Abstract
Objectives
We examined the effect of daily-moderate (2 drinks/day, 7 days/week) and weekend-binge (7 drinks/day, 2 days/week) patterns of alcohol consumption on plasma lipid levels and physiological parameters of atherosclerotic plaque development.
Methods
ApoE k/o mouse were fed (1) ‘daily-moderate’ (blood alcohol content: 0.07%) or (2) ‘weekend-binge’ (blood alcohol content: 0.23%), or (3) an isocaloric cornstarch mix. Then, to induce atherosclerotic plaque formation, all groups underwent partial carotid artery ligation, started on an atherogenic diet and continued on the alcohol feeding regimen. After 2 weeks plasma lipid levels and atherosclerotic plaque formation were assessed.
Results
While there was an increase in HDL-C levels in both binge and moderate groups, LDL-C levels were significantly decreased in the daily-moderate drinking mice and significantly elevated in the weekend-binge drinking mice. In the daily-moderate alcohol group there was a decrease in atherosclerotic plaque volume, concomitant with an increase in lumen volume and decreased macrophage accumulation, when compared to no alcohol mice. In contrast, after 4 weeks of weekend-binge alcohol there was an increase in plaque volume, concomitant with a decrease in lumen volume and increased deposition of macrophages.
Conclusion
These findings demonstrate for the first time a differential effect of daily-moderate vs. weekend-binge alcohol consumption on atherosclerotic plaque development and highlight the importance of patterns of alcohol consumption, as opposed to total amount consumed, in relation to the cardiovascular effects of alcohol.
Keywords: Alcohol, Atherosclerosis, Binge Drinking, Lipids, Macrophages
Introduction
According to the World Health Organization an estimated 17.1 million people died from cardiovascular diseases in 2004, which represents 29% of all deaths 1. There appears to be a complex association between alcohol consumption and cardiovascular health. Overall, the net effect of alcohol consumption on health is detrimental, with an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life-years and approximately 10% of cardiovascular disease-related deaths attributable to alcohol 2.
Moderate alcohol consumption is generally considered to be in the range 1-3 drinks/day, giving rise to blood alcohol levels of approximately 0.046 to 0.092 g% 3. Mortality and morbidity attributable to coronary heart disease is reportedly 20% to 40% lower in light-to-moderate drinkers compared with abstainers 4. However, the probability of coronary heart disease and cardiovascular mortality increases with heavier consumption 5. Binge drinking is a common pattern of excessive alcohol use and according to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) a ‘binge’ is a pattern of drinking that corresponds to consuming 5 or more drinks (male), or 4 or more drinks (female), in about 2 hours 6. Studies suggest that a binge pattern of drinking may precipitate myocardial ischemia or infarction 7 and evidence also exists of an association between binge alcohol consumption and a 2-fold greater mortality after acute myocardial infarction 8. It is thus increasingly apparent that in addition to the volume of consumption, the pattern of drinking must be considered.
The role of drinking pattern in the relation between alcohol consumption and cardiovascular disease has been considered using prospective data from epidemiological studies. However, the validity of self-reported data is an area of concern in such studies 9. To date, there is no evidence in the literature that directly links patterns of alcohol consumption to atherosclerotic plaque development. We show here that daily-moderate and weekend-binge patterns, decrease and increase the development of atherosclerosis, respectively, when compared with no alcohol controls.
Materials and Methods
Mouse Model of Atherosclerosis
6 week old male apoliprotein E knockout (apoE −/−) mice fed a normal chow diet were placed on an alcohol feeding regimen for 2 weeks. Mice then underwent partial carotid artery ligation to induce the formation of an atherosclerotic plaque and to further enhance atherosclerosis progression, animals were started on the Paigen atherogenic diet (D12336, Research Diets, Inc., New Brunswick, NJ). Mice were then continued on the appropriate alcohol feeding regimen for another 2 week period prior to vessel harvest. All alcohol and isocaloric cornstarch mixtures were administered by oral gavage in a final volume of 200 μl.
