Abstract
Steroidogenic acute regulatory protein (StAR) mediates the rate-limiting step in the synthesis of steroid hormones, essential to fetal development. We have reported that the StAR expression in fetal adrenal is inhibited in a rat model of nicotine-induced intrauterine growth retardation (IUGR). Here using primary human fetal adrenal cortex (pHFAC) cells and a human fetal adrenal cell line NCI-H295A, we show that nicotine inhibits StAR expression and cortisol production in a dose- and time-dependent manner, and prolongs the inhibitory effect on cells proliferating over 5 passages after termination of nicotine treatment. Methylation detection within the StAR promoter region uncovers a single site CpG methylation at nt −377 that is sensitive to nicotine treatment. Nicotine-induced alterations in frequency of this point methylation correlates well with the levels of StAR expression, suggesting an important role of the single site in regulating StAR expression. Further studies using bioinformatics analysis and siRNA approach reveal that the single CpG site is part of the Pax6 binding motif (CGCCTGA) in the StAR promoter. The luciferase activity assays validate that Pax6 increases StAR gene expression by binding to the glucagon G3-like motif (CGCCTGA) and methylation of this site blocks Pax6 binding and thus suppresses StAR expression. These data identify a nicotine-sensitive CpG site at the Pax6 binding motif in the StAR promoter that may play a central role in regulating StAR expression. The results suggest an epigenetic mechanism that may explain how nicotine contributes to onset of adult diseases or disorders such as metabolic syndrome via fetal programming.
Keywords: nicotine, StAR, CpG methylation, epigenetic regulation, gene expression, Pax6, IUGR
Introduction
Maternal cigarette smoking is the single largest modifiable risk factor attributable to intrauterine growth retardation (IUGR), which is defined as being less than 10th percentile of the body size distribution for a gestational age (Hofman et al., 1997; Delpisheh et al., 2008). Among more than 2000 compounds released from smoking, nicotine is the major aversive substance that perturbs fetal growth and development (Yildiz, 2004; Petre et al., 2011). Increasing epidemiological evidence supports the notion that adverse events in fetal life such as IUGR permanently alter the structure and physiology of the adult offspring, a phenomenon dubbed “fetal programming” (Guilloteau et al., 2009; Habib et al., 2011). In addition, prenatal nicotine exposure is associated with increased risk of IUGR and susceptibility to adult diseases, particularly cardiovascular diseases and metabolic syndrome (Feng et al., 2010; Pellanda et al., 2009; Geelhoed et al., 2011).
Fetal programming of key endocrine systems, especially the hypothalamic-pituitary-adrenal (HPA) axis, has been proposed as a potential intermediary that links IUGR to adult metabolic dysfunction (Kanaka-Gantenbein, 2010; Xita and Tsatsoulis, 2010). Long term consequences of low birth weight on adrenal cortisol secretion contribute to increased risks for the metabolic syndrome in later life, suggesting a central role for adrenocortical steroidogenesis in intrauterine fetal programming (Ong, 2005; Marciniak et al., 2011). Like adult, human fetal adrenal expresses the rate-limiting enzyme of steroidogenesis - acute regulatory protein (StAR) and cytochrome P450 cholesterol side chain cleavage (P450scc), which is essential for the production of steroid hormones including cortisol, aldosterone, dehydroepiandrosterone sulfate (DHEAS), etc (Wang et al., 2006). StAR mediates the translocation of cholesterol from the outer to the inner mitochondrial membrane, which is the initial and rate-limiting step in adrenocortical steroid biosynthesis, while P450scc cleaves the cholesterol side chain, converting cholesterol to pregnenolone, the precursor of steroid hormones (Stocco, 2001; Miller and Auchus, 2011). Our previous studies have shown that fetal adrenal could be an important xenobiotic-metabolizing organ in fetal development and may play a potential role in xenobiotic-induced fetal development toxicity (Wang et al., 2008). Furthermore, our results have shown that prenatal exposure to nicotine as well as ethanol can inhibit adrenal StAR expression and induce IUGR in fetal rats (Liang et al., 2010; Chen et al., 2007). However, the underlying mechanism concerning how nicotine alters StAR expression in fetal adrenal remains unknown.
Experimental data in rodents and recent observations in humans suggest that epigenetic changes in growth-related genes play a significant role in fetal programming (Gicquel et al., 2008; Martinez-Arguelles and Papadopoulos, 2010). DNA methylation as the major epigenetic modification persists throughout the process of fertilization and embryo development. The patterns of DNA methylation are heritable marks that ensure accurate transmission of the chromatin states and gene expression profiles over many cell generations. As alterations of DNA methylation are stable in tissues and body fluids and are suitable for sensitive detection, DNA methylation changes may serve as molecular biomarkers for disease diagnosis and therapeutic outcome (Deng et al., 2010). Studies have shown that aberrant DNA methylation occurs in human adrenocortical tumorigenesis that is often accompanied by abnormal hormone production (Liu et al., 2004). Maternal nicotine exposure increases fetal incidence of adult cardiomyopathy, which has a direct relationship with abnormal regulated gene expression caused by the altered pattern of DNA methylation (Meyer and Lubo, 2007). In addition, evidence has shown that DNA methylation inhibitor 5-aza-2′-deoxycytidine (Azad) affects StAR expression and cortisol secretion in human adrenocortical NCI-H295R cells (Liu et al., 2004). Therefore, DNA methylation may mediate how nicotine alters StAR expression in fetal adrenal, which increases the susceptibility to adult metabolic syndrome.
Although numerous publications regarding the mechanisms of nicotine-induced IUGR are available in the literature, the direct toxicity of nicotine on fetal adrenal is poorly understood, as are the inheritable epigenetic mechanisms that are responsible for fetal programming. We have shown that in a nicotine-induced IUGR rat model the expression of StAR and P450scc increases in the maternal adrenals, but decreases in the fetal adrenals (Chen et al., 2007). In this study, primary human fetal adrenal cortex (pHFAC) cells and a human fetal adrenal cell line NCI-H295A were used to understand how nicotine inhibits StAR expression and cortisol production. We have identified a nicotine-sensitive CpG site of methylation at a potential Pax6 binding motif in the StAR promoter that may down-regulate StAR expression. This study has uncovered a novel potential target that will be helpful for development of early diagnosis and therapeutics for IUGR-related diseases or disorders.
Materials and methods
Chemicals and reagents
Nicotine (N3876), collagenase I, deoxyribonuclease I (DNase I), selenium/insulin/transferrin (SIT) and bovine serum albumin (BSA) were obtained from Sigma-Aldrich Corp (St Louis, Mo). Dulbecco’s Modified Eagle’s Medium and Ham’s F12 Medium (DMEM/F12), Hanks balanced salt solution, RPMI-1640, Opti-MEM® I Reduced Serum Medium, fetal bovine serum (FBS), penicillin, streptomycin, and amphotericin B were purchased from Invitrogen (Carlsbad, Calif). All primers were synthesized by Integrated DNA Technologies (Coralville, Iowa). All chemicals and reagents were of analytical grade.
