Abstract
Percutaneous transhepatic portal access avoids surgery, but is rarely associated with bleeding or portal venous thrombosis. We herein report our large, single-center experience of percutaneous islet implantation, and evaluate risk factors of portal vein thrombosis and graft function.
Prospective data was collected on 268 intraportal islet transplants (122 subjects). A portal venous Doppler ultrasound was obtained on Days 1 and 7 days posttransplant.
Therapeutic heparinization, complete ablation of the portal catheter tract with Avitene paste, and limiting packed cell volume to < 5 ml completely prevented any portal thrombosis in the most recent 101 islet transplant procedures over the past 5 years. In the previous cumulative experience, partial thrombosis did not affect islet function. Standard liver volume correlated negatively (r=−0.257, P<0.001), and packed cell volume correlated positively with portal pressure rise (r=0.463, P<0.001). Overall, partial portal thrombosis occurred after 10 procedures (overall incidence 3.7%, most recent 101 patient incidence 0%). There were no cases of complete thrombosis, and no patient developed sequelae of portal hypertension.
In conclusion, portal thrombosis is a preventable complication in clinical islet transplantation, provided therapeutic anticoagulation is maintained, and packed cell volume is limited to <5 ml.
Keywords: Islet transplantation, risk factors, portal vein thrombosis, standard liver volume
Introduction
The outcomes and safety of clinical islet transplantation have improved considerably in the past decade (1–4). The attraction of a percutaneous, day-case procedure that avoids general anesthesia, prolonged hospitalization and pain may be preferential to the major alternative approach of vascularized whole pancreas transplantation, but this premise is dependent upon the percutaneous technique being both safe and free from complications. The risk of both bleeding and portal venous thrombosis (PVT) have been well described previously, but now may be entirely preventable with a combination of systemic heparin, limited islet cell volume and complete obliteration of the transhepatic catheter tract (5–8).
Complete thrombosis of the entire portal venous system is the most feared complication of intraportal islet transplantation (9, 10). However, complete thrombosis with sequelae of chronic portal hypertension has now proven to be an exceedingly rare complication.
We herein investigate the incidence of portal venous thrombosis in a large, single-center prospective series of 268 consecutive intraportal islet transplants conducted over 11 years. We further analyze contributory factors leading to partial thrombosis, including standard liver volume (SLV), packed cell volume and acute portal pressure rise after islet transplantation, assuming that a smaller liver volume would likely have less capacity for embolized intraportal tissue. We further explore whether partial portal thrombosis is associated with detrimental loss of islet functional reserve.
Methods
Patients
Between May 1999 and August 2010, a total of 278 percutaneous intraportal islet-alone transplants were carried out in 127 subjects with unstable Type 1 diabetes at the University of Alberta. Those participating in the Collaborative Islet Transplant CIT trials (10 procedures, 5 subjects) were excluded from this analysis, as this data will be reported collectively when the CIT trials are completed. Of note, we did not encounter any portal thrombosis in these 10 procedures. Therefore 268 islet-alone transplants were analyzed in 122 subjects. The study was approved by the Health Research Ethics Board of the University of Alberta and all patients gave written consent to participate.
Islet isolation and islet transplantation
Islets were prepared as described previously, using xenoprotein-free media with 35 units/kg of heparin (70 units/kg if packed cell volume > 5 ml), and infused under gravity into the main portal vein (1). Access to the portal vein was achieved using a percutaneous transhepatic technique under fluoroscopic guidance as described previously (12–14). Briefly, portal vein access was carried out using 22 gauge Chiba needle and exchanged over a 0.018 inch guidewire for a 4Fr cannula (13). Portal venous pressure (mmHg) was measured by using a pressure transducer (Medex, Hillard, OH) at intervals throughout the islet infusion. A Doppler ultrasound (Philips HDI 5000 color-flow doppler ultrasound machine or equivalent, with 2.0–2.5 MHz probe, Philips Healthcare, Andover MA) was performed routinely within 24 hours and repeated on Day 7 to look for portal vein thrombosis, hematoma or intraperitoneal bleeding.