Mouse Carotid Artery Partial Ligation
The carotid artery ligation model of vascular injury and remodeling was performed essentially as described previously 10. All procedures were approved by the University of Rochester Animal Care Committee. After induction of anesthesia using inhalational isoflurane, a midline cervical incision was made and with the aid of a dissecting microscope, the left external and internal carotid arterial branches were isolated and ligated with a 6-0 silk suture, reducing left carotid blood flow to flow via the patent occipital artery (Figure 1A). The neck incision (2 layers, muscle and skin) was closed with a running suture using 4-0 coated Vicryl. Ligation of the carotid artery in this manner results in a decrease in blood flow in the left carotid artery, concomitant with an increase in the right carotid artery, with an intact endothelial monolayer, when compared with a sham-operated control.
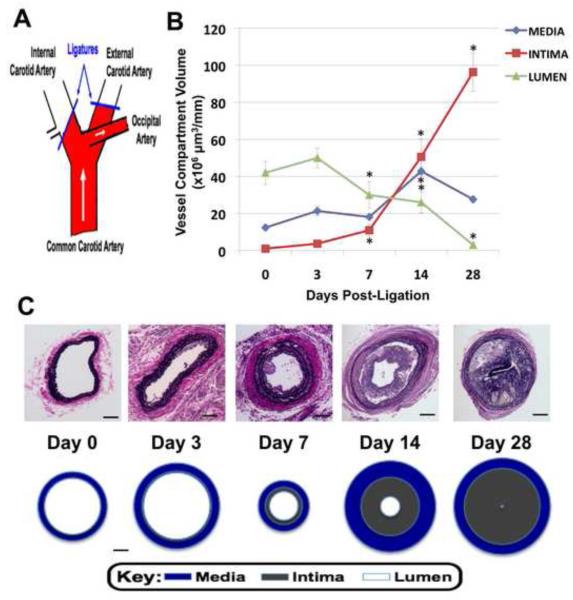
Figure 1.
Time-dependent effect of partial carotid artery ligation on vessel remodeling in apoE KO mice, *p<0.05 vs day 0. A: Schematic of partial ligation of carotid artery. B: Vessel cross-section area derived from cumulative data (n=4) calculated for 1 mm of carotid artery length as detailed in methods section (n=4 per timepoint). C: Representative photomicrographs of Van Gieson stained sections (magnification is 20x) and scale drawings of cross-sections through carotid arteries (n=4). Scale bars: 25 μm.
Histomorphometry and Immunohistochemistry
Mice were perfusion fixed with 10% paraformaldehyde in sodium phosphate buffer (pH 7.0), 0 - 28 days after ligation; and the carotids were harvested and embedded in paraffin. Starting 500 μm below the carotid bifurcation landmark, a series of cross-sections (10 × 5 μm) was made, every 200 μm through 2 mm length of carotid artery. Cross-sections were stained with Verhoeff–van Gieson stain for elastic laminae, and sections were imaged using a microscope (Nikon TE300) equipped with a digital camera (Spot RT). Digitized images were analyzed using microcomputer imaging device software. Assuming a circular structure in vivo, the circumference of the lumen was used to calculate the lumen area; the intimal area was defined by the luminal surface and internal elastic lamina; the medial area was defined by the internal elastic lamina and the external elastic lamina; and the adventitial area was the area between the external elastic lamina and the outer edge, essentially as previously described by Korshunov and Berk 11. For immunohistochemistry, sections were stained by Mac-3 (BD-Pharmingen, 1:50 dilution), or isotype control IgG, followed by biotinylated secondary antibody and streptavidin–horseradish peroxidase. Specimens were then developed with diaminobenzadine/water substrate and counterstained with Harris hematoxylin. Carotid sections were imaged using a microscope (Nikon TE300) equipped with a digital camera (Spot RT).
