Abstract
Objective
Sex differences have been demonstrated in the peripheral auditory system as well as in higher-level cognitive processing. Here, we aimed to determine if the subcortical response to a complex auditory stimulus is encoded differently between the sexes.
Method
Using electrophysiological techniques, we assessed the auditory brainstem response to a synthesized stop-consonant speech syllable [da] in 76 native-English speaking, young adults (38 female). Timing and frequency components of the response were compared between males and females to determine which aspects of the response are affected by sex.
Results
A dissimilarity between males and females was seen in the neural response to the components of the speech stimulus that change rapidly over time; but not in the slower changing, lower frequency information in the stimulus. We demonstrate that, in agreement with the click-evoked brainstem response, females have earlier peaks relative to males in the subcomponents of the response representing the onset of the speech sound. In contrast, the response peaks comprising the frequency-following response, which encode the fundamental frequency (F0) of the stimulus, as well as the spectral amplitude of the response to the F0, is not affected by sex. Notably, the higher-frequency elements of the speech syllable are encoded differently between males and females, with females having greater representation of spectrotemporal information above the F0.
Conclusions
Our results provide a baseline for interpreting the higher incidence of language impairment (e.g. dyslexia, autism, specific language impairment) in males, and the subcortical deficits associated with these disorders.
Significance
These results parallel the subcortical encoding patterns that are documented for good and poor readers in that poor readers differ from good readers on encoding fast but not slow components of speech. This parallel may thus help to explain the higher incidence of reading impairment in males compared to females.
Keywords: ABR, sex, speech, subcortical, brainstem
Introduction
Between the sexes, significant differences exist in the click-evoked auditory brainstem response (ABR), a response predominately representative of high frequency information (Eggermont and Don, 1980). This difference is reflected in the timing of the response, with females having earlier latencies (the time interval between the stimulus onset and the response peak) compared to males (Jerger and Hall, 1980). No sex differences, however, are seen in the phase-locked response to pure tones, a response termed the frequency following response (FFR) that encodes low frequency information (Batra et al., 1986, Hoormann et al., 1992). Because the click- and tone-evoked auditory responses represent the subcortical processing of the elemental components of more complex stimuli, namely onset and phase-locked responses to the acoustic features of speech, (Aiken and Picton, 2008, Akhoun et al., 2008, Basu et al., 2010, Chandrasekaran and Kraus, 2010, Hornickel et al., 2009a, Skoe and Kraus, 2010) we predict that a speech syllable should produce responses that show a non-uniform difference of encoding between the sexes. To address this question, we recorded speech-ABRs to the stop consonant speech-syllable [da] presented to the right ear in a normal learning, young adult population.
Stop consonant syllables, such as [da], have been shown to be difficult for certain populations to perceive, such as individuals with hearing impairments (Gordon-Salant et al., 2007, Townsend and Schwartz, 1981, Van Tasell et al., 1982) and children with specific language impairment and dyslexia (Bradlow et al., 1999, Merzenich et al., 1996, Serniclaes and Sprenger-Charolles, 2003, Tallal, 1980). To determine the biological underpinnings of these deficits, the brainstem response to the stop consonant syllable [da] has been investigated in individuals with speech in noise difficulties (Anderson and Kraus, 2010, Anderson et al., 2010a, Anderson et al., 2010b, Chandrasekaran et al., 2009, Hornickel et al., 2009b), normal hearing young adults (Dhar et al., 2009, Hornickel, Skoe, 2009a, Song et al., 2010, Vander Werff and Burns, 2011), auditory experts (Kraus and Chandrasekaran, 2010, Parbery-Clark et al., 2009a, Parbery-Clark et al., 2009b), typically-developing children, as well as with reading impairments (Banai et al., 2009, Banai et al., 2005, Chandrasekaran, Hornickel, 2009, Cunningham et al., 2001, Hornickel, Skoe, 2009b, Russo et al., 2004, Wible et al., 2004), and children with autism spectrum disorders (Russo et al., 2010, Russo et al., 2008). Although males tend to have a higher prevalence of both autism and language-based learning impairments than females (Katusic et al., 2001, Rutter et al., 2004), and children with autism and language impairments demonstrate impaired subcortical encoding of auditory stimuli (Banai, Hornickel, 2009, Banai, Nicol, 2005, Basu, Krishnan, 2010, McAnally and Stein, 1996), this is the first study to examine sex differences that are evident in the subcortical response to speech.
Distinct processing for fast and slow (low vs. high frequency) components of acoustic signals has been amply demonstrated (see Zatorre and Gandour, 2008 for review). Acoustic information is asymmetrically routed (Hickok and Poeppel, 2007, Poeppel, 2003) with faster cues lateralized to the left hemisphere (Abrams et al., 2006, Belin et al., 1998, Zatorre and Belin, 2001) and slower information lateralized to the right hemisphere (Abrams et al., 2008, Abrams, Nicol, 2006, Boemio et al., 2005). Because fast, rapid fluctuations are important for conveying linguistic meaning, speech is maximally processed by the left hemisphere (Zatorre et al., 2002). A corresponding right ear advantage (REA) has been demonstrated behaviorally for speech, although this advantage is dependent upon the temporal characteristics of the speech stimulus (Schwartz and Tallal, 1980). Subcortically, lateralization of signal processing is evident with rapid and transient stimuli being encoded more robustly when presented to the right ear and sustained, slower information being maximally encoded when presented to the left (Ballachanda et al., 1994, Sininger and Cone-Wesson, 2006). The speech-ABR to [da] demonstrates an REA for specific features characteristic of the fast elements of speech (Hornickel, Skoe, 2009a). These differences are also reflected in asymmetry in peripheral processing of auditory stimuli. Across both sexes, otoacoustic emissions generated in response to continuous tones are more robust in the left ear, whereas transient stimuli evoke larger responses in the right ear (Sininger and Cone-Wesson, 2004).
Sex differences that exist in the auditory system may interact with peripheral and hemispheric asymmetry for processing slow and fast elements of sound. Regardless of sex, right ears are more sensitive than left to auditory stimuli and females have, on average, greater hearing sensitivity than males (see McFadden, 1998 for review). Moreover, across both sexes, spontaneous otoacoustic emissions (SOAEs) are more prevalent in right ears than left ears but females have larger and more numerous SOAEs than males (Bilger et al., 1990, Lamprecht-Dinnesen et al., 2000). OAEs evoked by transient, rapidly presented stimuli such as clicks or tone bursts (TEOAEs) are also larger in females than males and are generally larger in right than left ears (Ismail and Thornton, 2003, McFadden et al., 2009). Across both sexes, infants demonstrate larger TEOAEs in the right ear while OAEs evoked by continuous tone pairs (DPOAEs) are larger in the left ear (Sininger and Cone-Wesson, 2004). Although adults demonstrate a weak sex difference in the amplitude of DPOAEs, with females having a larger amplitude response, they do not show an ear asymmetry (McFadden, Martin, 2009).