Insulin infusion and anti-coagulation treatment after islet transplantation
Intravenous insulin (1–2 units/hr) was infused together with dextrose 10% to maintain blood glucose levels between 4.1 and 7.0 mmol/L for 48 hr post transplant, as previously described (7). After discontinuing the intravenous insulin infusion, subjects monitored capillary blood glucose seven times per day and administered subcutaneous insulin aiming for preprandial glucose 4 to 6 mmol/L and postprandial glucose 5 to 8 mmol/L. Intravenous heparin was infused at a loading dose of 70 units per kg, and delivered intraportally mixed with the islet preparation, and a peripheral intravenous heparin infusion (3 units per kg per hour) was initiated immediately following ablation of the transhepatic islet catheter tract, while the patient was still in the radiology suite, and adjusted to keep the partial thromboplastin time between 70 and 90 sec for 48 hr after transplant. After this period, subjects were given subcutaneous low molecular weight heparin (enoxaparin 30 mg twice daily) for 7 days and aspirin 81 mg daily for 14 days. Before 2005, only 35 units per kg heparin was given intraportally, and no post-transplant infusion was given.
Calculation of body surface area, body mass index and standard liver volume
Body surface area (BSA) was calculated using Mosteller's formula: (15). Body mass index (BMI) was obtained by using the formula:BMI = weight (kg)/square height (meters) (16). SLV was calculated based on BSA by using formula: SLV= −794.41+1,267.28 × BSA (17).
Calculation of secretory unit of islet transplant objects index
Secretory unit of islet transplant objects (SUITO) index was calculated using the formula: 1500 × [fasting C-peptide immunoreactivity (F-CPR) (ng/ml) / (fasting blood glucose (mg/dl)-63)] (18). Average SUITO index in healthy subjects is designed as a score of 100.
Statistical analyses
Statistical comparisons between the groups were performed with the two-sample t-test or Mann-Whitney U-test, as appropriate for comparison of the two groups.
When both variable and outcome data were ordinal, correlations were performed using the Pearson's correlation test and expressed as Pearson's correlation coefficient. All comparisons were 2-tailed. A P value <0.05 was considered to be statistically significant for univariate analysis. Multiple regression analyses were performed with SLV, change in portal venous pressure, transplanted islet cells, transplanted islet cells/kg and packed cell volume, in order to account for confounding variable effect. The cutoff of packed cell volume was determined according to risk of portal vein thrombosis after islet transplantation of a receiver operating characteristic (ROC) curve, and was selected to achieve the best sensitivity/specificity combination.
All statistical calculations were performed using SPSS (Chicago, IL). Values for P less than 0.05 were considered statistically significant.
Results
Characteristics of islet transplant recipients and transplanted islet cells
Characteristics of recipients were summarized in Table 1. Recipients received a mean of 2.19 islet infusions per patient (one transplant 12, two transplants 80, three transplants 24, and four transplants 6). A mean islet mass of 404,999 islet equivalents (IE) were infused intraportally per transplant (5,908 IE/kg) in a mean packed cell volume of 4.1 ml (range: 1.5 – 15.0 ml). Mean subject height and weight were 169 cm and 67.5 kg, respectively. Mean calculated SLV of recipients was 1,484.4 cm3, ranging from 908.1 to 2,020.9 cm3.
Table 1.