Blood Alcohol Concentration
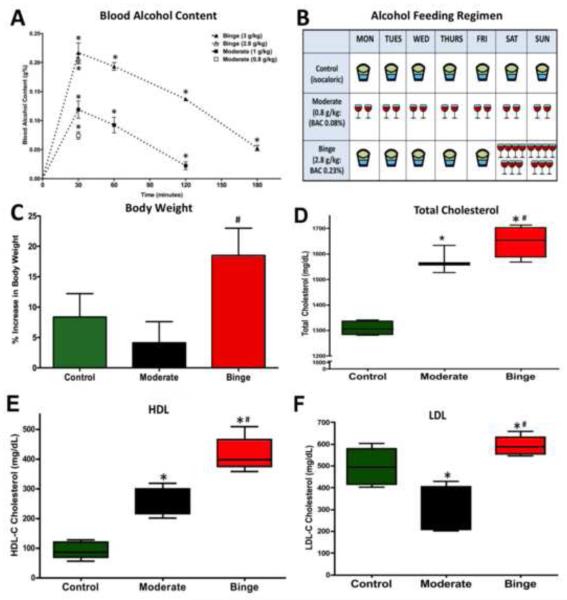
Whole blood samples, collected by submandibular bleeding or cardiac puncture, were diluted 1:5 with water (100 μL of blood plus 400 μL of water) and analyzed for EtOH using head-space gas chromatography on an instrument by the Strong Health Clinical Laboratories (University of Rochester Medical Center, Rochester, NY). The method uses N-propanol as the internal standard and is sensitive to 10 mg/dL of EtOH. The blood samples for plasma lipid analysis were collected at least 3 days after the last binge alcohol gavage, suggesting that the alterations in plasma lipid profiles observed in this group were not due to the acute effects of binge alcohol ingestion. In preliminary experiments 8 week old male apoE KO mice received 1 g/kg or 3 g/kg alcohol in 200 μl water by oral gavage. In mice receiving 1 g/kg ethanol, BAC was 0.12 g% after 30 minutes and 0.10 g% after 60 minutes, with levels becoming undetectable after 2 hours (Figure 2A). In mice receiving 3 g/kg ethanol, a peak BAC of ~0.23 g% was recorded after 30 minutes, with concentrations still remaining within the range of detection after 2 hours (Figure 2A). The mice were then divided into two groups with each group receiving the same weekly volume of alcohol in two different patterns (Figure 2B). The ‘daily-moderate’ group (n=7) received the equivalent of 2 drinks/day for 7 days/week, i.e., 0.8 g/kg alcohol per day, resulting in a BAC of 0.07 g% after 30 minutes (Figure 2A), equivalent to ‘moderate’ consumption. The ‘weekend-binge’ group (n=7) received, 7 drinks/day for 2 days/week, the same total weekly volume as the ‘daily-moderate’ group (5.6 g/kg per week), by the administration of 2.8 g/kg alcohol per day over 2 days, resulting in a peak BAC of 0.20 g% (Figure 2A). To adjust for the calorific content of alcohol (7.1 kcal/g) we ensured that mice in the control ‘no alcohol’ group (n=8) received an isocaloric water/cornstarch mixture by oral gavage.
Figure 2.
A: Effect of alcohol administration on blood alcohol content (BAC) in apoE knockout mice, *p<0.05 vs t0, n=3. B: Schematic representation of weekly alcohol feeding regimen. C: Effect of alcohol administration on % increase in body weight after 4 weeks in apoE knockout mice, *p<0.05 vs control. D-F: Effect of alcohol administration (4 weeks) on plasma total cholesterol, HDL and LDL in apoE knockout mice, *p<0.05 vs control; #p<0.05 vs moderate; n=6-8.
Plasma Lipid Analysis
For analysis of plasma samples, blood was centrifuged at 14,000 rpm for 5 minutes and plasma was collected from the upper layer. Following a 1:10 dilution with PBS total cholesterol content was measured according to the procedure of the Wako Cholesterol E kit (Wako Diagnostics, cat #: 439-17501), and L-type HDL and L-type LDL were measured using the L-type HDL-C and L-type LDL-C kits (Wako Diagnostics, L-Type HDL-C, cat #: 997-72591 and L-Type LDL-C: 997-72591).