Given the well-documented sex differences in the auditory system and their interaction with lateralization of auditory processing, we hypothesized that the transient (i.e., fast) aspects of the speech-ABR, like the click-ABR, would be affected by the sex of the subject. Specifically, we predicted that females would have faster response timing for the onset peaks in the speech-evoked response. Furthermore, the lack of a sex effect for the FFR to the fundamental frequency (F0) and second harmonic (Hoormann, Falkenstein, 1992) of tone burst stimuli led us to hypothesize that males and females would not differ in the encoding of the lowest frequency (i.e. slow) components of the speech syllable or the corresponding temporal interpeak intervals. Because the click-ABR is reflective of primarily high-frequency encoding, we also hypothesized that responses collected from males and females would differ in the spectral magnitude of the higher frequency (i.e., above the F0) information in the stimulus.
Methods
Participants
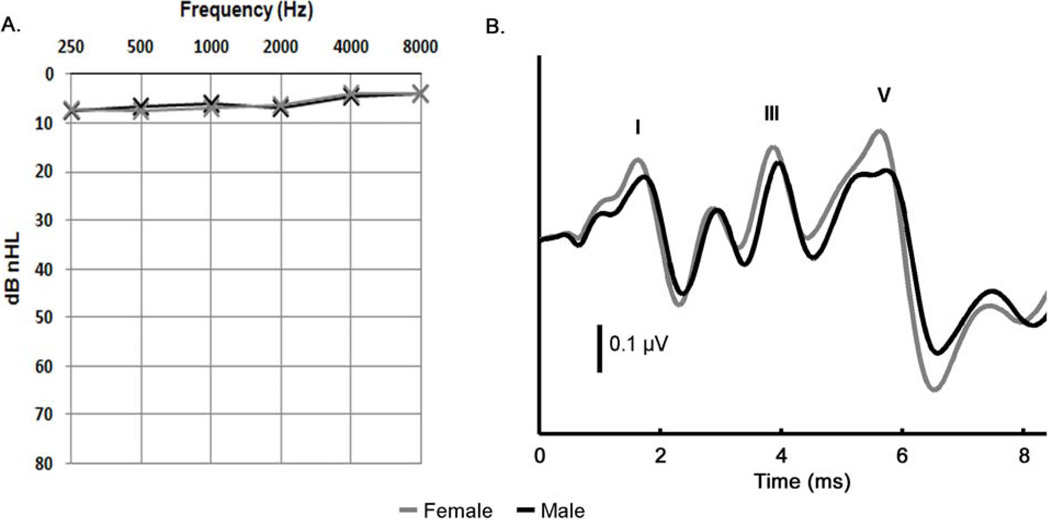
Seventy-six subjects, 38 female, aged 22 to 29 years (Females: mean = 24.21 years, SD = 2.02 years, Males: mean = 24.65 years, SD = 2.07 years) were recruited from Northwestern University and reported no history of language impairment. Subjects were included if their airconduction thresholds were ≤ 20 dB nHL at octave frequencies between 250–8000 Hz. Inclusion in the study also required that the subject’s wave V latency elicited by a 100-µs click (presented in rarefaction to the right ear at a presentation rate of 31.25 Hz and a level of 45 dB nHL) was within the range of normative values (mean +/− 1.5 standard deviations) previously established in the lab (5.41 – 5.96 ms). Figure 1 shows the average audiometric thresholds and click-evoked responses of the male and female groups.
Figure 1.
Comparison of the mean click-evoked auditory brainstem response and mean audiometric thresholds between males (black) and females (gray). (a) There was no difference in the pure-tone auditory thresholds between males and females between 250–8000 Hz. (b) As expected, the female click-evoked response had a significantly earlier and larger amplitude peak V.
Stimuli and Recording Parameters
Brainstem responses were elicited using a 40 ms speech sound, [da], presented at a rate of 10.9 Hz to the subject’s right ear. This five-formant synthesized speech sound (Klatt, 1980) comprises an initial noise burst and a formant transition between the consonant and the vowel. Although the stimulus does not contain a steady-state vowel, it is still perceived as the syllable [da]. The F0 and the first three formants (F1, F2, F3) change linearly over the duration of the stimulus: F0 from 103 to 125, F1 from 220–720, F2 from 1700-1240, and F3 from 2580-2500 Hz. F4 and F5 remain constant at 3600 and 4500 Hz, respectively. The speech stimulus was presented in alternating polarity monaurally to the right ear at 80 dB SPL through an insert earphone. Responses were collected with the Bio-logic Navigator Pro System (Natus Medical Incorporated, San Carlos, California) using a recording procedure previously described in detail (Banai, Hornickel, 2009, Dhar, Abel, 2009, Hornickel, Skoe, 2009a, Krizman et al., 2010, Song, Nicol, 2010). During data collection, the subject was seated comfortably in a soundproof room and watched a movie of his or her choice. The left ear remained unoccluded and the movie soundtrack was set to < 40 dB SPL so as to be heard at a level that would not mask the stimulus. Using Ag/AgCl electrodes, the evoked response was collected from the vertex (Cz) referenced to the ipsilateral (right) ear lobe and the forehead was used as ground. All procedures were approved by the Northwestern University Institutional Review Board. All participants gave their consent to participate and were compensated monetarily for their time.
Data Analysis
For each subject, peak latencies were visually identified and corresponding amplitudes were determined for seven peaks in the brainstem response, including the onset (V and A), consonant-to-vowel transition (C), offset (O) and FFR (D, E and F) peaks. The onset peaks, V and A, have been shown to be analogous to the click-evoked response peaks V and Vn (Song et al., 2006). Therefore, criteria similar to the standard peak-picking criteria of these click-evoked peaks (Hall, 2006) were used to identify the speech-evoked onset peaks. Peak V was selected as the largest peak near 6.5 ms immediately before the negative slope, and A was specified as the first point at the bottom of this downward slope as identified by a plateau or slope reversal that lasted over three points near the expected latency range for peak A. Peaks C, D, E, F, and O were identified as the deepest troughs within the expected latency range for each peak, as determined by previously established young adult normative data (Dhar, Abel, 2009, Hornickel, Skoe, 2009a). Typical latencies are: C ~ 18.5 ms, D ~ 22 ms, E ~ 10 ms following D, peak F ~ 10 ms after peak E, and O was centered around 48 ms. If two points were equivalent in amplitude within the trough, the one with the earlier latency was always chosen. If the peak was a plateau, such that multiple points (≥ 3) were of the same maximum amplitude, the center point was always chosen. Any ambiguities in peak selection in the averaged waveform (6000 trials) were resolved by comparison of the two sub-average waveforms (3000 trials each). Two experienced peak pickers, one blind to subject sex, separately identified the peak latencies in each subject’s response. If the two experts could not agree on a latency, the peak was marked as “not reliable” and excluded from analyses. Additionally, any peak smaller than the average amplitude of the pre-stimulus baseline activity (from −15.4 ms to 0 ms) was deemed “not reliable” and excluded from analyses. Across all peaks in the waveform, peak reliability was 96%. Three composite measures of neural synchrony to the onset of the stimulus were also analyzed: V to A interpeak latency, V to A peak-to-trough amplitude, and the slope of the VA complex as calculated by the change in peak amplitude over time. Frequency encoding was analyzed using a fast Fourier analysis of the FFR (11.4 – 40.6 ms), a region of the response that includes peaks C, D, E and F. Three ranges, corresponding to the major frequencies of the stimulus, were analyzed: the fundamental frequency (F0) 103–125 Hz, the first formant frequency range (F1) 180 – 755 Hz, and higher frequencies (HF) 756–1130 Hz, which are between the first formant and the second formants, but still within the phase-locking capabilities of the brainstem (Liu et al., 2006). Mean values for male and female participants in the current study are presented in Table 1.