Characteristics of recipients and transplanted islet cell data
| Recipient and islet cell data | |
|---|---|
| Recipient data | |
| Total transplant patients | 122 |
| Total number of transplant procedures | 268 |
| Mean height (cm) | 169 ± 9.1 (151 – 188) |
| Median transplants | 2,19 ± 0.68 (1–4): one transplant (n=12), two (n=80), three (n=24), four (n=6) |
| Mean weight (kg) | 67.5 ± 11.5 (44.2–100.0) |
| Mean BMI (kg/m2) | 23.6 ± 3.3 (16.5–40.0) |
| Mean standard liver volume (cm ) | 1484.4 ± 234.4 (908.1–2020.9) |
| Islet cell data | |
| Mean transplanted islet cells (IE) | 404,999 ± 121,764 (160,116 – 883,764) |
| Mean transplanted islet cells/kg (IE/kg) | 5,908 ± 1,541 (2,740 – 10,907) |
| Mean packed cell volume (ml) | 4.1 ± 1.7 (1.5–15.0) |
Factors affecting portal venous pressure after islet transplantation
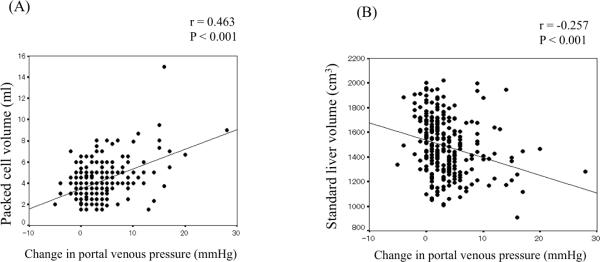
Packed cell volume correlated positively with change in portal venous pressure with r=0.463 and P <0.001 (Figure 1A). Also, SLV correlated negatively with change in portal pressure (r=−0.257 and P <0.001) (Figure 1B), with larger livers able to accommodate less perturbation in portal venous pressure.
Figure 1.
Factors affecting acute change of portal venous pressure after islet transplantation
A. Correlation between packed cell volume and change in portal vein pressure
B. Correlation between standard liver volume and change in portal vein pressure
Partial portal vein thrombosis after islet transplantation
There were no cases of complete thrombosis of the portal system, and none of our 127 subjects have manifested signs of portal hypertension in up to 11 years of follow-up. Among the 268 islet transplantation procedures, 10 cases (9 subjects) (3.7 %) developed partial portal vein thrombosis as summarized in Table 2 (case 3 and 9 represent the same subject, with partial thrombosis on two occasions – no underlying thrombophilia was uncovered). In all cases, the partial portal vein thrombosis resolved within a few weeks, and did not propagate. Anticoagulation with intravenous heparin then coumadin was continued for a duration of 6 months. Since 2005, we have ablated the hepatic catheter parenchymal tract routinely with Avitene™ paste. This single maneuver has substantially increased the safety of therapeutic heparinization after the procedure (8). After the introduction of therapeutic heparinization and limiting the packed cell volume to less than 5 ml for each islet infusion, we have not experienced any further cases of partial portal vein thrombosis in the subsequent 101 procedures over the most recent 4.5 years (Figure 2).
Table 2.
Characteristics of recipients with partial portal vein thrombosis after islet transplantation
| Cases | Weight (kg) | BMI (kg/m2) | BSA (m2) | SLV (cm3) | Transplanted islet cell (IE) | Transplanted islet cell (IE/kg) | PCV (ml) | Pre-PVP (mmHg) | Post-PVP (mmHg) | Change in PVP (mmHg) | PVT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77.0 | 24.9 | 1.940 | 1664.4 | 413,178 | 5366 | 3.0 | 6 | 8 | 2 | Right PVT |
| 2 | 57.6 | 20.5 | 1.637 | 1280.5 | 497,379 | 8633 | 9.0 | 8 | 36 | 28 | Complete left PVT |
| 3 | 60.7 | 23.1 | 1.654 | 1301.4 | 496,110 | 8173 | 5 | 12 | 20 | 8 | PVT on anterior branch of the right PV |
| 4 | 54.4 | 23.9 | 1.511 | 1119.9 | 298,802 | 5493 | 8.0 | 13 | 20 | 7 | Complete left PVT |
| 5 | 71.9 | 22.2 | 1.896 | 1608.1 | 349,930 | 4868 | 3.5 | 12 | 17 | 5 | Partial PVT in right PV |
| 6 | 55.7 | 24.1 | 1.533 | 1148.9 | 243,328 | 4369 | 5.5 | 13 | 19 | 6 | Left PVT |
| 7 | 67.0 | 23.7 | 1.768 | 1446.6 | 315,470 | 4708 | 2.7 | 4 | 5 | 1 | PVT on anterior branch of the right PV |
| 8 | 67.0 | 26.2 | 1.725 | 1392.2 | 511,621 | 7638 | 8.0 | 6 | 21 | 15 | Left PVT |
| 9 | 60.0 | 22.8 | 1.644 | 1289.2 | 290,210 | 4837 | 3.0 | 14 | 14 | 0 | Left PVT |
| 10 | 69.0 | 26.0 | 1.767 | 1445.5 | 372,100 | 5393 | 7.0 | 11 | 28 | 17 | PVT in the right anterior and posterior portal vein branch |
BMI, body mass index; BSA, body surface area; SLV, standard liver volume; IE, islet equivalent; PCV, packed cell volume; PVP, portal venous pressure; PVT, portal vein thrombosis
Figure 2.