Isolation of primary human fetal adrenal cortex cells (pHFAC) and cell culture
The pHFAC cells, consisting mainly (90% – 95%) of fetal zone cells, were isolated from clinically dead fetuses without cigarette smoke and tobacco exposure as previously described by our group (Wang et al., 2006). Use of human material was approved by the Human Medical Ethics Committee of Wuhan University. The gland was decapsulated to remove most of the definitive zone, and the remaining fetal zone was minced with fine scissors and incubated in the digestion mixture at 37°C for 30 minutes with gentle shaking. The digestion mixture consisted of 10 mL of Hanks balanced salt solution containing 1 mg/mL collagenase I, 5 mg/L DNase I, and 5 mg/mL BSA. After the cells were dispersed using a pipette, they were washed with DMEM/F12 media, filtered through a 100-μm strainer (Millipore, Bedford, Mass), and counted with a hemacytometer. For each adrenal gland, approximately 20 million pHFAC cells were obtained immediately after enzymatic digestion, and about 90% of the isolated cells were viable as determined with trypan blue exclusion. The cells were plated in 6-well polystyrene plates (Corning, NY) at 106 cells per well in 2 mL cell culture media: DMEM/F12 with 20% heat-inactivated FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL amphotericin B. Plates were placed in a humidified incubator with 10% CO2 at 37°C. Two days later, the medium was replaced by adding fresh medium containing nicotine (1–100 μM) and the culture continued for 24 hours. Then the medium and the cells were harvested and stored at −80°C for future use.
NCI-H295A cell culture and drug treatment
An adherent subline of human adrenocortical carcinoma cells (NCI-H295A) is the best model available for experimental research on human fetal adrenal, and produces the steroids and regulates the enzymes of human adrenal steroidogenesis in a manner similar to that of human fetal adrenal cells (Dardis and Miller, 2003; Kian Tee et al., 2011). NCI-H295A cells were kindly provided as gift by Prof. W L Miller and employed for all the mechanistic experiments of this study. Standard medium for NCI-H295A cells is RPMI-1640 supplemented with 2% FBS, 0.1% SIT and penicillin/streptomycin (Dardis and Miller, 2003). At 80% confluence, NCI-H295A cells were starved with serum-free media overnight, and then were treated with nicotine at the doses and for the days indicated. In some experiments, 100 μM nicotine treatment was withdrawn 10 days later and the cells were continually subcultured for up to 0, 15, and 30 days (or 0, 5, and 10 passages), respectively. The mRNA and genomic DNA were isolated and stored at −80°C for the future analysis.
Human cortisol and dehydroepiandrosterone (DHEAS) production
Culture medium collected from pHFAC cells and NCI-H295A cells with or without nicotine treatment was used to detect the levels of cortisol and DHEAS. Cortisol level was measured by human cortisol ELISA kit obtained from DRG Instruments GmbH (Marburg, Germany) following the manufacturer’s protocol. DHEAS levels were assayed using human DHEAS radioimmunoassay kit from NovaTec Immundiagnostica GmbH (Dietzenbach, Germany) following the manufacturer’s protocol. Hormone levels in all samples were measured simultaneously to avoid inter-assay variability.
RNA extraction and RT-PCR
Total RNAs were extracted using RNeasy Mini kit obtained from Qiagen (Hiden, Germany) following the protocol provided by the manufacturer. Extracted RNA was reverse transcribed to cDNA with the SuperScript ™ II RNase H Reverse Transcriptase (Invitrogen, Carlsbad, Calif). Synthesized cDNA was amplified by Platinum TaqPCRx DNA Polymerase (Invitrogen, Carlsbad, Calif). Specific primers for StAR: StAR forward TGAGCAGAAGGGTGTCATCAGG; StAR reverse CGCAGGTGGTTGGCAAAATC. PCR conditions for StAR were 30 s at 94°C, 30 s at 59°C, 25 s at 68°C for 25 cycles; Specific primers for Pax6 (paired box 6): Pax6 forward GTGCGACATTTCCCGAATTCTG; Pax6 reverse GCCAGGTTGCGAAGAACTCTG. PCR conditions for Pax6 were 30 s at 94°C, 30 s at 56°C, 30 s at 68°C for 40 cycles. The expression of a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was served as internal controls.
Genomic DNA extraction and sodium bisulfite modification
Genomic DNA samples prepared using DNeasy Blood & Tissue kit (Qiagen, Hiden, Germany) were subjected to bisulfite modification using EZ DNA methylation-direct kit (Zymo research corporation, Orange, CA) according to the manufacturer’s instruction. The basic principle of bisulfite modification of DNA is that in the bisulfite reaction, all unmethylated cytosines are deaminated and sulfonated, converting them to thymines, while methylated cytosines (5-methylcytosines) remain unaltered (Lorente et al., 2008). Modified DNA was used immediately or stored at −80°C for future use within six months.
Bisulfite Sequence PCR (BSP)
The methylation status of StAR gene was quantitated using Bisulfite Sequence PCR (BSP) as described previously (Habano et al., 2011). The bisulfite-treated genomic DNA was amplified by Takara’s Ex Taq™ DNA Polymerase purchased from Invitrogen (Carlsbad, Calif) using two primers StAR-1 and StAR-2 which cover almost the entire CpG rich region of the proximal StAR promoter. Primers of StAR-1 (−719 bp ~ −280 bp): forward ACGACTCACTCTAGGGATGGTTTTTATTGTTTGGTAAATATTTT and reverse AAAAAAAAAAAACTTCCCTTAACCAAAC; StAR-2 (−9 bp ~ +402 bp): forward ACGACTCACTCTAGGGGGTTAAAGTAGTAGTGTGAGGTAAT and reverse CAAAATTAAATAACCTAAACCTCATCC. The bisulfite PCR products were sequenced for 6–9 reactions by Biomedical Instrumentation Center (USUHS, USA). Methyl+ ACGACTCACTCTAGGG as an additional sequence primer.
Methylation Specific PCR (MSP)
The methylation status of the single CpG (−377 bp) within StAR promoter was evaluated by MSP as described previously (Csepregi et al., 2010). In brief, DNA was subjected to bisulfite modification and was amplified using two different primer pairs specific to either methylated or unmethylated sequences, respectively. Primer sequences for methylated reactions were forward primer: ATGGTTTTTATTGTTTGGTAAATATTTT and reverse primer: CAAAATAAACAAATCACTTAAAATCAAACG, which amplified a 373 bp product. Primer sequences for unmethylated reactions were forward primer: ATGGTTTTTATTGTTTGGTAAATATTTT and reverse primer: CCAAAATAAACAAATCACTTAAAATCAAACA, which amplified a 374 bp product. CpGenome ™ Universal Methylated DNA (Q-Biogene, Heidelberg, Germany) was used as positive control. PCR amplification was performed by Takara’s Ex Taq™ DNA Polymerase (Invitrogen, Carlsbad, Calif). Methylation specific PCR products were analyzed by a 2% agarose gel and stained with ethidium bromide.
Pax6 gene silence (siRNAs)
Small interfering RNAs (siRNAs) to the human Pax6, Pax6 siRNA (h) and the control siRNA-A were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Transfection was carried out using the Lipofectamine™ 2000 transfection reagent (Invitrogen, Carlsbad, Calif) according to the manufacturer’s instruction. In brief, NCI-H295A cells were grown to 30% confluence in complete medium without antibiotics in 60-mm plates. 200 pmol siRNAs resuspended in 500 μl Opti-MEM® I Reduced Serum Medium without serum was diluted at 1:50 in volume ratio with Opti-MEM® I Reduced Serum Medium. After 5-minute of incubation at room temperature, the diluted oligomer was combined with the diluted lipofectamine™ 2000 following the manufacturer’s instruction. After incubation for 20 minutes at room temperature, the Lipofectamine™ 2000 complexes were added to each well containing cells and medium. Incubate the cells at 37°C in a CO2 incubator for 72 hour. The transfection of siRNAs was repeated once before the cells were harvested for analysis.