Table 1.
The mean (standard deviation) latencies and amplitudes of the males and females are given for peak V of the click-evoked response and the seven peaks of the speech-ABR. Females had significantly earlier latencies at click-evoked peak V, and peaks V and A of the speech-evoked response. The slope of the onset response, and the spectral magnitude of the higher frequencies were larger in females t.han males. Significance (p < 0.0033) is indicated with asterisks. Due to the multiple factors influencing the amplitude of individual peaks in the temporal waveform, analyses were not performed on peak amplitude differences between males and females; however, mean values are provided in the table
| Male | Female | ||
|---|---|---|---|
| Latency (ms) | |||
| Click | |||
| ***V | 5.84 (0.15) | 5.72 (0.14) | |
| Speech-ABR | |||
| ***V | 6.78 (0.24) | 6.60 (0.24) | |
| ***A | 7.79 (0.26) | 7.54 (0.34) | |
| C | 18.60 (0.69) | 18.63 (0.67) | |
| D | 22.80 (0.56) | 22.75 (0.81) | |
| E | 31.18 (0.46) | 31.04 (0.62) | |
| F | 39.81 (0.63) | 39.50 (0.45) | |
| O | 48.50 (0.51) | 48.25 (0.36) | |
| Amplitude (µV) | |||
| Click | |||
| V | 0.19 (0.10) | 0.25 (0.13) | |
| Speech-ABR | |||
| V | 0.11 (0.05) | 0.13 (0.07) | |
| A | −0.19 (0.06) | −0.22 (0.07) | |
| C | −0.04 (0.05) | −0.06 (0.05) | |
| D | −0.13 (0.10) | −0.15 (0.08) | |
| E | −0.21 (0.06) | −0.25 (0.10) | |
| F | −0.13 (0.10) | −0.19 (0.11) | |
| O | −0.13 (0.07) | −0.17 (0.07) | |
| SNR | 2.76 (1.08) | 2.89 (0.92) | |
| Speech-ABR Onset Measures | |||
| Duration (ms) | 1.01 (0.22) | 0.97 (0.28) | |
| Amplitude (µV) | 0.30 (0.09) | 0.35 (0.11) | |
| **Slope(ms/µV) | −0.30 (0.10) | 0.39 (0.16) | |
| Spectral Magnitude (µV) | |||
| F0 | 0.0615 (0.031) | 0.0651 (0.023) | |
| F1 | 0.0078 (0.004) | 0.0103 (0.005) | |
| ***HF | 0.0033 (0.001) | 0.0043 (0.001) | |
Independent sample, two-tail t-tests were used to determine significant differences in the speech-evoked response between males and females for each of the dependent variables. Results are reported with a correction for multiple comparisons (α = 0.0033). Data processing was performed using custom routines coded in Matlab 2006b (The MathWorks, Inc., Natick, MA) and statistical analyses were performed in SPSS (SPSS Inc., Chicago, IL).
Results
Peak Timing
Consistent with sex differences reported for the click-evoked ABR, the timing of the onset peaks of the speech-evoked response were sex dependent with females having significantly earlier peak latencies at peaks V (t(75) = 3.496, p = 0.001) and A (t(76) = 3.326, p = 0.001) compared to males (Fig. 2). Timing of the other peaks in the speech-evoked response, when correcting for multiple comparisons, was not dependent on the sex of the subject, including the transition peak C (t(62) = -0.120, p = 0.905), the FFR peaks, D (t(73) = 0.501, p = 0.618), E (t(74) = 1.099, p = 0.275) and F (t(76) = 2.464, p = 0.016), and the offset peak, O (t(73) = 2.5, p = 0.015). The interpeak interval of the FFR peaks corresponding to the period of the fundamental frequency, D to E (t(69) = 0.964, p=0.338) and E to F (t(72) = 0.679, p=0.499) also did not differ significantly between males and females. This coincides with the absence of sex differences for the F0 in the frequency domain (see Spectral Magnitude section below).
Figure 2.
Differences in the grand average responses to the 40 ms speech stimulus [da] between males and females. (a) The grand average response of the males (black) and the females (gray) are plotted for comparison. The stimulus waveform is plotted above the male and female responses with a delay of 7 ms in order to emphasize the stimulus characteristics evoking the corresponding response peaks. (b) Mean latencies at each of the characteristic peaks of the speech-ABR are shown with error bars representing +/− 1 standard error of the mean. From these plots it is clear that not all peaks of the speech-evoked response are affected by sex. The latencies of the onset peaks, V and A, are sex dependent. At these peaks, females consistently demonstrate earlier latencies than males. In contrast, the transition peak, C the peaks of the frequency following response, peaks D, E, and F, and the offset peak O are sex invariant, with males and females having similar peak latencies at these peaks.
Composite Onset Measures
The response to the onset of [da] was further analyzed using composite measures. The slope from peak V to peak A differed between males and females (t(75) = 2.794, p = 0.007), with females having steeper slopes. Neither of the measures contributing to the VA slope were significant (VA peak to trough amplitude, t(75) = −2.351, p = 0.021; interpeak interval t(75) = 0.685, p = 0.495).
Spectral Magnitude
To evaluate the spectral encoding in the evoked response to the formant transition, a fast Fourier transform was performed over the 11 ms to 40 ms range of the response. Over this region, the encoding of the F0 (t(76) = −0.633, p = 0.529), and the first formant range (t(76) = −2.314, p = 0.023) were not different between the sexes while the higher frequency response components up to 1150 Hz (t(76) = −3.477, p = 0.001) were larger in females (Fig. 3). Taken together with the differences in the timing of the onset peaks, these results support the differential encoding of the fast (i.e. onset and high frequency) elements of speech between females and males.
Figure 3.
A fast Fourier analysis of the frequency following response revealed no differences in the response to the fundamental frequency (F0) and first formant (F1) of the speech stimulus. However, compared to males (black), females (gray) had more robust spectral encoding of higher frequency (HF) information (a) Boxes outline the three frequency ranges (F0, F1, HF) and dashed lines represent one standard error of the mean spectral magnitude. (b) Bar graphs represent the mean spectral amplitude (+/− 1 standard error) of the same three frequency regions for females and males.