Cumulative risk of portal vein thrombosis and number of islet transplant procedures per year. Since 2005, Intravenous heparin (70u/kg) has been infused to keep the partial thromboplastin time >60 sec or 48 hr after transplant and packed cell volume has been kept < 5 ml. No portal vein thrombosis has been observed in the most recent 5 years.
Functional assessment of transplanted islet cells by secretory unit of islet transplant objects (SUITO) index after portal vein thrombosis
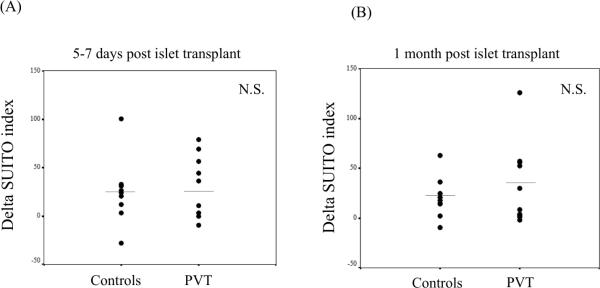
We assessed the potential detrimental impact of a partial portal thrombosis on islet function by the SUITO index, a tool to evaluate function of engrafted islet cells and predicts the possibility of insulin independence (18). We measured C-peptide and fasting blood glucose at 5–7 days and at one month after islet transplantation (Figure 3A and 3B).
Figure 3.
Evaluation of transplanted islet cells in the livers with or without portal vein thrombosis by SUITO index. No significant difference was observed between no portal vein thrombosis patients and portal vein thrombosis patients at 5–7 days (A) and 1 month (B) after islet transplantation. Each symbol represents an individual case.
As controls, we randomly selected a similar number of age, gender-matched and duration of diabetes matched non-portal vein thrombosis subjects undergoing similar islet transplant procedures at our center. There is no significant difference between controls (no PVT) group and PVT group in the number of transplanted islet cells and the number of transplanted islet cells/kg (data not shown). To standardize the SUITO indexes, we report delta SUITO index, calculated from the SUITO index in each time point (5–7 days or 1 month posttransplant) minus SUITO index just before the transplant. No differences were observed between the non-portal vein thrombosis subjects and those experiencing partial thrombosis at 5–7 days (Figure 3A; no PVT vs. PVT, 31.3 ± 10.2 vs. 29.9 ± 9.9: N.S.) or at 1 month (Figure 3B; no PVT vs. PVT, 20.4 ± 9.9 vs. 33.4 ± 12.8: N.S.) post transplant, indicating that partial thrombosis did not substantially affect the transplanted islet graft function.
Risk factors of portal vein thrombosis after islet transplantation
Univariate analysis revealed that the change in portal venous pressure and packed cell volume had significant correlations with portal vein thrombosis (P=0.001 and P=0.009, respectively) (Table 3). Interestingly, no significant correlation was observed in the number of transplanted islet cells. Subjects that developed portal thrombosis received higher packed cell volume (P<0.05) (Figure 4A), and had a significantly higher change of portal pressure (P<0.05) (Figure 4B), compared to subjects that did not develop partial portal thrombosis. We applied a multivariate analysis in a multiple logistic regression model (Table 4). The change in portal venous pressure was found to be an independent factor that was significantly predictive of portal thrombosis (Odds ratio 1.17, P=0.021).
Table 3.