Luciferase activity assay
Luciferase assay kit and pGL4 luciferase reporter vector were purchased from Promega. CpG Methyltransferase M.SssI was purchased from New England Biolab. DNA sequence (GCTGGTCTCGAACGCCTGACCTCAAGTGATCTG) in the human StAR gene promoter that contains the HD binding motif of Pax6 was subcloned into pGL4 luciferase reporter vector at Nhe I and Hind III sites. This reporter vector has a minimal promoter that ensures minimal expression of luciferase in the absence of a specific transcription activator. Luciferase activity was assayed by following the manufacturer’s protocol with the EnVision system (PerkinElmer). CpG methylation of the StAR/Pax6 reporter was generated by incubation of the reporter plasmid with M.SssI by following the enzyme instruction. The reaction induces complete CpG methylation of the reporter plasmids. The methylated reporter was then purified with MiniPrep DNA kit (Qiagen). 2×105 NCI-H295A cells/well were seeded in 24-well plate one day before transfection with Lipofectamine™ 2000 in growth RPMI 1640 medium containing 2% FBS and 1% ITS. For each well, 0.4 μg of StAR/Pax6 reporter with either 0.4 μg Pax6 or 0.4 μg of empty vector (mock) were mixed and then utilized for co-transfection. Two days after transfection, the cells were harvested for luciferase activity assay. All experiments were performed in duplicates.
Immunoprecipitation and western blot analysis
Cells were rinsed with ice-cold PBS, and then lysed for 30 minutes at 4°C in RIPA lysis buffer (25 mmol/L HEPES pH 7.5, 50 mmol/L NaCl, 1% NP40, 2.5 mmol/L EDTA, 10% glycerol, 1% triton X-100) containing protease inhibitors (2 mmol/L NaF, 2 mmol/L sodium orthovanadate, 2 mmol/L sodium pyrophosphate, and 1 mmol/L protein inhibitor). After centrifugation at 15,000 g for 15 minutes, the resulting supernatant was collected and used for SDS polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting analysis. Protein concentration was estimated with a Bio-Rad protein assay kit. For direct immunoblotting, aliquots of lysate were mixed with 5×loading buffer containing 2-mercaptoethanol and maintained at 100°C for 10 minutes before loading on 10% SDS-PAGE. Following SDS-PAGE separation, proteins were transferred to PVDF membrane. Membranes were blocked in TBST containing 5% (w/v) non-fat milk and dried for 1 hour at room temperature. Membrane strips were incubated overnight at 4°C with primary antibodies at the following dilutions: StAR (Santa Cruz Biotechnology, Santa Cruz, CA) 1:200, P450scc (Santa Cruz Biotechnology, Santa Cruz, CA) 1:200, GAPDH (Cell Signaling Technology, Beverly, MA) 1:1000. Following extensive washing, membrane strips were incubated in the dark for 1 hour with a fluorescent secondary antibody at 1:5000 dilutions in blocking solution containing 0.1% Tween-20. The fluorescent secondary antibodies were Alexa Fluor 680 goat anti-mouse Molecular Probes (Rockland Immunochemicals, PA), Alexa Fluor 800CW goat anti-rabbit Molecular Probes (Rockland Immunochemicals, PA). Images were acquired with the Odyssey Infrared Imaging System (LI-COR, Biosciences, NE, USA) and analyzed by the software program as speciWed in the Odyssey Software Manual.
Statistical analysis
All the values are expressed as mean ± standard error of the mean (S.E.M.). Statistical Package for Social Sciences (SPSS 11.5) was used for data analysis. Differences among multiple groups were assessed using analysis of variance (ANOVA). Differences in proportions were examined with Chi-square test or Fisher’s Exact Test. A probability value of P<0.05 was considered statistically significant.
Results
Nicotine decreases cortisol/DHEAS production in pHFAC cells
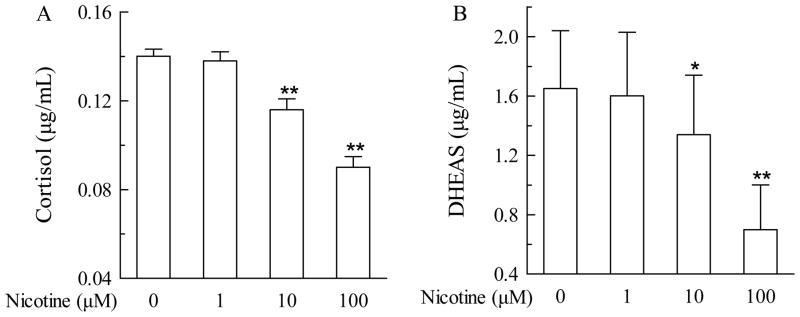
To determine the direct effect of nicotine on the fetal adrenal, the pHFAC cells were treated with nicotine by addition to the culture medium at various concentrations for 24 hours. Nicotine exhibited dose-dependent inhibition of accumulation of cortisol and DHEAS in medium (Figure 1). Nicotine at 10 μM suppressed the production of cortisol and DHEAS by 17.1% (P<0.01) and 18.8% (P<0.05) respectively, whereas at 100 μM, suppression was 35.7% (P<0.01) and 57.6% (P<0.01), respectively.
Fig. 1.
Effects of nicotine (0 to 100 μM) on cortisol and dehydroepiandrosterone (DHEAS) levels in primary human fetal adrenal cortex cells. Cortisol (A) and DHEAS (B) levels after nicotine exposure at different concentrations (0, 1, 10, and 100 μM) for 24 hours are expressed as mean ± S.E.M. n=6. *P<0.05, **P<0.01 vs their corresponding controls.
The decrease of cortisol/DHEAS production associates with suppression of StAR/P450scc expression in nicotine-treated pHFAC cells
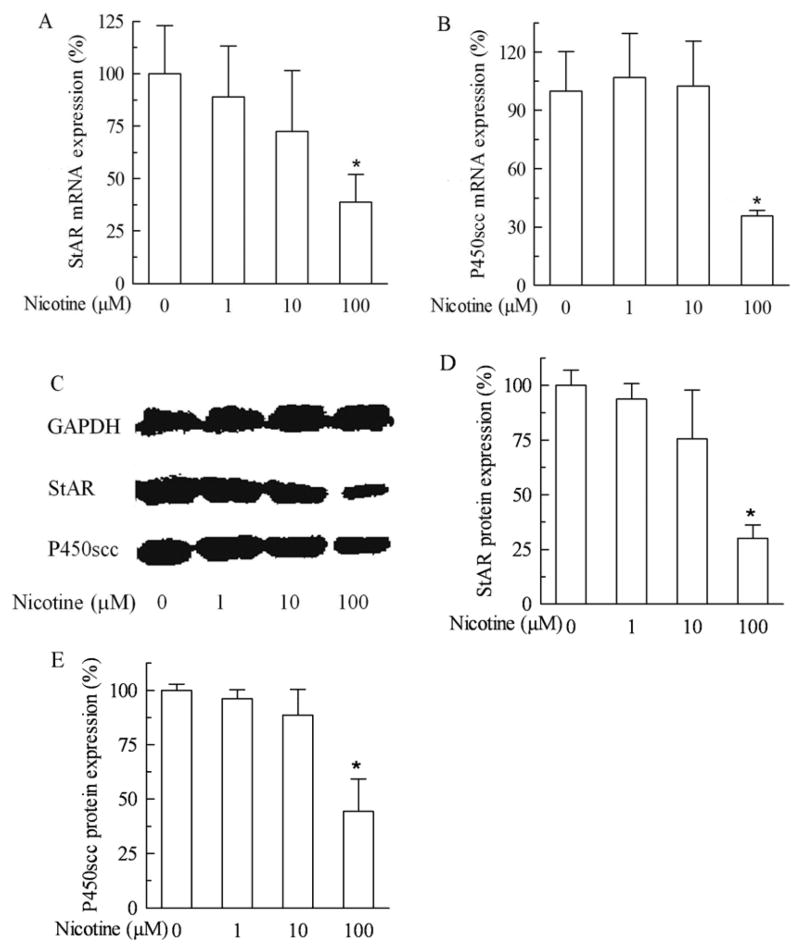
Fig 2A shows that the mRNA expression of fetal adrenal StAR had a tendency of dose-dependent decrease. Nicotine at 100 μM decreased the mRNA expression of StAR by 61.2% (P<0.05). Nicotine at 100 μM also decreased P450scc mRNA expression by 64.3% (P<0.05), although a tendency of dose-dependent decrease was not seen (Fig 2B). Moreover, the protein expression of fetal adrenal StAR and P450scc as shown in Fig 2C was in agreement with the results of mRNA expression. Nicotine at 100 μM decreased the StAR and P450scc protein expression by 70.0% (P<0.01, Fig 2D) and 55.7% (P<0.05, Fig 2E), respectively.