Discussion
The purpose of this study was to identify the aspects of the speech-evoked brainstem response of adult subjects that show sex differences. We hypothesized that differences would exist, with females having faster and larger responses than males, and that these differences would be restricted to the encoding of the rapid features of the speech syllable such as the onset of the noise burst and formant-related frequencies. Indeed, females had significantly earlier encoding of the stimulus onset and greater subcortical representation of the higher frequencies in the stimulus. As predicted, no difference was demonstrated in the encoding of the F0 (neither spectral magnitude nor the timing between F0 peaks).
Behaviorally, morphologically, and physiologically, sex differences have been demonstrated to exist throughout the auditory system (Jerger and Hall, 1980, McFadden, 1993b, 1998, McFadden, Martin, 2009, Michalewski et al., 1980). What is of interest here, however, is that the sex differences in the evoked brainstem response to a complex speech stimulus are not pervasive in that they are not uniformly present in every aspect of the response. Aspects of the response that encode the faster elements of speech differed between males and females while slower elements, specifically the F0, did not. As is discussed below, our findings are in agreement with selective sex differences observed for click-evoked and tone-evoked responses (Hoormann, Falkenstein, 1992) as well as behavioral and cognitive processing differences in humans (Burman et al., 2008, Cahill, 2006, Camarata and Woodcock, 2006) and animals (Clark et al., 2000a, b, Fitch et al., 1993b, Herman et al., 1997). Moreover, our results parallel differences in the encoding of the fast elements of speech that is seen in the subcortical encoding of speech in good and poor readers (Banai, Hornickel, 2009).
Peripheral and anatomical sex differences
In the click-evoked ABR, females have, on average, earlier peak latencies than males (Jerger and Hall, 1980). An initial explanation for this latency difference was the difference in head size between males and females, with males having a larger diameter, on average, than females (Aoyagi et al., 1990, Church et al., 1984). However, others have found the functional-anatomical correlation to be too weak to be considered a valid explanation (Durrant et al., 1990, Sabo et al., 1992), especially given that latency differences persist when comparing males and females of equal head size (Trune et al., 1988). Our results support the notion that head size is not an exclusive contributor to sex differences given that the speech-ABR sex differences are selective to specific aspects of the response.
Although gross morphological differences such as head size may not account for the subcortical dissimilitude between males and females, sexual dimorphism within the inner ear maybe a contributing factor. Indeed, males and females differ in cochlear size, with males having longer cochlear ducts than females, resulting in longer cochlear travel times in males (Bowman et al., 2000, Don et al., 1993, Sato et al., 1991). The travel time difference, likely affected by the greater stiffness of the female basilar membrane resulting from its shorter length, may contribute to females having earlier latencies relative to males in the speech-ABR. Moreover, these peripheral differences may be the consequence of differential activation of the oliviocochlear system, suggesting sex differences in efferent modulation (Bilger, Matthies, 1990, Ismail and Thornton, 2003, McFadden, 1993b, McFadden, Martin, 2009).
Cortical sex differences in the auditory system
In addition to the differences in the male and female peripheral hearing mechanism, behavioral and imaging studies have indicated that male and female cortices process acoustic stimuli differently. For example, fMRI studies have identified stronger activation of cortical language processing areas in females, as compared to males, throughout development (Burman, Bitan, 2008), which may account for advantages in verbal and written language development seen in females (Bauer et al., 2002, Bornstein et al., 2000, Martin and Hoover, 1987). The planum temporale, which is important in auditory processing (Binder et al., 1996), is symmetric in females but larger in the left hemisphere of males, (Kulynych et al., 1994) and this asymmetry has been shown to be reversed or nonexistent in dyslexic males (Hynd et al., 1990). Moreover, a study looking at sex differences in cortical language processing found that males and females differ in the functional organization of the brain for phonological processing such that females showed bilateral activation during phonological processing tasks while male activation was strongest in the left hemisphere (Shaywitz et al., 1995).
Differences in hemispheric laterality during language processing tasks have been observed behaviorally as well. In a dichotic listening test, in which two different words were presented, one to each ear simultaneously, males demonstrated a significant right ear advantage (REA) for performance on the task while females performed similarly on words presented to either ear (Kimura and Harshman, 1984, Lake and Bryden, 1976). However, because slowing down or speeding up the formant transitions within a speech syllable alters the magnitude of the REA for speech (Schwartz and Tallal, 1980), this may explain why other studies have reported an REA regardless of sex (i.e, both sexes showed an REA) (Bryden, 1988). Similarly in rodents, sex differences to tone sequences have been demonstrated, with male rodents having a stronger REA than female rodents (Fitch et al., 1993a).
Additional rodent work investigating the effects of bilateral cortical lesions shortly following birth demonstrates a difficulty for early-cortical lesioned male, but not female, rodents to discriminate rapidly presented tones (< 350 ms), while both sexes could reliably discriminate tones presented at longer interstimulus intervals (ISIs) (Herman, Galaburda, 1997). The cortical lesions resulted in significantly smaller cells in the medial geniculate nucleus (MGN) in early-lesioned male, but not female rodents (Herman, Galaburda, 1997).
Role of estrogen in sex differences in the auditory system
Larger and more frequent spontaneous otoacoustic emissions (OAEs) (Bilger, Matthies, 1990, McFadden, 1993b) and stronger click-evoked OAEs exist in healthy, normal hearing young adult females than males (McFadden, Martin, 2009). Females also demonstrate more acute sound sensitivity than males (Rogers et al., 2003, Sagi et al., 2007). These differences are reduced during menopause (Hultcrantz et al., 2006, Murphy and Gates, 1997, Wharton and Church, 1990), in females taking oral contraception (McFadden, 2000), and in females who have a male twin (McFadden, 1993a, McFadden et al., 1996), which suggests a role of hormones, including estrogen, in improving auditory function in females (McFadden, Martin, 2009). In support of this hormonal explanation, females with Turner syndrome, a chromosomal abnormality resulting in estrogen deficiency, demonstrate longer click-ABR latencies and earlier age-related hearing loss, similar to males (Beckman et al., 2004, Güngör et al., 2000, Hultcrantz et al., 1994). It has also been suggested that auditory thresholds vary with the menstrual cycle in females. For example, in some females with Meniere’s disease, auditory symptoms are exacerbated during the premenstrual phase, when estrogen levels are lowest (Andrews et al., 1992, Andrews and Honrubia).