Univariate predictors of portal vein thrombosis in allogeneic islet transplantation
| Portal vein thrombosis | |
|---|---|
| P value | |
| Recipient data | |
| Weight (kg) | 0.229 |
| BMI (kg/m2) | 0.449 |
| Standard liver volume (cm3) | 0.550 |
| Body surface area (m2) | 0.975 |
| Change in portal venous pressure (mmHg) | 0.001* |
| Islet cell data | |
| Transplanted islet cells (IE) | 0.991 |
| Transplanted islet cells/kg (IE/kg) | 0.844 |
| Packed cell volume (mL) | 0.009* |
Figure 4.
Comparison between the patients without portal vein thrombosis and those with portal vein thrombosis. The subjects that developed portal thrombosis had larger packed cell volume (A), and had a significantly increased portal venous pressure during the islet transplant (B). Each symbol represents an individual case.
Table 4.
Multivariable logistic regression of risk of portal vein thrombosis after allogeneic islet transplantation
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Standard liver volume (cm3) | 1.08 | (0.99, 1.01) | 0.603 |
| Change in portal venous pressure (mmHg) | 1.17 | (1.02, 1.32) | 0.021 * |
| Transplanted islet cells (IE) | 1.00 | (1.00, 1.00) | 0.783 |
| Transplanted islet cells/kg (IE/kg) | 1.00 | (0.99, 1.00) | 0.778 |
| Packed cell volume (ml) | 1.08 | (0.78, 1.49) | 0.634 |
Selection of cutoff value of packed cell volume and change in portal venous pressure for the risk of portal vein thrombosis
ROC curves were constructed to examine the optimal cut-off values both for packed cell volume and for acute change in portal pressure. We found that 5 ml of tissue, and a portal pressure change of less than 5 mmHg protected against thrombosis (Figure 5A and 5B).
Figure 5.
Receiver operating characteristic curve assessment of (A) the packed cell volume and (B) portal venous pressure change to predict portal vein thrombosis after islet transplantation A. A cut off 5.5 ml was selected, providing sensitivity 50 %, specificity 84.5 % and area under the curve (AUC) 0.667. B. A cut off 4.5 mmHg was selected, providing sensitivity 70 %, specificity 73.2 % and area under the curve 0.716
Discussion
The strength of the current report lies in the large number of percutaneous intraportal islet transplants carried out at a single institution (268 procedures in 122 patients), with prospective documentation of portal pressure and procedural complications, expanding the previous preliminary report from our group in 26 subjects (19). Both portal vein thrombosis and elevated packed cell volume infused into the portal system have been suggested previously as potential risk factors for portal vein thrombosis in allogeneic islet transplantation. We previously observed an increasing risk for acutely elevated portal pressures in patients requiring two or three infusions (19). Since then, we, along with most other islet transplant institutions, set limits on the safe volume of tissue delivered intraportally, recommending that this should be maintained below 5 ml, and that therapeutic intraportal and systemic anticoagulation with intravenous heparin should be initiated in the radiology suite, in order to minimize the risk of thrombosis, standards that were set by discussion amongst leading islet transplant centers in consensus. Therefore our current report reflects the impact of institution of these guidelines, and demonstrates that percutaneous intraportal islet transplantation may be accomplished with exceedingly low risk – an essential component if islet transplantation is to be more broadly and applied earlier in patients with Type 1 diabetes.
We acknowledge that the current findings represent a much more definitive analysis of that previous preliminary experience, but importantly emphasize that with attention both to islet purity and quality, and to therapeutic anticoagulation in the immediate peritransplant period, that in the largest experience of intraportal islet transplants performed at a single centre, both avoidance of thrombosis and avoidance of intraperitoneal bleeding are achievable goals. This procedure may be regarded as safe and associated with minimal complications from the current experience. The only portal thrombosis complication of any major potential clinical significance – complete thrombosis of the entire portal vein, has never occurred in our series to date. This is an important point from a safety perspective.
Specifically, the expanded series to 268 intraportal procedures has allowed us to complete in depth multivariate analyses, the clear finding confirming that acute portal venous pressure rise is a clear risk factor for partial portal vein thrombosis. The calculation of receiver operating characteristics are new to the analysis, confirming that cut-off values of packed cell volume and portal venous pressure rise are meaningful guidelines to other investigators in order to avoid portal vein thrombosis in islet transplantation. In current study, we have clearly demonstrated the impact of institution of these guidelines, and demonstrated that percutaneous intraportal islet transplantation may be accomplished with exceedingly low risk. The relationship between packed cell volume and standard liver volume is also new, and not described previously, and further highlights to other investigators the importance of lowering set limits of intraportal tissue mass for patients with small livers. This may become especially important in future clinical islet transplantation as and when this procedure is expanded to include children with small livers.