Fig. 2.
Effects of nicotine (0 to 100 μM) on acute regulatory protein (StAR) and cytochrome P450 cholesterol side chain cleavage (P450scc) expression in primary human fetal adrenal cortex cells. A. StAR mRNA expression after nicotine treatment for 24 hours; B. P450scc mRNA expression after nicotine treatment for 24 hours; C. Western blot detection of protein expression of StAR and P450scc after nicotine exposure for 24 hours; D: Quantitative presentation of StAR protein expression. Data are expressed as mean ± S.E.M. n=3. *P<0.05, **P<0.01 vs their corresponding controls.
The nicotine-induced alterations are reproducible in NCI-H295A cells
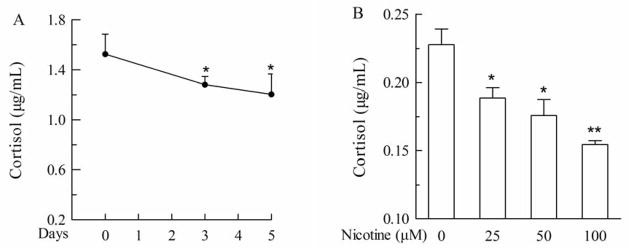
Chronic treatment of NCI-H295A cells with nicotine resulted in lower levels of cortisol in a time- and concentration-dependent manner, than in the corresponding controls (Fig 3A). Nicotine decreased cortisol level by 16% (P<0.05) and 21% (P<0.05) at 50 μM for 3 and 5 days (Fig 3A), and by 17.1% (P<0.05), and 32.1% (P<0.01) at 25 and 100 μM for 5 days, respectively, as compared with the control (Fig 3B). These data in NCI-H295A cells reproduced nicotine-induced inhibition of accumulation of cortisol and DHEAS in medium.
Fig. 3.
Effect of nicotine chronic treatment on cortisol level in NCI-H295A cells. A. Cortisol produced by 3×104 cells treated with 50 μM nicotine for different days (3 and 5 days) were detected with ELISA kit. B. Cortisol produced by 4×103 cells treated with different concentrations (0, 25, 50, and 100 μM) of nicotine for 5 days were detected with ELISA kit. Data are expressed as mean ± S.E.M. n=5. *P<0.05, **P<0.01 vs control.
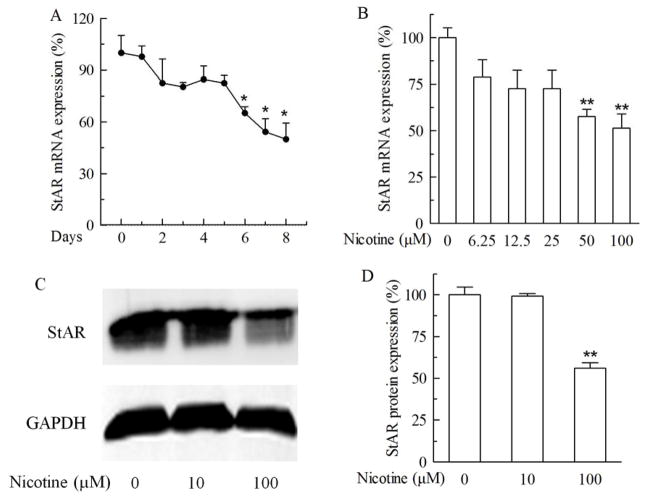
Consistent to the decrease of cortisol level, nicotine treatment also inhibited the expression of StAR in a dose-dependent manner (Fig 4). Chronic treatment of NCI-H295A cells with 100 μM nicotine for 5, 6, 7 and 8 days significantly decreased StAR mRNA expression by 17.4%, 34.8%, 45.7% and 50.0% (P>0.05 or P<0.05) respectively, as compared with their corresponding control treatments (Fig 4A). On day 3, nicotine at 100 μM had already caused a 19.6% decrease of StAR mRNA expression, equivalent to the magnitude seen on day 5. When various concentrations were applied, treatment with nicotine at 50 and 100 μM for 7 days decreased StAR mRNA expression by 42.4% (P<0.01) and 48.5% (P<0.01), respectively (Fig 4B). In agreement with the mRNA expression, chronic treatment with 100 μM nicotine for 7 days decreased StAR protein expression by 44.0% (P<0.01) as compared with the control treatment (Fig 4C).
Fig. 4.
Effect of chronic nicotine treatment on acute regulatory protein (StAR) expression in NCI-H295A cells. A. StAR mRNA expression after 100 μM nicotine exposure for different days. B. StAR mRNA expression after different doses of nicotine treatment for 7 days. C. StAR protein expression after 100 μM nicotine treatment for 7 days. D. Quantitative presentation of StAR protein expression. Data are expressed as mean ± S.E.M. n=3. *P<0.05, **P<0.01 vs control.
To determine whether cell proliferation and viability played any role in the nicotine-induced decreases of StAR mRNA and protein expression, NCI-H295A cells were treated with nicotine at 1, 10, and 100 μM for 7 days and the cell proliferation/viability was measured with the MTT assay. The data revealed no significant difference among various treatments (Fig S1 in supplemental information).
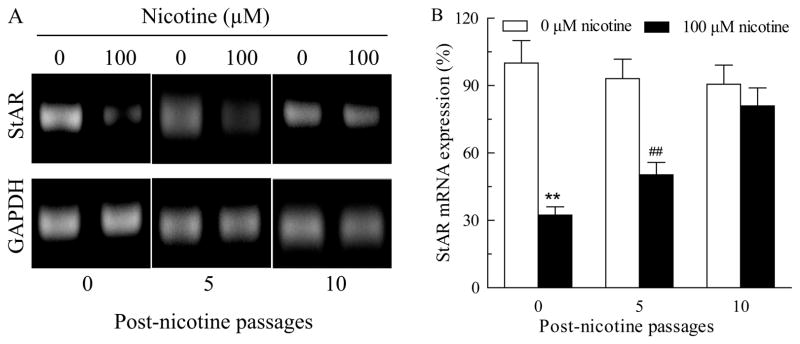
The alteration in StAR expression persists for more than 5 passages after termination of nicotine treatment
To determine for how long the depressed StAR expression can last after withdrawal of nicotine from the culture medium, NCI-H295A cells treated with 100 μM of nicotine for 10 days were continued to grow in the absence of nicotine for up to 30 days or about 10 passages. As shown in Fig 5, the StAR mRNA expression at post-nicotine day 0 (passage 0), day 15 (passage 5), and day 30 (passage 10) were decreased by 67.6% (P<0.01), 46.0% (P<0.01) and 10.7%, respectively, as compared with their corresponding control. These data revealed an almost linear daily recovery (1.4%-1.9%) of the depressed StAR mRNA expression after termination of the nicotine treatment. In theory, a full recovery might be achievable on day 35 after the nicotine treatment is terminated.