Data Analysis
All data is shown as mean ± SEM. Differences among multiple data sets were analyzed by one-way ANOVA followed by a Tukey test. In all analyses, a P value ≤0.05 denoted the presence of a statistically significant difference.
Results
Development of Atherosclerosis
There was a time-dependent atherosclerotic plaque development as determined by increased neointima volume when compared to sham controls, 50.6 ± 8.7 × 106 vs. 1.0 ± 0.3 × 106 μm3/mm (n=4, p<0.05) at 14 days, concomitant with a decrease in lumen volume, 26.0 ± 5.7 × 106 vs. 42.0 ± 6.1 × 106 μm3/mm (n=4, p<0.05) (Figure 1B and 1C).
Patterns of Alcohol Consumption Differentially Affect Body Weights
Body weights of apoE −/− mice were similar among all groups at the beginning of the study (20.47 ± 0.23 g, n=7-8) and all groups consumed similar amounts of food over the experimental period. Control, no alcohol mice, gained ~8.5% body weight in 4 weeks, with mice on the ‘daily-moderate’ alcohol feeding regimen showing slightly smaller weight gains of ~5% body weight (Figure 2C). However, mice fed ‘weekend-binge’ alcohol gained significantly more weight ie. 17.5% over the same period of time (Figure 2C).
Patterns of Alcohol Consumption Differentially Affect Plasma Lipids
After 4 weeks of daily-moderate or weekend-binge patterns of alcohol consumption there was a 20% and 26% increase, respectively, in total cholesterol when compared to control mice (1568 ± 12 and 1648 ± 27 vs 1309 ± 12 mg/dL, n=5-6, p<0.05) (Figure 2D). While there was a 280% and 450% increase in HDL-C levels in the moderate and binge groups, respectively (260 ± 16 and 415 ± 26 vs 92 ± 11 mg/dL, n=5-6, p<0.05) (Figure 2E), LDL-C levels were significantly decreased in the moderate drinking mice and significantly elevated in the binge drinking mice, when compared to no alcohol controls (293 ± 38 and 591 ± 20 vs 496 ± 31 mg/dL, n=5-6, p<0.05) (Figure 2F).
Patterns of Alcohol Consumption Differentially Affect Atherosclerotic Plaque Development
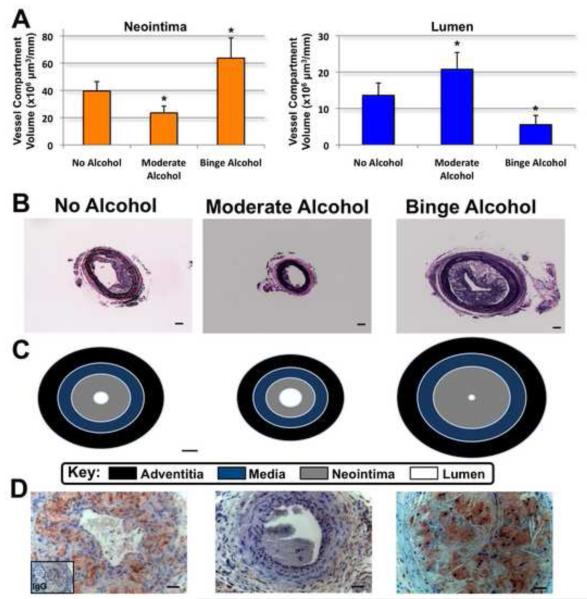
In mice fed daily-moderate alcohol, while there was no change in adventitia and media volume, there was a 40% decrease in atherosclerotic plaque neointima volume concomitant with a 50% increase in patent lumen volume, when compared to control mice (23.5 ± 4.9 vs 40 ± 7.0 × 106 μm3/mm and 20.8 ± 4.6 vs 13.6 ± 3.4 × 106 μm3/mm, n=7-8, p<0.05) (Figures 3A, B and C). In contrast, mice fed a weekend-binge pattern of alcohol exhibited a 60% increase in atherosclerotic plaque neointima volume concomitant with a 60% decrease in patent lumen volume, when compared to control mice (63.7 ± 14.8 vs 40 ± 7.0 × 106 μm3/mm and 5.6 ± 4.6 vs 13.6 ± 3.4 × 106 μm3/mm, n=7-8, p<0.05) Figures (3A, B and C).