Based on historical reports, it is surprising that the incidence of portal thrombosis is not considerably higher in islet autotransplantation, where previously several cases of portal thrombosis, variceal bleeding and disseminated intravascular coagulation were reported, including the occasional patient death (9, 20–22). Typically, unpurified islets are infused in the autotransplant setting of chronic pancreatitis. The introduction of the Ricordi technique for mass islet isolation, the use of purified collagenase enzymes low in endotoxin, the development of standard operating procedures and clinical good manufacturing practice (cGMP) ultra-clean room facilities, coupled with stringent islet product release criteria have all contributed to the substantial reduction of portal thrombosis and sequelae in clinical islet autotransplantation (2). In contrast to our current findings in allogeneic islet transplantation, Bucher et al found no association between portal thrombosis and raised intraportal pressure in their combined experience of both islet autograft and allogeneic transplants, where they limited the tissue volume infused to a mean of 13 mls (23). However in that report, while the rate of portal thrombosis was low (3.2%), the risk of intraperitoneal hemorrhage was high (11.3%). Bleeding post transplantation is now an entirely avoidable complication with intraparenchymal liver tract ablation (8). The 6th annual report of the CITR showed that partial branch-vein occlusions were identified in 2.2 % of transplanted patients (11). In our own prospective experience, partial portal venous thrombosis occurred in 3.7 % of our 268 procedures in 122 subjects overall, but the rate has been 0% in the most recent 4.5 years (in the most recent 101 percutaneous procedures).
A previous report from an international multicenter trial suggested that covert, underlying thrombophilic tendencies (protein C, S, antithrombin III, factor v Leiden deficiencies, or presence of antiphospholipid antibodies) may potentially exacerbate the risk of portal thrombosis after islet transplantation (24). However, with therapeutic anticoagulation initiated both intraportally and systemically at the time of islet infusion, and a prolonged period of low molecular weight heparin and aspirin, and based on the extremely low risk of this complication in our most recent four year experience, it has not been our routine practice to screen for these abnormalities.
Interestingly, we found that despite partial portal thrombosis, there was no detectable loss of islet graft function as measured by the SUITO index or insulin requirements. One might have anticipated that thrombosis of a major vessel supplying the islet graft, or entrapment of islets within a macro-clot, would lead to loss of islet mass, but this does not appear to have been the case. This observation is limited however to just 10 cases, and may therefore be underpowered.
The calculation of ROC curves in the current study corresponds entirely with the current recommendations of limiting the packed islet cell volume below 5 ml, and limiting the final elevation in portal pressure after islet infusion to less than 5 mmHg. Analysis of the cases of partial portal thrombosis shown in Figure 2 demonstrate that most, but not all, cases of partial portal thrombosis were associated with transplants outside of the above limits – and the occasional thrombus occurred despite an optimal islet infusion suggests that there may still be other factors unaccounted for, that could have contributed to the thrombosis, including increased tissue factor or monocyte chemoattractant protein (MCP-1) expression that may trigger a thrombotic and inflammatory reaction (25–28). One report showed that islet surface heparinization prevented the IBMIR without increasing bleeding risk, and it might be useful to prevent portal thrombosis (29).