Fig. 5.
Expression of acute regulatory protein (StAR) mRNA in NCI-H295A cells after withdrawal of nicotine treatment. A. After chronic treatment of NCI-H295A cells with 100 μM nicotine for 10 days, nicotine treatment was stopped and the cells were further cultured for up to 5, 10 passages. Then the StAR mRNA expression was detected using semi-quantitative RT-PCR; B. Quantitative presentation of StAR mRNA expression. Data are expressed as mean ± S.E.M. n=3. **P<0.01, ##P<0.01 vs their corresponding controls.
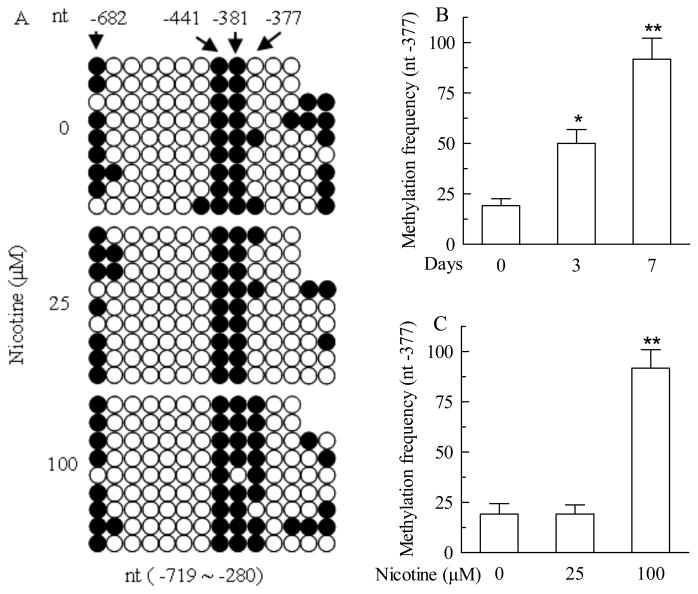
A single CpG site of nicotine-sensitive methylation is identified in StAR promoter
To determine whether CpG methylation is involved in the nicotine-induced decrease of StAR expression, the proximal promoter of the human StAR gene was selected for methylation analysis. We first analyzed human StAR gene with a web promoter scan service (http://www-bimas.cit.nih.gov/molbio/proscan/) and found that it has one promoter. As shown in Fig S2 (supplemental information), the StAR promoter and the adjacent regions contain many CpG dinucleotides, potential sites of DNA methylation, but no CpG islands. The methylation status within such a region (nt −719 to −280 and −9 to 402) was detected using BSP and MSP assays. Figure 6A shows that the three CpG sites at nt −682, −441, and −381 of the promoter region are methylated in the absence of nicotine treatment. Treatment with 100 μM nicotine for 7 days resulted in a substantially increased frequency of methylation of a single CpG site at nt −377, but little effect on the methylation status of the three CpG sites. No significant changes were seen in NCI-H295A cells treated with 25 μM of nicotine for 7 days. No CpG methylation was detected within the region of −9 to 402 bp in both the absence and presence of nicotine treatment (data not shown).
Fig. 6.
Nicotine-induced changes in methylation status of acute regulatory protein (StAR) gene promoter (nt −719 ~ −280) in NCI-H295A cells detected with bisulfite sequence PCR (BSP) and methylation specific PCR (MSP) methods. A. Methylation map of StAR promoter in NCI-H295A cells treated with 25, 100 μM nicotine for 7 days detected with BSP. B. Methylation frequency of StAR promoter at nt −377 single site in NCI-H295A cells treated with 100 μM nicotine for 3, 7 days detected with MSP. C. Methylation frequency of StAR promoter at nt −377 single site in NCI-H295A cells treated with 25, and 100 μM nicotine for 7 days detected with MSP. Data are expressed as mean ± S.E.M. n=3. *P<0.05, **P<0.01 vs their corresponding controls.
Consistently, the frequency of the single point methylation at nt −377 increased in a time-dependent manner when the cells were treated with 100 μM nicotine (Fig 6B). On day 3 and 7, 100 μM nicotine increased the frequencies of this site methylation by 2.6 fold (50%, or 30.8% above the basal frequency) and 4.8 fold (91.8%, or 72.6% above the corresponding basal frequency), respectively, as assayed using the MSP method (Fig 6B). However, nicotine at a lower concentration (25 μM) for 7 days did not increase the frequency of this point methylation (Fig 6C), suggesting that this nicotine-sensitive CpG site is more sensitive to nicotine dose than the treatment duration. Interestingly, a basal frequency (19.2%) of the point methylation in the absence of nicotine treatment was also observed (Fig 6B and 6C). This is consistent to the result obtained using the BSP assay (Fig 6A).
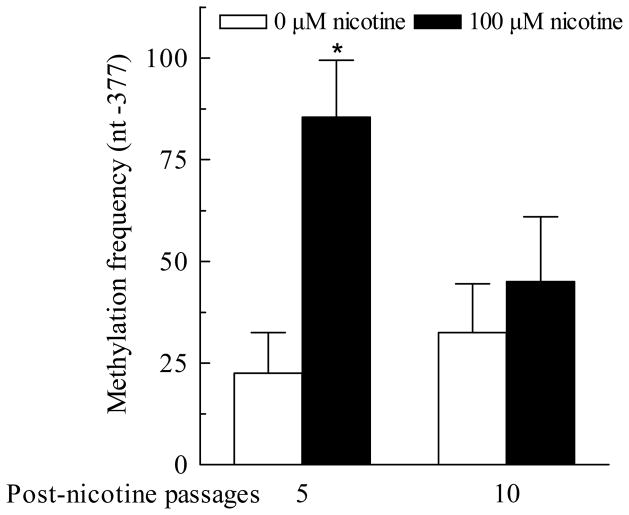
The single site methylation persists for more than 5 passages after termination of nicotine treatment
We have shown in Fig 5 that the suppression of StAR expression can continue for more than 15 days or 5 passages after the nicotine treatment is terminated. To determine whether the nicotine-sensitive point methylation at nt −377 in StAR promoter undergoes a similar time course as StAR expression does, the NCI-295A cells pre-treated with 100 μM nicotine for 10 days were cultured for 5 and 10 more passages (equivalent to about 15 and 30 more days, respectively) in the absence of nicotine treatment. At the end of passage 5 and 10, the genomic DNA samples were isolated and the point methylation was examined using the MSP assay. Fig 7 shows that the frequency of the single point CpG methylation at nt −377 remained high level (85.5%) over the first 5 passages (15 days) and dropped to 45% over the second 5 passages after treatment with nicotine for 10 days. These MSP results were largely consistent to BSP data on the methylation frequency (data not shown).
Fig. 7.
Prolonged methylation status of nt −377 single site in acute regulatory protein (StAR) promoter in the NCI-H295A cells after withdrawal of nicotine treatment. After chronic treatment of NCI-H295A cells with 100 μM nicotine for 10 days, nicotine treatment was stopped and the cells were further cultured for up to 5, 10 passages. Then the methylation frequency at nt −377 single site in StAR promoter was detected using MSP. Data are expressed as mean ± S.E.M. n=3. *P<0.05 vs their corresponding controls.