Figure 3.
Effect of 4 weeks of alcohol administration on atherosclerotic plaque development. A: Cumulative data for neointima and lumen volume, calculated for 1 mm of carotid artery length as detailed in methods section. n=7-8; *p<0.05 vs no alcohol. B: Representative photomicrographs of Van Gieson stained sections. (magnification is 10x). Scale bars: 10 μm. C: Scale drawings (20x) of cross-sections through carotid arteries (n=7-8). Scale bars: 10 μm. D: Photomicrographs of Mac-3 staining (magnification is 40x). Scale bars: 25 μm.
Patterns of Alcohol Consumption Differentially Affect Macrophage Accumulation
Mac-3 antibody staining demonstrated increased macrophage accumulation in the neointima of the weekend-binge drinking mice when compared to the no alcohol group, whereas there was virtually no evidence of macrophage staining in the daily-moderate group (Figure 3D).
Discussion
This is the first controlled study on the effects of patterns of alcohol consumption on atherosclerotic plaque development. While epidemiological studies support an association between alcohol and cardiovascular disease there is lack of evidence-based data, free of artifacts or confounding variables often found in human population studies and where the validity of self-reported data is an area of concern. We report here, for the first time, a differential effect of daily-moderate vs. weekend-binge alcohol consumption on atherosclerotic plaque development. Animal models are essential for testing hypotheses in a controlled manner and mouse models in particular have proved useful to study atherosclerotic lesion development. We established a low-flow hypercholesterolemic model of atherosclerosis using partial carotid artery ligation in the apolipoprotein E knockout (apoE KO) mouse (recently described by Nam, et al. 12), in which the left external and internal carotid arterial branches are isolated and ligated, reducing left carotid blood flow to flow via the patent occipital artery (Figure 1A) 10. This model exhibits several key pathological characteristics similar to those seen in humans, in particular a large number of infiltrated monocytes/macrophages, increased MMP-9 expression and cholesterol deposits 13.
The blood alcohol levels observed in these mice over time followed a similar pattern to that observed in humans following moderate and binge alcohol consumption 14. Alcohol delivery by gavage allowed us to unequivocally ensure that each animal received the desired amount over a short period of time as opposed to ad libitum in drinking water. Coupled with blood alcohol measurement this ensured that the levels reached were those associated with moderate and binge alcohol consumption, and therefore allowed us to accurately replicate these drinking patterns in the mouse.
The majority of studies of alcohol and body weight have generally only examined average volumes of alcohol consumption. In the present study, greater increases in body weight were observed in the binge-drinking group when compared to the moderate group, despite the fact that both groups consumed the same total weekly volume of alcohol. These effects of different patterns of alcohol consumption on body weight are in agreement with a study demonstrating that persons who consumed the smallest quantity of alcohol the most frequently were leanest, and those who consumed the greatest quantity of alcohol the least frequently were heaviest 15. Our results support the idea that alcohol may contribute to excess body weight among certain drinkers, especially those in the binge group.
