA-induced thermal hyperalgesia was not significantly different in PKC α knockout mice compared with wild-type littermates (fig. 5 A). In contrast, compared with their respective wild type littermates, PKC β, γ, or δ knockout mice developed statistically significantly less thermal hyperalgesia (group main effect; no statistically significant differences in group × time effects) following CFA injection (fig. 5 B, C, D). These results indicate that deletion of PKC β, γ, or δ, but not PKC α, attenuates the thermal hyperalgesia induce by CFA.
Fig. 5.
CFA-induced thermal hyperalgesia in PKC knockout mice. (A) PKCα knockout mice developed CFA-induced thermal hyperalgesia which is not significantly different from wildtype littermate control mice. (B–D) PKC β, γ, and δ, knockout mice develop attenuated CFA-induced thermal hyperalgesia compared with wildtype littermate control mice (* p <0.05, ** p <0.01). For all experiments, the experimenter blind to genotype of the animals. Note: * and ** represent group main effects and the group × time effects are not statistically significant.
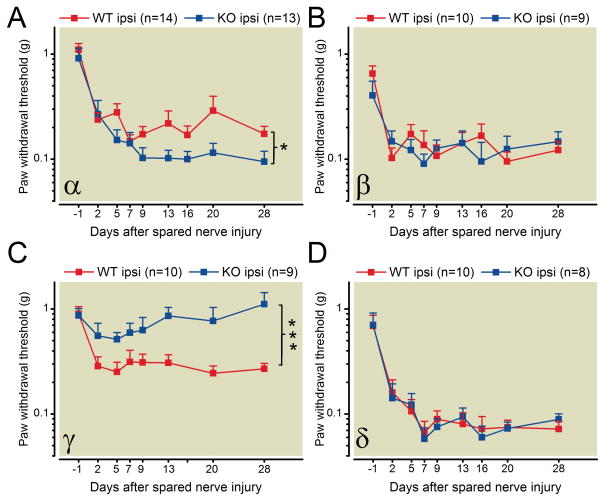
Deletion of PKC α and PKC γ differentially modulate the induction of mechanical allodynia in the SNI model of neuropathic pain
The SNI model of neuropathic pain was employed to investigate the effect of PKC α, β, γ, and δ on the development of neuropathic pain. Paw withdrawal thresholds to mechanical stimuli were measured with von Frey filaments before and after SNI. After tibial and femoral nerve injury, the paw withdrawal thresholds measured in the lateral sides of mouse hind paws were statistically significantly decreased in the isplilateral paws of wild-type mice (fig. 6). This result demonstrates the development of mechanical allodynia induced by SNI. Nerve injury-induced mechanical allodynia was not significantly different in PKC β or δ knockout mice compared with wild type littermate control mice (fig. 6 B and D). Nerve injury-induced mechanical allodynia was significantly increased in PKC α knockout mice compared with their respective wild type littermates (fig. 6 A). In contrast, nerve injury-induced mechanical allodynia was significantly decreased in PKCγ knockout mice compared with littermate control mice (fig. 6 C). The above results suggest different roles for individual PKC isozymes in the development of nerve injury-induced pain. PKCγ attenuates nerve injury-induced mechanical allodynia while deletion of PKC α has an opposite effect. Deletion of PKC β or δ does not alter nerve injury-induced mechanical allodynia.
Fig. 6.
Spared nerve injury-induced mechanical allodynia in PKC knockout mice. (A) SNI-induced allodynia is enhanced in PKCα knockout mice (* p <0.05), attenuated in PKC γ knockout mice (*** p <0.01)(C), and unchanged in PKC β and PKC δ knockout mice (B and D) compared to respective wildtype littermate control mice. Details of the SNI procedure are provided in “materials and methods.” For all experiments, the experimenter blind to genotype of the animals. Note: * and ** represent group main effects and the group × time effects are not statistically significant.