Rodent studies have shown that when the estrogen receptor (ER-beta) is knocked out, severe and early onset presbycusis is evident (Wang et al., 2001) and that ovariectomizing female rodents results in delayed electrophysiological responses from cochlear and brainstem structures (Coleman et al., 1994). Furthermore, prenatal stress more adversely affects male rodents, resulting in cortical symmetry, similar to female rodents (Fleming et al., 1986, Power and Moore, 1986). Taken together with evidence that lesioning the MGN results in cell size reduction in male, but not female rodents, these sex-specific effects support a role of neuroendocrine systems in the development of the functional specialization of the brain (Berrebi et al., 1988, Herman, Galaburda, 1997). For these reasons, if estrogen does influence the efficacy of the auditory system, it may do so by enhancing synaptic transmission and improving neural conduction (Tremere et al., 2009, Tremere and Pinaud, 2011). These enhancements of the auditory system, in conjunction with the differences in size of the auditory periphery may contribute to the earlier and larger responses seen in the speech-ABRs of females compared to males. Additionally, the corticofugal system, which acts in tandem with peripheral (Don, Ponton, 1993, Eggermont and Don, 1980) and experience-dependent factors (Kraus and Chandrasekaran, 2010, Krishnan et al., 2005, Parbery-Clark, Skoe, 2009a, Strait et al., 2009), is known to modify brainstem processing (Bajo et al., 2009, Gao and Suga, 1998, 2000, Ma and Suga, 2001a, b, Suga, 2008, Suga and Ma, 2003, Tzounopoulos and Kraus, 2009). The speech-ABR sex differences likely reflect an interaction between peripheral and cortical factors that are mediated by a reciprocal network of afferent and efferent synapses that are all likely influenced by estrogen activity.
Parallels with reading impaired population
Between the sexes, disparities in encoding of auditory information are apparent in the response to transient cues or rapidly presented stimuli. These differences may relate to differences in temporal encoding between normal and impaired readers. It has been demonstrated that an ISI of ~ 330 ms or a formant transition of ~80 ms is the behavioral discrimination threshold for individuals with language or reading disabilities (Tallal and Newcombe, 1978, Tallal and Piercy, 1973, 1974, 1975). Stop consonant speech syllables, such as [da], which contain rapidly changing formant transitions, which contain timing cues on the order of milliseconds, demonstrate a strong REA and are exceedingly difficult for language-impaired children to discriminate (Bradlow, Kraus, 1999, Kraus et al., 1996, Tallal, 1976, Tallal and Piercy, 1974, 1975). The stimulus presentation rate and the length of the dynamic formant transition used here (50 ms ISI and 40 ms formant transition) are well within the range of rates that are difficult for language-impaired children to differentiate behaviorally. Even in presumably normal males and females, differences in encoding of slow versus fast elements of a stimulus are seen in the auditory brainstem response (Hornickel, Skoe, 2009a, Krizman, Skoe, 2010). Interestingly, these rate-sensitive (i.e. fast) components of the speech-ABR correspond both to the components of the response that demonstrate sex differences and those that are deficient in impaired readers (Banai, Hornickel, 2009). Males are significantly more likely to have a language or reading impairment, such as dyslexia (Nass, 1993) and this disability may result from a general difficulty in processing rapidly presented, dynamic stimuli (Farmer and Klein, 1995, Tallal, 1975, 1976, 1979, Temple et al., 2000). Consistent with “extreme male brain” (EMB) theory, our results suggest that dyslexia may represent an extreme form of what is observed in the typical male brain (Baron-Cohen et al., 2005).
Conclusions
This study demonstrated sex differences in the encoding of the fast, but not the slow elements of speech, with females having significantly faster and larger magnitude responses to only the transient aspects of the stimulus compared to males. Although we tested a normal population and cannot speak directly to language impairments, it is interesting that within this population, sex differences in the encoding of a speech stimulus are still apparent. Both the faster timing of the transient peaks and the larger spectral magnitude of the higher frequency components likely reflect more synchronous neural activity in females in response to the rapidly changing features of the acoustic stimulus. No sex differences, however, were seen in response to the slower, sustained components, indicating similar neural phase-locking between males and females to these components. The prevalence of language and reading impairments, including dyslexia, in males combined with the demonstrated disparity between males and females in the subcortical encoding of a rapidly transitioning stop-consonant speech syllable ([da]), provide insight into the biological and genetic processes that influence language processing. This study serves as a foundation for studying sex differences in an impaired population. Future research should explore the potential of the speech-ABR to be used as a neural marker for assessing individuals with auditory-based disorders.
Highlights.
Male and female young adults have measurably different neural responses to speech; to the fast acoustic components of speech, female are generally earlier and more robust than male responses.
The differences observed between males and females in the nervous system’s response to speech parallel those previously reported for poor, relative to good, readers.
These sex differences provide a baseline for interpreting the higher incidence of language impairment in males, and the neural deficits associated with these disorders.
Acknowledgements
The authors thank the members of the Auditory Neuroscience Laboratory, who helped collect the data, and to Dawna Bagherian and Winnie Lin for their help in preparing and processing the data. Thank you to Catherine Warrier, Karen Chan, and Trent Nicol for their comments on the manuscript. This work was supported by R01 DC01510.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DA, Nicol T, Zecker S, Kraus N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. J Neurosci. 2008;28:3958–3965. doi: 10.1523/JNEUROSCI.0187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams DA, Nicol T, Zecker SG, Kraus N. Auditory Brainstem Timing Predicts Cerebral Asymmetry for Speech. J Neurosci. 2006;26:11131–11137. doi: 10.1523/JNEUROSCI.2744-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear Res. 2008;245:35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Akhoun I, Gallego S, Moulin A, Menard M, Veuillet E, Berger-Vachon C, et al. The temporal relationship between speech auditory brainstem responses and the acoustic pattern of the phoneme /ba/ in normal-hearing adults. Clin Neurophysiol. 2008;119:922–933. doi: 10.1016/j.clinph.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Anderson S, Kraus N. Objective Neural Indices of Speech-in-Noise Perception. Trends Amplif. 2010;14:73–83. doi: 10.1177/1084713810380227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. J Neurosci. 2010a;30:4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Zecker S, Kraus N. Brainstem Correlates of Speech-in-Noise Perception in Children. Hear Res. 2010b;270:151–157. doi: 10.1016/j.heares.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JC, Ator GA, Honrubia V. The exacerbation of symptoms in Meniere's disease during the premenstrual period. Arch Otolaryngol Head Neck Surg. 1992;118:74–78. doi: 10.1001/archotol.1992.01880010078020. [DOI] [PubMed] [Google Scholar]