The mechanisms by which moderate alcohol consumption decreases coronary artery disease are likely complex and multiple. A major beneficial effect of alcohol on atherosclerosis is explained by its action on plasma lipids. Plasma HDL and LDL levels are increased and decreased, respectively, by moderate alcohol consumption 16. In the current study, both patterns of alcohol consumption increased plasma HDL levels, with a more pronounced effect observed in the binge-drinking group than in the moderate group. These findings are in agreement with studies that have reported an association between alcohol intake and serum lipid levels, most notably an increase in HDL cholesterol 16. Despite both patterns having a favorable effect on plasma HDL levels, it was observed that while daily-moderate alcohol consumption lowered LDL levels by 40%, weekend-binge alcohol consumption elevated LDL levels by 20%, compared to control mice. LDL is pro-atherogenic increasing the risk of heart disease, whereas HDL is anti-atherogenic and decrease the risk of disease 17. A comprehensive review of previous studies indicates that LDL levels are increased by heavy drinking episodes 18 and a recent study in rats has shown that heavy ethanol ingestion caused increased LDL-C levels 19. Our results highlight the importance of drinking pattern on plasma lipid levels. It is well documented that a 10% increase in plasma LDL levels results in a 20% increase in atherosclerosis risk 17. Our data indicate that daily-moderate drinking favorably changes the plasma lipid profile ie., increasing HDL-C and decreasing LDL-C plasma levels. On the other hand, weekend-binge drinking resulted in elevated plasma HDL-C levels but also elevated plasma LDL-C levels, the latter an established risk factor for atherosclerosis.
The inhibitory effect of daily-moderate alcohol on plaque development shown here is in agreement with meta-analysis of the relationship between alcohol consumption and coronary heart disease; ie., compared with abstinence from alcohol, low-to-moderate daily consumption of alcohol is associated with the lowest risk for coronary heart disease incidence 4. Ours is the first controlled study demonstrating a differential effect of daily-moderate versus weekend-binge alcohol consumption on atherosclerotic plaque development and it highlights the importance of considering patterns of alcohol consumption, as opposed to total amount consumed, in relation to the cardiovascular effects of alcohol. Our findings are also in agreement with a recent prospective cohort study which demonstrated that regular and moderate alcohol intake throughout the week, the typical pattern in middle aged men in France, is associated with a low risk of ischemic heart disease, whereas a binge drinking pattern more prevalent in Belfast confers a higher risk 20.
In addition to exerting favorable effects on plasma lipoproteins it has also been suggested that moderate alcohol consumption may exhibit anti-inflammatory properties 21, 22. Our data suggest that the two patterns of alcohol consumption compared differentially affect the inflammatory component (i.e., infiltrated monocytes) and this may explain the differences in plaque development that were observed between the two groups. We have previously reported that ethanol in a range spanning both moderate and binge levels (10 - 100 mM) inhibits interleukin-1β-stimulated vascular endothelial cell MCP-1 secretion and subsequent monocyte adhesion in a dose-dependent manner 21. However, it must be noted that in those in vitro experiments with endothelial cells there is no significant metabolism of ethanol occurring. More recently we have demonstrated that in contrast to ethanol, incubation with its metabolite acetaldehyde, increases monocyte adhesion and the expression of endothelial cell inflammatory mediators 21, 23. Co-incubation of endothelial cells with ethanol, 10 mM and 25 mM, completely reversed acetaldehyde-mediated monocyte adhesion 23. Taken together, the results from our in vitro studies suggest that following alcohol consumption there may be a delicate interplay between ethanol and acetaldehyde on monocyte recruitment within the vasculature. If the quantity of alcohol consumed is great enough to result in acetaldehyde levels that remain elevated after ethanol levels have been diminished, it is likely that the acetaldehyde present may have detrimental effects on the vasculature by promoting monocyte recruitment. These differential effects of alcohol and its primary metabolite, acetaldehyde, observed on vascular cells in vitro may underlie the contrasting effects of daily-moderate and weekend-binge alcohol consumption on atherosclerotic plaque development in vivo.