PKCγ has no effect on the development of morphine tolerance
There is evidence suggesting that PKC may be involved in the development of morphine tolerance 17,18, but the effect of PKCγ on morphine tolerance is still controversial 6,7. We investigated the effect of PKCγ on the development of morphine tolerance using PKC γ knockout mice.
We initially used subcutaneous implantation of 75mg morphine pellets to induce morphine tolerance. As table 1 shows, this dose resulted in a statistically significant increase in the mortality of PKCγ knockout mice compared with their wild type littermate mice within the first day after implantation of morphine pellets.
Table 1.
Morphine Induces Higher Mortality in PKCγ Knockout Mice
| Alive | Dead | |
|---|---|---|
| Wild type | 11 | 1 |
| knockout | 3 | 5 |
Fisher’s exact test: p < 0.05
PKC = protein kinase c.
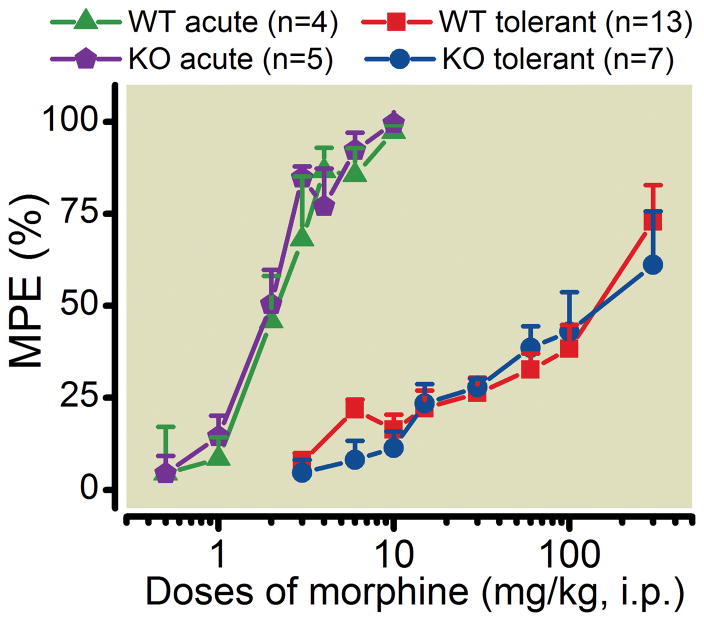
We next tried a novel method to induce morphine tolerance by subcutaneously implanting one 25 mg morphine pellet followed by implantation of another 25 mg pellet 4days later. The tail-flick latency was determined prior to implantation of the first morphine pellet (baseline) and again four days after the implantation of the second pellet. The tail-flick latency after the implantation of morphine pellets was not statistically significantly different compared with their respective baselines in PKC γ knockout mice and their wild type littermates (data not shown). The dose-response curves of morphine analgesia 4 days after the implantation of the second morphine pellet in PKC γ knockout mice and their wildtype littermates were significantly shifted to the right compared to that of naïve PKC γ knockout mice and their wildtype littermates (fig. 7), which indicates the development of morphine tolerance in both PKC γ knockout mice and their wild type littermates. There was no statistically significant difference in the dose-response curves of morphine analgesia between PKC γ knockout mice and their wild-type littermate mice either before or after the implantation of morphine pellets, indicating that deletion of PKC γ does not alter morphine analgesia or the development of morphine tolerance. However, the absence of PKC γ led to increased mortality at high doses of morphine, indicating that PKC γ may play a role in opposing the toxic effects of morphine that are not directly related to analgesia.
Fig. 7.
Morphine-induced analgesia is not significantly different in naïve PKCγ knockout mice relative to their wild type littermate control mice. Implantation of controlled-release morphine pellets induced a right shift of the morphine analgesic dose-response curve in both PKC γ knockout mice and wild type littermate control mice. No statistically significant difference in acute morphine-induced analgesia was found between morphine tolerated PKC γ knockout mice and wild type littermate control mice. For all experiments, the experimenter blind to genotype of the animals.