- Andrews JC, Honrubia V. Premenstrual Exacerbation of Meniere's Disease Revisited. Otolaryngol Clin North Am. 43:1029–1040. doi: 10.1016/j.otc.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Aoyagi M, Kim Y, Yokoyama J, Kiren T, Suzuki Y, Koike Y. Head size as a basis of gender difference in the latency of the brainstem auditory-evoked response. Int J Audiol. 1990;29:107–112. doi: 10.3109/00206099009081652. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci. 2009;13:253–260. doi: 10.1038/nn.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballachanda BB, Rupert A, Moushegian G. Asymmetric frequency-following responses. J Am Acad Audiol. 1994;5:133–137. [PubMed] [Google Scholar]
- Banai K, Hornickel J, Skoe E, Nicol T, Zecker S, Kraus N. Reading and Subcortical Auditory Function. Cereb Cortex. 2009;19:2699–2707. doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Nicol T, Zecker SG, Kraus N. Brainstem Timing: Implications for Cortical Processing and Literacy. J Neurosci. 2005;25:9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex Differences in the Brain: Implications for Explaining Autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Develop Sci. 2010;13:77–91. doi: 10.1111/j.1467-7687.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Maher VL. The frequency-following response to continuous tones in humans. Hear Res. 1986;21:167–177. doi: 10.1016/0378-5955(86)90037-7. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Goldfield BA, Reznick JS. Alternative approaches to analyzing individual differences in the rate of early vocabulary development. Appl Psycholinguist. 2002;23:313–335. [Google Scholar]
- Beckman A, Conway GS, Cadge B. Audiological features of Turner's syndrome in adults. Int J Audiol. 2004;43:533–544. doi: 10.1080/14992020400050068. [DOI] [PubMed] [Google Scholar]
- Belin P, Zilbovicius M, Crozier S, Thivard L, Fontaine A, Masure MC, et al. Lateralization of speech and auditory temporal processing. J Cogn Neurosci. 1998;10:536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Fitch RH, Ralphe DL, Denenberg JO, Friedrich VL. Corpus callosum: region-specific effects of sex, early experience and age. Brain Res. 1988;438:216–224. doi: 10.1016/0006-8993(88)91340-6. [DOI] [PubMed] [Google Scholar]
- Bilger RC, Matthies ML, Hammel DR, Demorest ME. Genetic implications of gender differences in the prevalence of spontaneous otoacoustic emissions. J Speech Hear Res. 1990;33:418–432. doi: 10.1044/jshr.3303.418. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996;119:1239–1247. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- Boemio A, Fromm S, Braun A, Poeppel D. Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nat Neurosci. 2005;8:389–395. doi: 10.1038/nn1409. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Haynes OM, Painter KM, Genevro JL. Child language with mother and with stranger at home and in the laboratory: A methodological study. J Child Lang. 2000;27:407–420. doi: 10.1017/s0305000900004165. [DOI] [PubMed] [Google Scholar]
- Bowman DM, Brown DK, Kimberley BP. An examination of gender differences in DPOAE phase delay measurements in normal-hearing human adults. Hear Res. 2000;142:1–11. doi: 10.1016/s0378-5955(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Bradlow AR, Kraus N, Nicol TG, McGee TJ, Cunningham J, Zecker SG, et al. Effects of lengthened formant transition duration on discrimination and neural representation of synthetic CV syllables by normal and learning-disabled children. J Acoust Soc Am. 1999;106:2086–2096. doi: 10.1121/1.427953. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Correlates of the dichotic right-ear effect. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 1988;24:313–319. doi: 10.1016/s0010-9452(88)80039-x. [DOI] [PubMed] [Google Scholar]
- Burman DD, Bitan T, Booth JR. Sex differences in neural processing of language among children. Neuropsychologia. 2008;46:1349–1362. doi: 10.1016/j.neuropsychologia.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Camarata S, Woodcock R. Sex differences in processing speed: Developmental effects in males and females. Intelligence. 2006;34:231–252. [Google Scholar]
- Chandrasekaran B, Hornickel J, Skoe E, Nicol T, Kraus N. Context-dependent encoding in the human auditory brainstem relates to hearing speech in noise: Implications for developmental dyslexia. Neuron. 2009;64:311–319. doi: 10.1016/j.neuron.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: Neural origins and plasticity. Psychophysiology. 2010;47:236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MW, Willaims HL, Holloway JA. Brain-stem auditory evoked potentials in the rat: Effects of gender, stimulus characteristics and ethanol sedation. Electroencephalogr Clin Neurophysiol. 1984;59:328–339. doi: 10.1016/0168-5597(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Clark MG, Rosen GD, Tallal P, Fitch RHA. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria: An animal model of developmental language disabilities. J Cogn Neurosci. 2000a;12:828–839. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- Clark MG, Rosen GD, Tallal P, Fitch RH. Impaired two-tone processing at rapid rates in male rats with induced microgyria. Brain Res. 2000b;871:94–97. doi: 10.1016/s0006-8993(00)02447-1. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–215. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: Deficits and strategies for improvement. Clin Neurophysiol. 2001;112:758–767. doi: 10.1016/s1388-2457(01)00465-5. [DOI] [PubMed] [Google Scholar]
- Dhar S, Abel R, Hornickel J, Nicol T, Skoe E, Zhao W, et al. Exploring the relationship between physiological measures of cochlear and brainstem function. Clin Neurophysiol. 2009;120:959–966. doi: 10.1016/j.clinph.2009.02.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M, Ponton CW, Eggermont JJ, Masuda A. Gender differences in cochlear response time: An explanation for gender amplitude differences in the unmasked auditory brain stem response. The Journal of the Acoustical Society of America. 1993;94:2135. doi: 10.1121/1.407485. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Sabo DL, Hyre RJ. Gender, head size, and ABRs examined in large clinical sample. Ear Hear. 1990;11:210. doi: 10.1097/00003446-199006000-00008. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Don M. Analysis of click-evoked brainstem auditory electric potentials using high-pass noise masking and its clinical application. Ann N Y Acad Sci. 1980;338:471–486. doi: 10.1111/j.1749-6632.1982.tb50810.x. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Klein RM. The evidence for a temporal processing deficit linked to dyslexia: A review. Psychon Bull Rev. 1995;2:460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Brown CP, O'Connor K, Tallal P. Functional lateralization for auditory temporal processing in male and female rats. Behav Neurosci. 1993a;107:844–850. doi: 10.1037//0735-7044.107.5.844. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Brown CP, Tallal P. Left hemisphere specialization for auditory temporal processing in rats. Ann N Y Acad Sci. 1993b;682:346–347. doi: 10.1111/j.1749-6632.1993.tb22989.x. [DOI] [PubMed] [Google Scholar]