Alcohol has many effects on the cardiovascular system. Although most vascular research has focused on the cardioprotective effects of moderate alcohol consumption, some effects are unfavorable and may explain the increase in cardiovascular events following episodes of binge drinking. The Behavioral Risk Factor Surveillance System shows the binge drinking rate among all adults to be 14.8% 24. This type of drinker may take longer for a health care professional to become concerned about enough to consider an intervention. One study 25 has shown that a positive response to the question “On any single occasion during the past 3 months, have you had more than 5 drinks containing alcohol?” accurately identifies patients who meet either NIAAA’s criteria for at-risk drinking or the criteria for alcohol abuse or dependence specified in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) 26. It has recently been reported that alcohol screening and counseling is one of the highest ranked preventative services among 25 effective services that were evaluated 27. However, the current levels of delivery for alcohol screening and counseling were the lowest of comparably ranked services. In a national survey of problem drinkers, only 8.7% reported having been asked and counseled about their alcohol use in the last 12 months 28. Health psychologists argue that motivating people to change their drinking behavior depends upon belief surrounding issues such as their vulnerability to harm as a result of their behavior, the benefits of change, and whether people believe they can implement strategies for change 29. Researchers point to the ‘lack of longitudinal studies to determine the relationship between patterns of alcohol consumption and the development of diseases’ 30. Individuals have yet to be convinced of the dangers of binge drinking as opposed to long-term alcohol misuse.
Our findings demonstrate a differential effect of daily-moderate vs. weekend-binge alcohol consumption on atherosclerotic plaque development, and highlight the importance of considering patterns of alcohol consumption, in addition to total amount consumed, in relation to the cardiovascular effects of alcohol. The results obtained in the present study provide strong evidence to support a health message discouraging binge drinking. Providing scientific evidence to healthcare professionals that binge drinking can accelerate atherosclerosis, may encourage them to perform brief interventions for individuals with at-risk drinking behavior.
Acknowledgements
This study was supported in part by a Grant-in-Aid 0855865D from the American Heart Association (JPC) and by funds from the National Institutes of Health (R21AA-020012 (JPC), R01AA-12610 (EMR) and K99HL095650 (DM)).
We thank Dr. Slava Korshunov for his assistance with the animal surgery and the Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, for assistance with tissue processing and staining.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(WHO) WHO . Who global infobase for cardiovascular diseases. Geneva, Switzerland: https://apps.who.int/infobase. [Google Scholar]
- 2.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 3.Zakhari S. Overview: How is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 4.Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol consumption and cardiovascular mortality among u.S. Adults, 1987 to 2002. J Am Coll Cardiol. 2010;55:1328–1335. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers and nondrinkers. Am J Cardiol. 1990;66:1237–1242. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- 6.NIAAA Niaaa council approves definition of binge drinking. NIAAA Newsletter. 2004;3:1. [Google Scholar]
- 7.McElduff P, Dobson AJ. How much alcohol and how often? Population based case-control study of alcohol consumption and risk of a major coronary event. Bmj. 1997;314:1159–1164. doi: 10.1136/bmj.314.7088.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukamal KJ, Maclure M, Muller JE, Mittleman MA. Binge drinking and mortality after acute myocardial infarction. Circulation. 2005;112:3839–3845. doi: 10.1161/CIRCULATIONAHA.105.574749. [DOI] [PubMed] [Google Scholar]
- 9.Whitford JL, Widner SC, Mellick D, Elkins RL. Self-report of drinking compared to objective markers of alcohol consumption. Am J Drug Alcohol Abuse. 2009;35:55–58. doi: 10.1080/00952990802295212. [DOI] [PubMed] [Google Scholar]