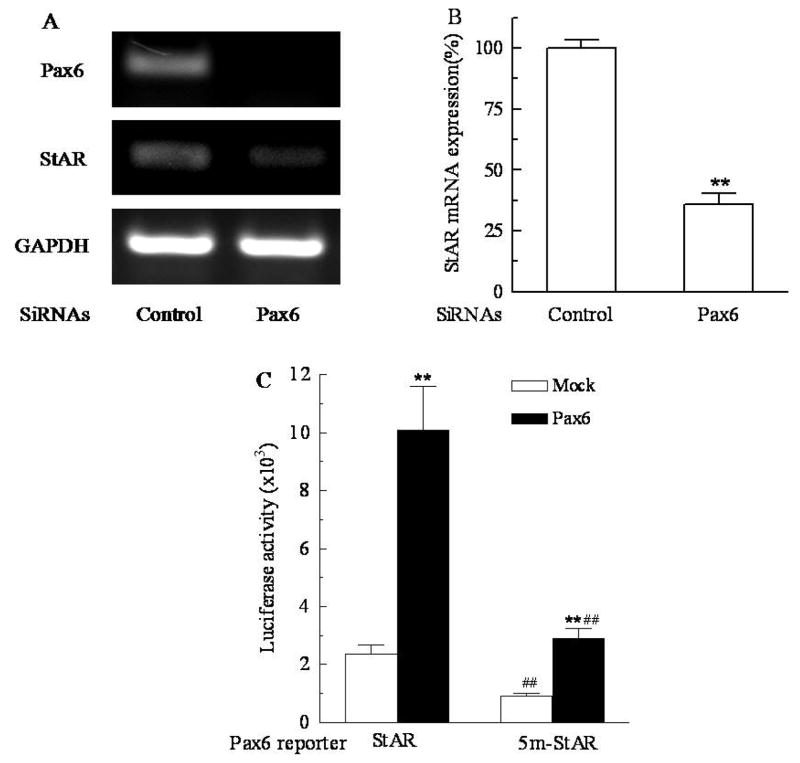
Pax6 promotes StAR gene expression
Human cDNA array data showed that nicotine treatment could not alter the expression of Pax6 in pHFAC cells (data not shown). To determine whether Pax6 binding could promote the StAR gene expression and the methylation at nt −377 could block Pax6 binding, the siRNA approach and luciferase activity assay were employed. Fig 8A and 8B show that the two consecutive transfections of NCI-H295A cells with Pax6 siRNA on day 1 and day 3 decrease Pax6 mRNA expression by more than 98% as assayed on day 6. As expected, the StAR mRNA expression is also decreased by about 64.2% (P<0.01) in the absence of nicotine treatment. This magnitude of decrease in StAR expression caused by Pax6 knock-down is even greater than that caused by treatment with 100 μM nicotine for 7 days. Fig 8C shows that pre-treatment of the StAR/Pax6 reporters with M.SssI significantly reduced the luciferase activities in both absence (62%) and presence (71%) of Pax6 expression (P<0.01), suggesting that CpG methylation in the binding motif of Pax6 regulates StAR expression. Expression of Pax6 significantly increased luciferase activities to 4 and 2.9 folds from baseline (mock) for the StAR/Pax6 reporters pre-treated with and without M.SssI, respectively (P<0.01), suggesting that Pax6 activates StAR gene expression by binding to the glucagon G3-like motif (CGCCTGA) and the methylation of this site partially blocks StAR expression.
Fig. 8.
Pax6 increased acute regulatory protein (StAR) gene expression by binding to the glucagon G3-like motif (CGCCTGA) in StAR promoter. A. Effect of Pax6 siRNAs on StAR mRNA expression. NCI-H295A cells were transfected with Pax6 siRNAs in lipofectamine 2000 for 72 hours, and then the transfection was repeated once. Then StAR and Pax6 mRNA expression were detected on day 6 after initial transfection using RT-PCR; B. Quantitative presentation of StAR mRNA expression. Data are expressed as mean ± S.E.M. n=3. **P<0.01 vs control. C. Luciferase activity assay of the StAR/Pax6 reporter. NCI-H295A cells were co-transfected with StAR/Pax6 reporter and Pax6 or empty vector (mock) in lipofectamine 2000 for 48 hours. The cells were harvested for luciferase activity assay. Data are expressed as mean ± S.E.M. n=3. 5m-StAR, the StAR/Pax6 reporter pretreated with M.SssI; **P<0.01 vs their corresponding mocks; ##P<0.01 vs their corresponding unmethylated StAR/Pax6 reporters.
Discussion
StAR mediates the initial and rate-limiting step in adrenocortical steroidogenesis, and plays a critical role in the maintenance of normal pregnancy and promotion of fetal growth (Ramanjaneya et al., 2011; Stocco, 2001). In the present study, we found that nicotine treatment inhibited StAR/P450scc expressions and cortisol production in both pHFAC cells and NCI-H295A cells. A correlation between cortisol production and StAR expression suggests that StAR is critical and sensitive in regulating steroidogenesis in the presence of nicotine. Moreover, the expression of StAR remained suppressed over 15 days (5 passages) after termination of nicotine treatment, which suggests the hereditability of nicotine-induced inhibition of StAR gene expression.
The nicotine concentration of 100 μM used in this cell culture study is much higher than the reported plasma levels of cigarette smokers, ranging from 0.1–0.6 μM (Russell et al., 1975; Satta et al., 2008). Nicotine levels in fetal circulation, amniotic fluid, and fetal tissues can be higher largely due to free penetration of placenta and lipophilic accumulation of nicotine over the course of 10-month pregnancy, and low enzymatic activity of fetal CYP2A6 (Lambers and Clark, 1996; Dempsey et al., 2000; Machado Jde et al., 2011). Thus, much higher concentrations up to 100 or even 300 μM have been utilized in many short-term (several days) in vitro cell culture studies (Klapproth et al., 1998; Fang and Svoboda, 2005; Serres and Carney, 2006; Kawakita et al., 2008; Naha et al., 2009; Klettner et al., 2011; Laytragoon-Lewin et al., 2011; Papaioannou et al., 2011). However, interpretation of these results generated from in vitro studies should be cautious in the context of in vivo conditions. Future study will be necessary to determine if the CpG methylation at the Pax6 binding motif of StAR gene promoter would also occur in smoking humans with typically long term exposure at physiologically relevant plasma levels.
StAR expression is tightly regulated at multiple levels including transcriptional, post-transcriptional, translational, and even post-receptor levels (Stocco et al., 2005; Zhao et al., 2005). In this study, we have demonstrated that a single CpG site methylation at nt −377 of StAR promoter associates highly with suppression of the gene expression. This CpG is sensitive to nicotine treatment. We also noticed that this single CpG methylation appeared on day 3 of 100 μM nicotine treatment and before the decrease of StAR mRNA expression, suggesting that the single CpG methylation is one of the causes for attenuation of expression of the StAR gene. Recently, more and more literatures support the viewpoint that single CpG site methylation can play an important role on regulation of gene expression. Expression of hypoxic marker CA IX is regulated by site-specific DNA methylation and is associated with the histology of gastric cancer (Nakamura et al., 2011). Methylation of a single intronic CpG mediates expression silencing of the PMP24 gene in prostate cancer (Zhang et al., 2010b). A variant in the CHEK2 promoter at a methylation site relieves transcriptional repression and confers reduced risk of lung cancer (Zhang et al., 2010a). Although the mechanism remains largely unknown and poorly explored, reports have shown that single site methylation requires additional transcription factors or methyl binding domain-containing proteins for regulation of gene expression (Kim et al., 2003; Kitazawa and Kitazawa, 2007; Zhang et al., 2010a). The obvious correlation between nicotine-induced alterations in StAR expression and the single CpG site methylation suggested that methylation-sensitive factor binding and competition binding between transcription factors to CpG-containing recognition motifs are possible mechanisms for the regulation of growth-related gene expression both during development and in the mature organism (Kitazawa and Kitazawa, 2007).