- Fleming DE, Anderson RH, Rhees RW. Effects of prenatal stress on sexually dimorphic asymmetries in the cerebral cortex of the male rat. Brain Res Bull. 1986;16:395–398. doi: 10.1016/0361-9230(86)90062-6. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci U S A. 1998;95:12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci U S A. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ, Friedman SA. Recognition of Time-Compressed and Natural Speech With Selective Temporal Enhancements by Young and Elderly Listeners. J Speech Lang Hear Res. 2007;50:1181–1193. doi: 10.1044/1092-4388(2007/082). [DOI] [PubMed] [Google Scholar]
- Güngör N, Böke B, Belgin E, Tunçbilek E. High frequency hearing loss in Ullrich-Turner syndrome. Eur J Pediatr. 2000;159:740–744. doi: 10.1007/pl00008338. [DOI] [PubMed] [Google Scholar]
- Hall JW. New handbook of auditory evoked responses. Boston Mass: Pearson; 2006. [Google Scholar]
- Herman AE, Galaburda AM, Fitch RH, Carter AR, Rosen GD. Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cereb Cortex. 1997;7:453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hoormann J, Falkenstein M, Hohnsbein J, Blanke L. The human frequency-following response (FFR): Normal variability and relation to the click-evoked brainstem response. Hear Res. 1992;59:179–188. doi: 10.1016/0378-5955(92)90114-3. [DOI] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Kraus N. Subcortical laterality of speech encoding. Audiol Neurootol. 2009a;14:198–207. doi: 10.1159/000188533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Nicol T, Zecker S, Kraus N. Subcortical Differentiation of Voiced Stop Consonants: Relationships to Reading and Speech in Noise Perception. Proc Natl Acad Sci U S A. 2009b;106:13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol (Stockh) 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Sylvén L, Borg E. Ear and hearing problems in 44 middle-aged women with Turner's syndrome. Hear Res. 1994;76:127–132. doi: 10.1016/0378-5955(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D. Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Arch Neurol. 1990;47:919–926. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- Ismail H, Thornton ARD. The interaction between ear and sex differences and stimulus rate. Hear Res. 2003;179:97–103. doi: 10.1016/s0378-5955(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106:387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort, 1976–1982, Rochester, Minn. Mayo Clinic. 2001:1081. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- Kimura D, Harshman RA. Sex differences in brain organization for verbal and non-verbal functions. Sex differences in the brain: the relation between structure and function: proceedings of the 13th International Summer School of Brain Research, Royal Netherlands Academy of Arts and Sciences, Amsterdam, The Netherlands. 1983 August 22–26;1984:423–441. doi: 10.1016/S0079-6123(08)64452-0. [DOI] [PubMed] [Google Scholar]
- Klatt D. Software for cascade/parallel formant synthesizer. J Acoust Soc Am. 1980;67:971–975. [Google Scholar]
- Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nat Rev Neurosci. 2010;11:599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Xu Y, Gandour J, Cariani P. Encoding of pitch in the human brainstem is sensitive to language experience. Cogn Brain Res. 2005;25:161–168. doi: 10.1016/j.cogbrainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Krizman J, Skoe E, Kraus N. Stimulus rate and subcortical auditory processing of speech. Audiol Neurootol. 2010;15:332–342. doi: 10.1159/000289572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl's gyrus and the planum temporale. Cereb Cortex. 1994;4:107. doi: 10.1093/cercor/4.2.107. [DOI] [PubMed] [Google Scholar]
- Lake DA, Bryden MP. Handedness and sex differences in hemispheric asymmetry. Brain Lang. 1976;3:266–282. doi: 10.1016/0093-934x(76)90022-5. [DOI] [PubMed] [Google Scholar]
- Lamprecht-Dinnesen A, Pohl M, Hartmann S, Heinecke A, Ahrens S, Müller E, et al. Effects of age, gender and ear side on SOAE parameters in infancy and childhood. Audiol Neurootol. 2000;3:386–401. doi: 10.1159/000013808. [DOI] [PubMed] [Google Scholar]
- Liu LF, Palmer AR, Wallace MN. Phase-Locked Responses to Pure Tones in the Inferior Colliculus. J Neurophysiol. 2006;95:1926–1935. doi: 10.1152/jn.00497.2005. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Corticofugal modulation of duration-tuned neurons in the midbrain auditory nucleus in bats. Proc Natl Acad Sci U S A. 2001a;98:14060–14065. doi: 10.1073/pnas.241517098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Plasticity of bat's central auditory system evoked by focal electric stimulation of auditory and/or somatosensory cortices. J Neurophysiol. 2001b;85:1078–1087. doi: 10.1152/jn.2001.85.3.1078. [DOI] [PubMed] [Google Scholar]
- Martin DJ, Hoover HD. Sex differences in educational achievement: A longitudinal study. The Journal of Early Adolescence. 1987;7:65–83. [Google Scholar]
- McAnally KI, Stein JF. Auditory Temporal Coding in Dyslexia. Proceedings: Biological Sciences. 1996;263:961–965. doi: 10.1098/rspb.1996.0142. [DOI] [PubMed] [Google Scholar]
- McFadden D. A masculinizing effect on the auditory systems of human females having male co-twins. Proc Natl Acad Sci U S A. 1993a;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. A speculation about the parallel ear asymmetries and sex differences in hearing sensitivity and otoacoustic emissions. Hear Res. 1993b;68:143–151. doi: 10.1016/0378-5955(93)90118-k. [DOI] [PubMed] [Google Scholar]
- McFadden D. Sex differences in the auditory system. Dev Neuropsychol. 1998;14:261–298. [Google Scholar]
- McFadden D. Masculinizing effects on otoacoustic emissions and auditory evoked potentials in women using oral contraceptives. Hear Res. 2000;142:23–33. doi: 10.1016/s0378-5955(00)00002-2. [DOI] [PubMed] [Google Scholar]
- McFadden D, Loehlin JC, Pasanen EG. Additional findings on heritability and prenatal masculinization of cochlear mechanisms: click-evoked otoacoustic emissions. Hear Res. 1996;97:102–119. [PubMed] [Google Scholar]
- McFadden D, Martin GK, Stagner BB, Maloney MM. Sex differences in distortion-product and transient-evoked otoacoustic emissions compared. The Journal of the Acoustical Society of America. 2009;125:239–246. doi: 10.1121/1.3037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM, Johnston P, Schreiner C, Miller SL, Tallal P. Temporal processing deficits of language-learning impaired children ameliorated by training. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Thompson LW, Patterson JV, Bowman TE, Litzelman D. Sex differences in the amplitudes and latencies of the human auditory brain stem potential. Electroencephalogr Clin Neurophysiol. 1980;48:351–356. doi: 10.1016/0013-4694(80)90271-0. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Gates GA. Hearing loss: Does gender play a role. Medscape Womens Health. 1997;2:2. [PubMed] [Google Scholar]
- Nass RD. Sex differences in learning abilities and disabilities. Annals of Dyslexia. 1993;43:61–77. doi: 10.1007/BF02928174. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Kraus N. Musical experience limits the degradative effects of background noise on the neural processing of sound. J Neurosci. 2009a;29:14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery-Clark A, Skoe E, Lam C, Kraus N. Musician enhancement for speech in noise. Ear Hear. 2009b;30:653–661. doi: 10.1097/AUD.0b013e3181b412e9. [DOI] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as 'asymmetric sampling in time'. Speech Communication. 2003;41:245–255. [Google Scholar]