We recognize that a major limitation to the interpretation of causality in this study is the limited number of 10 occurrences of partial portal thrombosis, and the fact that most of these occurred historically in our series, with none occurring in the past 5 years. While we surmise that limiting the intraportal tissue mass and using therapeutic anticoagulation is the primary factor protecting against this complication, we recognize that substantial changes in islet manufacture, collagenase enzyme and peritransplant management have also occurred, and clearly may have contributed at least in part to the avoidance of portal thrombotic complications in our recent series. The fact that three minor portal thromboses occurred subsequent to the packed cell volume and full heparinization protocols in 2005, indicates that factors other than tissue volume and anticoagulation may contribute, but with such a low incidence, and none in the past 5 years, we cannot resolve causality completely. Furthermore, we recognize that the resolution of the Doppler ultrasound interrogation of the portal venous system is operator, hardware and software dependent, and that with improvements in technology and experience, the sensitivity of detection of peripheral portal thrombosis could increase over era. Nonetheless, detection of main, left, right or peripheral segmental main branches of the portal system are readily detectable when present, and these are the potentially clinically relevant thromboses.
Finally, the question may be reasonably asked whether partial thrombosis of the intrahepatic portal vein has any serious clinical sequelae in islet transplantation. The complication is clearly iatrogenic, and is best avoided, but the current study provides reassuring data that partial portal thrombosis does not lead to clot propagation, and does not progress to complete thrombosis of the entire porto-mesenteric system, which would clearly have serious clinical consequences.
We conclude that excess packed cell volume and a change of portal venous pressure are critical factors for portal thrombosis after intraportal islet transplantation. We recommend packed cell volume < 5 mL and change in portal venous pressure < 5 mmHg to mitigate this risk. If the packed cell volume is substantially elevated above 5 ml, consideration may be given to further attempts at islet repurification, or further prolongation of the culture period, which may results in further differential loss of exocrine tissue and increased tissue purification. If the recipient's portal pressure is substantially elevated at baseline, and the measurement is determined to be accurate, then serious consideration of abandoning the islet infusion procedure must be given, with the possibility of infusing the islet preparation into an alternative recipient. The occurrence of partial portal thrombosis does not seem to degrade clinical islet function, and has not been associated with clinical sequelae of portal hypertension. In patients with low standard liver volume, and especially in children for future consideration, more stringent requirements for islet purification will be needed, combined with more cautious monitoring of portal pressure is strongly recommended, and full anticoagulation should be routinely applied. Portal thrombosis is a potentially avoidable complication in clinical islet transplantation.
Acknowledgments
This work was supported by a Clinical Centre Grant from the Juvenile Diabetes Research Foundation, and from a National Institutes of Health (NIH) Clinical Islet Transplantation Consortium (CIT) Grant. (Note – no data for subjects enrolled in the CIT Consortium trials are included in this analysis.)
T Kawahara is supported by a Clinical Fellowship Grant from Alberta Innovates-Healthcare Solutions (AIHS). AMJS is supported by a Senior Clinical Scholarship from AIHS, and through the JDRF and NIH Centre Grants. NMK is supported by a Pfizer-Wyeth grant, and the Canadian Institutes of Health Research. AMJS, PS and AK are members of the Alberta Diabetes Institute.
Non-standard Abbreviations used in this paper
- AUC
area under the curve
- BSA
body surface area
- IE
islet equivalents
- PCV
packed cell volume
- PVT
Portal vein thrombosis
- SLV
standard liver volume
- SUITO
secretory unit of islet transplant objects
Footnotes
Disclosure The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4(4):259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 3.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 4.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8(11):2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froud T, Yrizarry JM, Alejandro R, Ricordi C. Use of D-STAT to prevent bleeding following percutaneous transhepatic intraportal islet transplantation. Cell Transplant. 2004;13(1):55–59. doi: 10.3727/000000004772664897. [DOI] [PubMed] [Google Scholar]
- 6.Koh A, Imes S, Kin T, Dinyari P, Malcolm A, Toso C, et al. Supplemental islet infusions restore insulin independence after graft dysfunction in islet transplant recipients. Transplantation. 2010;89(3):361–365. doi: 10.1097/TP.0b013e3181bcdbe8. [DOI] [PubMed] [Google Scholar]
- 7.Koh A, Senior P, Salam A, Kin T, Imes S, Dinyari P, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89(4):465–471. doi: 10.1097/TP.0b013e3181c478fd. [DOI] [PubMed] [Google Scholar]
- 8.Villiger P, Ryan EA, Owen R, O'Kelly K, Oberholzer J, Al Saif F, et al. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005;5(12):2992–2998. doi: 10.1111/j.1600-6143.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Eggleston JC, Cameron JL. Portal hypertension, hepatic infarction, and liver failure complicating pancreatic islet autotransplantation. Surgery. 1982;91(4):485–487. [PubMed] [Google Scholar]
- 10.Shapiro AM, Lakey JR, Rajotte RV, Warnock GL, Friedlich MS, Jewell LD, et al. Portal vein thrombosis after transplantation of partially purified pancreatic islets in a combined human liver/islet allograft. Transplantation. 1995;59(7):1060–1063. doi: 10.1097/00007890-199504150-00027. [DOI] [PubMed] [Google Scholar]
- 11.Report CITR. CITR Annual Report. 2009 Available from: https://web.emmes.com/study/isl/reports/reports.htm.