Discussion
To investigate the effects of different PKC isoforms on pain modulation, we used a variety of pain models to study multiple aspects of pain. The behavior tests used to evaluate thermal and mechanical sensitivity depend upon the ability of the mouse to withdraw from a stimulus, requiring intact motor reflexes. Using the accelerating rotarod test, we evaluated gross motor function in PKC mutant mice to ensure that deletion of individual PKC isoforms does not impair gross motor function or simple motor learning. Our results from the rotarod test show that deletion of PKC α, β, or δ did not result in any statistically significant impairment of gross motor function. It has been reported that PKC γ knockout mice displayed impaired motor coordination 19, but no statistically significant difference of drop latencies was found between PKCγ knockout mice and their wildtype littermates in our experiments. The lack of effect of knockout of PKCα, β, γ, or δ on locomotor function supports the use of these animals in research on pain using motor-reflex pain models in these mice.
PKC is present at multiple sites of the nervous system that contribute to pain transmission and modulation. It has been reported that PKC is involved in multiple aspects of pain modulation3. We first explored whether knockout of PKC α, β, γ, or δ alters basal pain perception. Our results from hot-plate, Hargreaves and tail-flick tests indicate that PKC α, β, γ, and δ are not required for noxious heat perception; our results using the von Frey Test indicate that PKC α, β, γ, and δ are also not required for mechanical sensation. Our results indicate that these individual PKC isoforms are not required for normal acute noxious heat or mechanical sensitivity. This agrees with the literature reporting that PKC inhibitors have no effect on normal acute noxious heat and mechanical sensitivity 3,20,21. It is also possible that multiple PKC isoforms subserve similar functions in noxious heat and mechanical sensation, thus preventing observable phenotypes in mice where an individual isoform has been ablated.
The formalin test is a well-established model which has been used extensively to study the mechanisms of inflammation-induced pain. The intraplantar injection of formalin induces characteristic nociceptive behaviors, which appear in two distinct phases. The first phase is thought to result from direct activation of nociceptive afferent fibers. The second phase is believed to be mediated by the activation of central neurons that are sensitized due to peripheral inflammation, as well as ongoing activity of primary afferents 22–24. Intrathecal administration of PKC inhibitors has been shown to decrease the second phase of nociceptive behavior in the formalin test 25,26. Our results show that deletion of individual PKC isoforms α, β, γ, or δ in mice had no effect on formalin-induced nociceptive behavior, suggesting that none of the PKC α, β, γ, or δ isoforms is individually required for formalin-induced pain. This may suggest that other PKC isoforms that were not tested in this study might be candidate modifiers of the formalin second phase. An alternative possibility is that the individual PKC isoforms have redundant functions, and that simultaneous knockout of combinations of these would reveal dependence on multiple PKC isoforms. Yet another possibility is that the PKC inhibitors used in previous studies do not act in a PKC-specific manner at the doses used in vivo. Our observation that PKCγ is not required for formalin-induced pain behavior is in contrast with the report of Malmberg, et. al. that the second phase of the formalin test was significantly reduced in PKCγ knockout mice compared with wildtype mice27. The reason for this difference is unclear.
Chronic inflammatory pain conditions, like rheumatoid arthritis, are among the most common health problems and are difficult to treat. CFA-induced inflammation in the mouse is widely used as a model to study the mechanisms and management of chronic inflammatory pain. Our results indicate that PKC β, γ and δ, but not PKCα, are involved the development of CFA-induced thermal hyperalgesia and that none of PKC isoforms are individually necessary in the development of CFA-induced mechanical allodynia.