Like many genes, the expression of StAR can be regulated by a variety of transcription factors. These transcription factors include steroidogenic factor-1 (SF-1), CCAAT/enhancer binding proteins (C/EBP), sterol regulatory element-binding proteins (SREBPs), and GATA-4 (Stocco et al., 2005). Pax6 is a critical transcription factor in the development of eye, pancreas, and central nervous system and variations in the Pax6 binding motif are reported (Grapp et al., 2009; Umeda et al., 2010). Studies have shown that Pax6 binds the glucagon-G3 promoter consensus (CGCCTGA) (Grapp et al., 2009). Pax6 has two DNA binding domains, the amino-terminal paired domain (PD) followed by a homeodomain (HD). It is the PD domain that binds the glucagon-G3 consensus CGCCTGA (Grapp et al., 2009). Interestingly, the identified single CpG site of nicotine-sensitive methylation (nt −377) is part of the glucagon-G3 consensus. In this study, we identify using bioinformatic analysis (Fig S3 in supplemental information) and siRNA approach that nicotine causes methylation of the single CpG site at which the potential Pax6 binding motif is positioned. However, nicotine does not alter much the Pax6 expression in pHFAC cells (unpublished data). The luciferase activity assays validate the notion that Pax6 increases StAR gene expression by binding to the glucagon G3-like motif (CGCCTGA). This data with M.SssI pretreated reporter also confirms that Pax6 binding to StAR promoter is sensitive to CpG methylation. Similar to methylation-sensitive restriction endonuclease AscI, Pax6 may be a methylation-resistant transcription factor that binds only to its unmethylated motifs (Dai et al., 2002). CpG methylation at the minimal binding motif of Pax6 (CGCCTGA) caused inhibition of StAR expression to certain degree, but not complete abolishment. Although methylation at −377 CpG site correlates well with the suppressed StAR expression and reporter luciferase assay validates the role of Pax6 in regulating StAR expression, the suppression of StAR expression induced by nicotine treatment may also involve other unidentified mechanisms. Interestingly, using bioinformatic analysis, we also identify that the Pax6 motif identical to the glucagon-G3 consensus (CGCCTGA) in the StAR promoter is also present in other genes including component genes consisting of hypothalamic-pituitary-adrenal (HPA) axis, such as corticotrophin-releasing hormone receptor type-1 (CRHR1), melanocortin 2 receptor (MC2R), and glucocorticoid receptor (GR). Whether Pax6 binds to these genes consisting of HPA axis and whether nicotine induces single CpG methylation in these gene motifs are unclear. However, nicotine may alter intrauterine programming of HPA axis by interfering with the expression of these component genes, to induce collectively IUGR and onset of adult diseases or disorders (Simard et al., 2010).
Our results showed the nicotine-induced long-term suppression of StAR expression over 15 days (5 passages) after termination of the treatment largely matched up with the demethylation pattern of the single site methylation at nt −377 of the StAR promoter, which suggests that the DNA methylation mechanism induced by nicotine are heritable. Identification and correction of critical epigenetic modification may hold key for developing early diagnosis and treatment of some adult diseases or disorders (Deng et al., 2010). It is well established that CpG methylation in the proximal promoter inhibits the gene expression (Mund and Lyko, 2010). In our studies, nicotine-induced CpG methylation of Pax6 binding motif in StAR promotor reduces the StAR gene expression and cortisol production. These compelling data suggest that this nicotine-sensitive CpG methylation site at Pax6 binding motif in StAR promoter may be useful for the early diagnosis and treatment of prenatal nicotine exposure-related adult diseases or disorders.
In summary, in this study, we not only demonstrated that nicotine inhibited StAR/P450scc expressions and cortisol production in both pHFAC cells and NCI-H295A cells, but also for the first time identified a single nicotine-sensitive CpG methylation site at the Pax6 binding motif in StAR promoter that prevent Pax6 from binding the motif and down-regulate StAR gene expression. The close correlation between sustained suppression of StAR expression and persistent CpG methylation at nt −377 after termination of nicotine treatment provided an inheritable epigenetic mechanism for nicotine-induced intrauterine programming of HPA axis and onset of adult diseases or disorders.
Supplementary Material
Research Highlights.
Nicotine-induced StAR inhibition in two human adrenal cell models.
Nicotine-induced single CpG site methylation in StAR promoter.
Persistent StAR inhibition and single CpG methylation after nicotine termination.
Single CpG methylation located at Pax6 binding motif regulates StAR expression.
Acknowledgments
This work was supported by grants (#30830112, 81072709 and #30672566) to HW and (#30901213) to YY from the Chinese Nature Science Foundation, a NIH R01 grant (HL065492) to YHF, and a visiting scholarship (#20073020) to TW from China Scholarship Council.
We are grateful to Dr. W L Miller (Department of Pediatrics and the Metabolic Research Unit, University of California, San Francisco, California) for the NCI-H295A cells used in this study. We thank Dr. Charles Vincent Smith for critical reading of this manuscript and comment. Some of the authors are employees of the U.S. Government, and this manuscript was prepared as part of their official duties. Title 17 U.S.C. §105 provides that Copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C §101 defined a U.S. Government work as a work prepared by a military service member or employees of the U.S. Government as part of that person’s official duties. The views in this article are those of the authors and do not necessarily reflect the views, official policy, or position of the Uniformed Services University of the Health Sciences, Department of the Navy, Department of Defense, or the U.S. Federal Government.
Abbreviations
- StAR
Steroidogenic acute regulatory protein
- IUGR
intrauterine growth retardation
- pHFAC
primary human fetal adrenal cortex
- HPA
hypothalamic-pituitary-adrenal
- P450scc
cytochrome P450 cholesterol side chain cleavage
- DHEAS
dehydroepiandrosterone sulfate
- BSP
bisulfite Sequence PCR
- MSP
methylation Specific PCR
Footnotes
An abstract was submitted in June 2009 and presented at the 16th North American ISSX meeting, Baltimore, Maryland, USA October 18–22, 2009.