- 12.Goss JA, Soltes G, Goodpastor SE, Barth M, Lam R, Brunicardi FC, et al. Pancreatic islet transplantation: the radiographic approach. Transplantation. 2003;76(1):199–203. doi: 10.1097/01.TP.0000073976.26604.96. [DOI] [PubMed] [Google Scholar]
- 13.Owen RJ, Ryan EA, O'Kelly K, Lakey JR, McCarthy MC, Paty BW, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology. 2003;229(1):165–170. doi: 10.1148/radiol.2291021632. [DOI] [PubMed] [Google Scholar]
- 14.Weimar B, Rauber K, Brendel MD, Bretzel RG, Rau WS. Percutaneous transhepatic catheterization of the portal vein: A combined CT- and fluoroscopy-guided technique. Cardiovasc Intervent Radiol. 1999;22(4):342–344. doi: 10.1007/s002709900403. [DOI] [PubMed] [Google Scholar]
- 15.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341(6):427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 17.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8(3):233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Noguchi H, Hatanaka N, Shimoda M, Kobayashi N, Jackson A, et al. SUITO index for evaluation of efficacy of single donor islet transplantation. Cell Transplant. 2009;18(5):557–562. doi: 10.1177/096368970901805-611. [DOI] [PubMed] [Google Scholar]
- 19.Casey JJ, Lakey JR, Ryan EA, Paty BW, Owen R, O'Kelly K, et al. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation. 2002;74(7):913–915. doi: 10.1097/00007890-200210150-00002. [DOI] [PubMed] [Google Scholar]
- 20.Cameron JL, Mehigan DG, Broe PJ, Zuidema GD. Distal pancreatectomy and islet autotransplantation for chronic pancreatitis. Annals of surgery. 1981;193(3):312–317. doi: 10.1097/00000658-198103000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehigan DG, Bell WR, Zuidema GD, Eggleston JC, Cameron JL. Disseminated intravascular coagulation and portal hypertension following pancreatic islet autotransplantation. Annals of surgery. 1980;191(3):287–293. doi: 10.1097/00000658-198003000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memsic L, Busuttil RW, Traverso LW. Bleeding esophageal varices and portal vein thrombosis after pancreatic mixed-cell autotransplantation. Surgery. 1984;95(2):238–242. [PubMed] [Google Scholar]
- 23.Bucher P, Mathe Z, Bosco D, Becker C, Kessler L, Greget M, et al. Morbidity associated with intraportal islet transplantation. Transplantation proceedings. 2004;36(4):1119–1120. doi: 10.1016/j.transproceed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Brennan DC, Shannon MB, Koch MJ, Polonsky KS, Desai N, Shapiro J. Portal vein thrombosis complicating islet transplantation in a recipient with the Factor V Leiden mutation. Transplantation. 2004;78(1):172–173. doi: 10.1097/01.tp.0000128332.71657.ea. [DOI] [PubMed] [Google Scholar]
- 25.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 26.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun. 2003;308(3):474–479. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 27.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51(1):55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 28.Titus TT, Horton PJ, Badet L, Handa A, Chang L, Agarwal A, et al. Adverse outcome of human islet-allogeneic blood interaction. Transplantation. 2003;75(8):1317–1322. doi: 10.1097/01.TP.0000064517.98252.00. [DOI] [PubMed] [Google Scholar]
- 29.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Kallen R, et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]