Neuropathic pain is a devastating consequence of injury or diseases of the peripheral or central nervous system and characterized by spontaneous pain, hyperalgesia, and allodynia. The mechanisms that underlie neuropathic pain are incompletely understood. It has been reported that PKCγ knockout mice do not develop nerve injury-induced mechanical allodynia in the partial sciatic nerve ligation model of neuropathic pain 27. In addition, intrathecal lentiviral-mediated RNA interference targeting PKC γ attenuates chronic constriction injury-induced neuropathic pain in rats28. These studies suggest that PKC γ is required for the full expression of nerve injury-induced pain. The SNI model of neuropathic pain involves tightly ligating and transecting two of the three terminal branches of the sciatic nerve (tibial and common peroneal nerves), leaving the remaining sural nerve intact 15. SNI induces very stable, robust and long-lasting mechanical allodynia 29,30. Using the SNI model, we found that PKCγ, but not PKCβ and δ, plays a critical role in the development of nerve injury-induced mechanical allodynia. Further, our results show that deletion of PKC α enhances the mechanical allodynia induced by SNI. This may be caused by potential adaptive changes in the nerve system, such as up-regulation of PKCγ expression, induced by PKCα knockout, or by a loss of an inhibitory effect of PKC α on neuropathic mechanical allodynia. All these possibilities need to be further investigated.
The effect of PKCγ on the development of morphine tolerance has been investigated intensively 6,7,17,18,31. Zeitz, et al. reported that there was reduced development of morphine tolerance in PKCγ knockout mice 6, but Yukhananov and Kissin reported that they did not find any difference in the development of morphine tolerance in PKCγ knockout mice compared with wildtype control mice. We tried to use the same 75 mg morphine pellets as reported by the above two studies to induce morphine tolerance.
Interestingly, we found the mortality caused by morphine pellet implantation in PKC γ knockout mice was very high, which made our further research using the same morphine pellets unpractical. Our two 25 mg morphine pellets implantation paradigm did not cause death of either PKC γ knockout mice or in their wild type littermates, and induced very robust morphine tolerance. Our results indicate that PKC γ is not individually required for morphine analgesia or in the development of morphine tolerance.
Other PKC isoforms may also be involved in different aspects of pain modulation. For example, PKCε in primary afferent nociceptors plays multiple crucial roles in hyperalgesic priming32. It is necessary to further investigate the effects of other PKC isoforms in the modulation of pain.
In conclusion, our study examines the specific roles of different PKC isoforms (α, β, γ, and δ) in different aspects of pain modulation. These studies suggest a limited and modality-specific role of these PKC isoforms in inflammatory pain. However, our findings extend earlier reports and substantially support the hypothesis that PKC γ might be effectively targeted for the management of multiple forms of neuropathic pain.
Summary.
What we already know about this topic
Protein kinase C (PKC) plays an important role in the generation and maintenance of hypersensitivity after inflammation and nerve injury, but the roles of specific PKC isoenzymes have not been clearly defined
What this article tells us that is new
In mice with deletion of specific PKC isoenzymes there was no effect on baseline sensory testing but differing reliance of isoenzymes on behavioral responses to inflammation and nerve injury
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke, Bethesda, Maryland, Grant no. R01NS48602.
The authors thank Sherri Vogt and Changshen Qiu, M.D. for assistance with the animal colonies, and Judith Golden, Ph. D., Benedict Kolber, Ph. D. and Steve Davidson, Ph. D. (St Louis, Missouri) for assistance with the manuscript.
Footnotes
The work should be attributed to Washington University Pain Center and Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri.