- 10.Morrow D, Cullen JP, Liu W, Cahill PA, Redmond EM. Alcohol inhibits smooth muscle cell proliferation via regulation of the notch signaling pathway. Arterioscler Thromb Vasc Biol. 2010;30:2597–2603. doi: 10.1161/ATVBAHA.110.215681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: A model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 12.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivan E, Khatri JJ, Johnson C, Magid R, Godin D, Nandi S, Lessner S, Galis ZS. Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: The murine model of macrophage-rich carotid artery lesions. Circulation. 2002;105:2686–2691. doi: 10.1161/01.cir.0000016825.17448.11. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson PK, Sedman AJ, Sakmar E, Kay DR, Wagner JG. Pharmacokinetics of ethanol after oral administration in the fasting state. J Pharmacokinet Biopharm. 1977;5:207–224. doi: 10.1007/BF01065396. [DOI] [PubMed] [Google Scholar]
- 15.Breslow RA, Smothers BA. Drinking patterns and body mass index in never smokers: National health interview survey, 1997-2001. Am J Epidemiol. 2005;161:368–376. doi: 10.1093/aje/kwi061. [DOI] [PubMed] [Google Scholar]
- 16.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–1317. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- 17.Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyorala K. Prevention of coronary heart disease in clinical practice: Recommendations of the second joint task force of european and other societies on coronary prevention. Atherosclerosis. 1998;140:199–270. doi: 10.1016/s0021-9150(98)90209-x. [DOI] [PubMed] [Google Scholar]
- 18.McKee M, Britton A. The positive relationship between alcohol and heart disease in eastern europe: Potential physiological mechanisms. J R Soc Med. 1998;91:402–407. doi: 10.1177/014107689809100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Yao T, Song Z. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: Down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol Clin Exp Res. 2010;34:471–478. doi: 10.1111/j.1530-0277.2009.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruidavets JB, Ducimetiere P, Evans A, Montaye M, Haas B, Bingham A, Yarnell J, Amouyel P, Arveiler D, Kee F, Bongard V, Ferrieres J. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: The prospective epidemiological study of myocardial infarction (prime) BMJ. 2010;341:c6077. doi: 10.1136/bmj.c6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullen JP, Sayeed S, Jin Y, Theodorakis NG, Sitzmann JV, Cahill PA, Redmond EM. Ethanol inhibits monocyte chemotactic protein-1 expression in interleukin-1{beta}-activated human endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289:H1669–1675. doi: 10.1152/ajpheart.00106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imhof A, Koenig W. Alcohol inflammation and coronary heart disease. Addict Biol. 2003;8:271–277. doi: 10.1080/13556210310001602176. [DOI] [PubMed] [Google Scholar]
- 23.Redmond EM, Morrow D, Kundimi S, Miller-Graziano CL, Cullen JP. Acetaldehyde stimulates monocyte adhesion in a p-selectin- and tnfalpha-dependent manner. Atherosclerosis. 2008;204:372–380. doi: 10.1016/j.atherosclerosis.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson TF, Naimi TS, Brewer RD, Wechsler H. The state sets the rate: The relationship among state-specific college binge drinking, state binge drinking rates, and selected state alcohol control policies. Am J Public Health. 2005;95:441–446. doi: 10.2105/AJPH.2004.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taj N, Devera-Sales A, Vinson DC. Screening for problem drinking: Does a single question work? J Fam Pract. 1998;46:328–335. [PubMed] [Google Scholar]
- 26.Association AP. Diagnostic and statistical manual of mental disorders. fourth edition APA; Washington, DC: 1984. [Google Scholar]
- 27.Solberg LI, Maciosek MV, Edwards NM. Primary care intervention to reduce alcohol misuse ranking its health impact and cost effectiveness. Am J Prev Med. 2008;34:143–152. doi: 10.1016/j.amepre.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 28.D’Amico EJ, Paddock SM, Burnam A, Kung FY. Identification of and guidance for problem drinking by general medical providers: Results from a national survey. Med Care. 2005;43:229–236. doi: 10.1097/00005650-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Ahron V, White D, Phillips K. Encouraging drinking at safe limits on single occasions: The potential contribution of protection motivation theory. Alcohol Alcohol. 1995;30:633–639. [PubMed] [Google Scholar]
- 30.Plant M, Plant M, Mason W. People who enjoy drinking: Findings from a survey of british adults. The Drug and Alcohol Professional. 2002;2:26–37. [Google Scholar]