- Power KL, Moore CL. Prenatal stress eliminates differential maternal attention to male offspring in Norway rats. Physiol Behav. 1986;38:667–671. doi: 10.1016/0031-9384(86)90262-3. [DOI] [PubMed] [Google Scholar]
- Rogers DS, Harkrider AW, Burchfield SB, Nabelek AK. The influence of listener's gender on the acceptance of background noise. J Am Acad Audiol. 2003;14:372–382. [PubMed] [Google Scholar]
- Russo N, Nicol T, Musacchia G, Kraus N. Brainstem responses to speech syllables. Clin Neurophysiol. 2004;115:2021–2030. doi: 10.1016/j.clinph.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav Brain Funct. 2010;6:60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, et al. Deficient brainstem encoding of pitch in children with Autism Spectrum Disorders. Clin Neurophysiol. 2008;119:1720–1731. doi: 10.1016/j.clinph.2008.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Fergusson D, Horwood LJ, Goodman R, Maughan B, et al. Sex differences in developmental reading disability: new findings from 4 epidemiological studies. JAMA. 2004;291:2007–2012. doi: 10.1001/jama.291.16.2007. [DOI] [PubMed] [Google Scholar]
- Sabo DL, Durrant JD, Curtin H, Boston JR, Rood S. Correlations of neuroanatomical measures to auditory brain stem response latencies. Ear Hear. 1992;13:213–222. doi: 10.1097/00003446-199208000-00001. [DOI] [PubMed] [Google Scholar]
- Sagi E, D'Alessandro LM, Norwich KH. Identification Variability as a Measure of Loudness: An Application to Gender Differences. Can J Exp Psychol. 2007;61:64–70. doi: 10.1037/cjep2007007. [DOI] [PubMed] [Google Scholar]
- Sato H, Sando I, Takahashi H. Sexual dimorphism and development of the human cochlea: computer 3-D measurement. Acta Otolaryngol (Stockh) 1991;111:1037–1040. doi: 10.3109/00016489109100753. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Tallal P. Rate of acoustic change may underlie hemispheric specialization for speech perception. Science. 1980;207:1380–1381. doi: 10.1126/science.7355297. [DOI] [PubMed] [Google Scholar]
- Serniclaes W, Sprenger-Charolles L. Categorical perception of speech sounds and dyslexia. Curr Psychol Lett: Behav Brain Cogn. 2003;10 (published online) [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Cone-Wesson B. Asymmetric cochlear processing mimics hemispheric specialization. Science. 2004;305:1581. doi: 10.1126/science.1100646. [DOI] [PubMed] [Google Scholar]
- Sininger YS, Cone-Wesson B. Lateral asymmetry in the ABR of neonates: evidence and mechanisms. Hear Res. 2006;212:203–211. doi: 10.1016/j.heares.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Skoe E, Kraus N. Auditory brain stem response to complex sounds: A tutorial. Ear Hear. 2010;31:302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Banai K, Russo NM, Kraus N. On the relationship between speech- and nonspeech-evoked auditory brainstem responses. Audiol Neurootol. 2006;11:233–241. doi: 10.1159/000093058. [DOI] [PubMed] [Google Scholar]
- Song JH, Nicol T, Kraus N. Test-retest reliability of the speech-evoked auditory brainstem response. Clin Neurophysiol. 2010;122:346–355. doi: 10.1016/j.clinph.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait DL, Kraus N, Skoe E, Ashley R. Musical experience and neural efficiency - effects of training on subcortical processing of vocal expressions of emotion. Eur J Neurosci. 2009;29:661–668. doi: 10.1111/j.1460-9568.2009.06617.x. [DOI] [PubMed] [Google Scholar]
- Suga N. Role of corticofugal feedback in hearing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:169–183. doi: 10.1007/s00359-007-0274-2. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Tallal P. Perceptual and linguistic factors in the language impairment of developmental dysphasics: an experimental investigation with the token test. Cortex. 1975;11:196–205. doi: 10.1016/s0010-9452(75)80002-5. [DOI] [PubMed] [Google Scholar]
- Tallal P. Rapid auditory processing in normal and disordered language development. J Speech Hear Res. 1976;19:561–571. doi: 10.1044/jshr.1903.561. [DOI] [PubMed] [Google Scholar]
- Tallal P. Performance of children with auditory perceptual disorders on a time-compressed speech discrimination measure. J Speech Hear Disord. 1979;44:136–137. doi: 10.1044/jshd.4401.136. [DOI] [PubMed] [Google Scholar]
- Tallal P. Language disabilities in children: a perceptual or linguistic deficit? J Pediatr Psychol. 1980;5:127–140. doi: 10.1093/jpepsy/5.2.127. [DOI] [PubMed] [Google Scholar]
- Tallal P, Newcombe F. Impairment of auditory perception and language comprehension in dysphasia. Brain Lang. 1978;5:13–34. doi: 10.1016/0093-934x(78)90003-2. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Defects of non-verbal auditory perception in children with developmental aphasia. Nature. 1973;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: rate of auditory processing and selective impairment of consonant perception. Neuropsychologia. 1974;12:83–93. doi: 10.1016/0028-3932(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: the perception of brief vowels and extended stop consonants. Neuropsychologia. 1975;13:69–74. doi: 10.1016/0028-3932(75)90049-4. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Protopapas A, Nagarajan S, Salz T, Tallal P, et al. Disruption of the neural response to rapid acoustic stimuli in dyslexia: evidence from functional MRI. Proc Natl Acad Sci U S A. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TH, Schwartz DM. Error analysis on the California Consonant Test by manner of articulation. Ear Hear. 1981;2:108–111. doi: 10.1097/00003446-198105000-00004. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-Generated Estradiol Drives Long-Term Optimization of Auditory Coding to Enhance the Discrimination of Communication Signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Mitchell C, Phillips DS. The relative importance of head size, gender and age on the auditory brainstem response. Hear Res. 1988;32:165–174. doi: 10.1016/0378-5955(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kraus N. Learning to encode timing: mechanisms of plasticity in the auditory brainstem. Neuron. 2009;62:463–469. doi: 10.1016/j.neuron.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tasell DJ, Hagen LT, Koblas LL, Penner SG. Perception of short-term spectral cues for stop consonant place by normal and hearing-impaired subjects. J Acoust Soc Am. 1982;72:1771–1780. doi: 10.1121/1.388650. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Burns KS. Brain Stem Responses to Speech in Younger and Older Adults. Ear Hear. 2011;32:168–180. doi: 10.1097/AUD.0b013e3181f534b5. [DOI] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson J. Morphological abnormalities in the brains of estrogen β receptor knockout mice. Proc Natl Acad Sci U S A. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton JA, Church GT. Influence of menopause on the auditory brainstem response. Int J Audiol. 1990;29:196–201. doi: 10.3109/00206099009072850. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biol Psychol. 2004;67:299–317. doi: 10.1016/j.biopsycho.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Gandour JT. Neural specializations for speech and pitch: moving beyond the dichotomies. Phil Trans R Soc B. 2008;363:1087. doi: 10.1098/rstb.2007.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]