References
- 1.Yonezawa T, Kurata R, Kimura M, Inoko H. PKC delta and epsilon in drug targeting and therapeutics. Recent Pat DNA Gene Seq. 2009;3:96–101. doi: 10.2174/187221509788654205. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka C, Saito N. Localization of subspecies of protein kinase C in the mammalian central nervous system. Neurochem Int. 1992;21:499–512. doi: 10.1016/0197-0186(92)90081-2. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol Res. 2007;55:578–89. doi: 10.1016/j.phrs.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igwe OJ, Chronwall BM. Hyperalgesia induced by peripheral inflammation is mediated by protein kinase C betaII isozyme in the rat spinal cord. Neuroscience. 2001;104:875–90. doi: 10.1016/s0306-4522(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 5.Yajima Y, Narita M, Shimamura M, Kubota C, Suzuki T. Differential involvement of spinal protein kinase C and protein kinase A in neuropathic and inflammatory pain in mice. Brain Res. 2003;992:288–93. doi: 10.1016/j.brainres.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC[gamma] mutant mice. Pain. 2001;94:245–53. doi: 10.1016/S0304-3959(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 7.Yukhananov RY, Kissin I. Comment on Zeitz, K.P., et al., reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC mutant mice, PAIN 94 (2002) 245–253. Pain. 2003;102:309–10. doi: 10.1016/S0304-3959(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 8.Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–58. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- 9.Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–91. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 10.Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–62. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 11.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest. 2001;108:1505–12. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 13.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia inthe rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 14.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 15.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 16.Alter BJ, Zhao C, Karim F, Landreth GE, Gereau RW. Genetic targeting of ERK1 suggests a predominant role for ERK2 in murine pain models. J Neurosci. 30:11537–47. doi: 10.1523/JNEUROSCI.6103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao J, Price DD, Phillips LL, Lu J, Mayer DJ. Increases in protein kinase C gamma immunoreactivity in the spinal cord of rats associated with tolerance to the analgesic effects of morphine. Brain Res. 1995;677:257–67. doi: 10.1016/0006-8993(95)00161-i. [DOI] [PubMed] [Google Scholar]
- 18.Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain. 2007;127:129–39. doi: 10.1016/j.pain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, Hashimoto K, Thompson RF, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC gamma mutant mice. Cell. 1995;83:1233–42. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 20.Souza ALS, Moreira FA, Almeida KR, Bertollo CM, Costa KA, Coelho MM. In vivo evidence for a role of protein kinase C in peripheral nociceptive processing. BrJ Pharmacol. 2002;135:239–47. doi: 10.1038/sj.bjp.0704434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dina OA, Messing RO, Levine JD. Ethanol withdrawal induces hyperalgesia mediated by PKC epsilon. Eur J Neurosci. 2006;24:197–204. doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- 22.Dubner R, Ren K. Endogenous mechanisms of sensory modulation. Pain. 1999;82:S45–53. doi: 10.1016/S0304-3959(99)00137-2. [DOI] [PubMed] [Google Scholar]
- 23.Hunskaar S, Hole K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–14. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 24.Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: Systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–55. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 25.Wajima Z, Hua XY, Yaksh TL. Inhibition of spinal protein kinase C blocks substance P-mediated hyperalgesia. Brain Res. 2000;877:314–21. doi: 10.1016/s0006-8993(00)02714-1. [DOI] [PubMed] [Google Scholar]
- 26.Sweitzer SM, Wong SM, Peters MC, Mochly-Rosen D, Yeomans DC, Kendig JJ. Protein kinase C epsilon and gamma: Involvement in formalin-induced nociception in neonatal rats. J Pharmacol Exp Ther. 2004;309:616–25. doi: 10.1124/jpet.103.060350. [DOI] [PubMed] [Google Scholar]
- 27.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–83. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 28.Zou W, Song Z, Guo Q, Liu C, Zhang Z, Zhang Y. Intrathecal lentiviral-mediated RNA interference targeting PKCγ attenuates chronic constriction injury-induced neuropathic pain in rats. Hum Gene Ther. 2011;22:465–75. doi: 10.1089/hum.2010.207. [DOI] [PubMed] [Google Scholar]
- 29.Zhao C, Chen L, Tao Y-X, Tall JM, Borzan J, Ringkamp M, Meyer RA, Raja SN. Lumbar sympathectomy attenuates cold allodynia but not mechanical allodynia and hyperalgesia in rats with spared nerve injury. J Pain. 2007;8:931–7. doi: 10.1016/j.jpain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Tall JM, Meyer RA, Raja SN. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004;100:905–11. doi: 10.1097/00000542-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Song Z, Zou W, Liu C, Guo Q. Gene knockdown with lentiviral vector-mediated intrathecal RNA interference of protein kinase C gamma reverses chronic morphine tolerance in rats. J Gene Med. 12:873–80. doi: 10.1002/jgm.1514. [DOI] [PubMed] [Google Scholar]
- 32.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–8. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]