Disclosure summary: The authors have no interest of conflict.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen M, Wang T, Liao ZX, Pan XL, Feng YH, Wang H. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Exp Toxicol Pathol. 2007;59:245–251. doi: 10.1016/j.etp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Csepregi A, Ebert MP, Rocken C, Schneider-Stock R, Hoffmann J, Schulz HU, Roessner A, Malfertheiner P. Promoter methylation of CDKN2A and lack of p16 expression characterize patients with hepatocellular carcinoma. BMC Cancer. 2010;10:317. doi: 10.1186/1471-2407-10-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Weichenhan D, Wu YZ, Hall JL, Rush LJ, Smith LT, Raval A, Yu L, Kroll D, Muehlisch J, Fruhwald MC, de Jong P, Catanese J, Davuluri RV, Smiraglia DJ, Plass C. An AscI boundary library for the studies of genetic and epigenetic alterations in CpG islands. Genome Res. 2002;12:1591–1598. doi: 10.1101/gr.197402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardis A, Miller WL. Dexamethasone does not exert direct intracellular feedback on steroidogenesis in human adrenal NCI-H295A cells. J Endocrinol. 2003;179:131–142. doi: 10.1677/joe.0.1790131. [DOI] [PubMed] [Google Scholar]
- Delpisheh A, Brabin L, Drummond S, Brabin BJ. Prenatal smoking exposure and asymmetric fetal growth restriction. Ann Hum Biol. 2008;35:573–583. doi: 10.1080/03014460802375596. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67:458–465. doi: 10.1067/mcp.2000.106129. [DOI] [PubMed] [Google Scholar]
- Deng D, Liu Z, Du Y. Epigenetic alterations as cancer diagnostic, prognostic, and predictive biomarkers. Adv Genet. 2010;71:125–176. doi: 10.1016/B978-0-12-380864-6.00005-5. [DOI] [PubMed] [Google Scholar]
- Fang Y, Svoboda KK. Nicotine inhibits myofibroblast differentiation in human gingival fibroblasts. J Cell Biochem. 2005;95:1108–1119. doi: 10.1002/jcb.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Caiping M, Li C, Can R, Feichao X, Li Z, Zhice X. Fetal and offspring arrhythmia following exposure to nicotine during pregnancy. J Appl Toxicol. 2010;30:53–58. doi: 10.1002/jat.1471. [DOI] [PubMed] [Google Scholar]
- Geelhoed J, El Marroun H, Verburg B, van Osch-Gevers L, Hofman A, Huizink A, Moll H, Verhulst F, Helbing W, Steegers E, Jaddoe V. Maternal smoking during pregnancy, fetal arterial resistance adaptations and cardiovascular function in childhood. BJOG. 2011;118:755–762. doi: 10.1111/j.1471-0528.2011.02900.x. [DOI] [PubMed] [Google Scholar]
- Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab. 2008;22:1–16. doi: 10.1016/j.beem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Grapp M, Teichler S, Kitz J, Dibaj P, Dickel C, Knepel W, Kratzner R. The homeodomain of PAX6 is essential for PAX6-dependent activation of the rat glucagon gene promoter: evidence for a PH0-like binding that induces an active conformation. Biochim Biophys Acta. 2009;1789:403–412. doi: 10.1016/j.bbagrm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Guilloteau P, Zabielski R, Hammon HM, Metges CC. Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. J Physiol Pharmacol. 2009;60(Suppl 3):17–35. [PubMed] [Google Scholar]
- Habano W, Gamo T, Terashima J, Sugai T, Otsuka K, Wakabayashi G, Ozawa S. Involvement of promoter methylation in the regulation of Pregnane × receptor in colon cancer cells. BMC Cancer. 2011;11:81. doi: 10.1186/1471-2407-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib S, Gattineni J, Twombley K, Baum M. Evidence that prenatal programming of hypertension by dietary protein deprivation is mediated by fetal glucocorticoid exposure. Am J Hypertens. 2011;24:96–101. doi: 10.1038/ajh.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman PL, Cutfield WS, Robinson EM, Bergman RN, Menon RK, Sperling MA, Gluckman PD. Insulin resistance in short children with intrauterine growth retardation. J Clin Endocrinol Metab. 1997;82:402–406. doi: 10.1210/jcem.82.2.3752. [DOI] [PubMed] [Google Scholar]
- Kanaka-Gantenbein C. Fetal origins of adult diabetes. Ann N Y Acad Sci. 2010;1205:99–105. doi: 10.1111/j.1749-6632.2010.05683.x. [DOI] [PubMed] [Google Scholar]
- Kawakita A, Sato K, Makino H, Ikegami H, Takayama S, Toyama Y, Umezawa A. Nicotine acts on growth plate chondrocytes to delay skeletal growth through the alpha7 neuronal nicotinic acetylcholine receptor. PLoS One. 2008;3:e3945. doi: 10.1371/journal.pone.0003945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kian Tee M, Huang N, Damm I, Miller WL. Transcriptional Regulation of the Human P450 Oxidoreductase Gene: Hormonal Regulation and Influence of Promoter Polymorphisms. Mol Endocrinol. 2011;25:715–731. doi: 10.1210/me.2010-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kollhoff A, Bergmann A, Stubbs L. Methylation-sensitive binding of transcription factor YY1 to an insulator sequence within the paternally expressed imprinted gene, Peg3. Hum Mol Genet. 2003;12:233–245. doi: 10.1093/hmg/ddg028. [DOI] [PubMed] [Google Scholar]
- Kitazawa R, Kitazawa S. Methylation status of a single CpG locus 3 bases upstream of TATA-box of receptor activator of nuclear factor-kappaB ligand (RANKL) gene promoter modulates cell- and tissue-specific RANKL expression and osteoclastogenesis. Mol Endocrinol. 2007;21:148–158. doi: 10.1210/me.2006-0205. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Racke K, Wessler I. Acetylcholine and nicotine stimulate the release of granulocyte-macrophage colony stimulating factor from cultured human bronchial epithelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:472–475. doi: 10.1007/pl00005195. [DOI] [PubMed] [Google Scholar]
- Klettner AK, Doths J, Roider J. Nicotine reduces VEGF-secretion and phagocytotic activity in porcine RPE. Graefes Arch Clin Exp Ophthalmol. 2011:21. doi: 10.1007/s00417-011-1776-8. [DOI] [PubMed] [Google Scholar]
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- Laytragoon-Lewin N, Bahram F, Rutqvist LE, Turesson I, Lewin F. Direct effects of pure nicotine, cigarette smoke extract, Swedish-type smokeless tobacco (Snus) extract and ethanol on human normal endothelial cells and fibroblasts. Anticancer Res. 2011;31:1527–1534. [PubMed] [Google Scholar]
- Liang G, Chen M, Pan XL, Zheng J, Wang H. Ethanol-induced inhibition of fetal hypothalamic-pituitary-adrenal axis due to prenatal overexposure to maternal glucocorticoid in mice. Exp Toxicol Pathol. 2010:3. doi: 10.1016/j.etp.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Liu J, Li XD, Vaheri A, Voutilainen R. DNA methylation affects cell proliferation, cortisol secretion and steroidogenic gene expression in human adrenocortical NCI-H295R cells. J Mol Endocrinol. 2004;33:651–662. doi: 10.1677/jme.1.01560. [DOI] [PubMed] [Google Scholar]
- Lorente A, Mueller W, Urdangarin E, Lazcoz P, von Deimling A, Castresana JS. Detection of methylation in promoter sequences by melting curve analysis-based semiquantitative real time PCR. BMC Cancer. 2008;8:61. doi: 10.1186/1471-2407-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado Jde B, Plinio Filho VM, Petersen GO, Chatkin JM. Quantitative effects of tobacco smoking exposure on the maternal-fetal circulation. BMC Pregnancy Childbirth. 2011;11:24. doi: 10.1186/1471-2393-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak B, Patro-Malysza J, Poniedzialek-Czajkowska E, Kimber-Trojnar Z, Leszczynska-Gorzelak B, Oleszczuk J. Glucocorticoids in Pregnancy. Curr Pharm Biotechnol. 2011;12:750–757. doi: 10.2174/138920111795470868. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Papadopoulos V. Epigenetic regulation of the expression of genes involved in steroid hormone biosynthesis and action. Steroids. 2010;75:467–476. doi: 10.1016/j.steroids.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Lubo Z. Fetal programming of cardiac function and disease. Reprod Sci. 2007;14:209–216. doi: 10.1177/1933719107302324. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mund C, Lyko F. Epigenetic cancer therapy: Proof of concept and remaining challenges. Bioessays. 2010;32:949–957. doi: 10.1002/bies.201000061. [DOI] [PubMed] [Google Scholar]
- Naha N, Lee HY, Hwang JS, Bahk JY, Park MS, Lee SY, Kim SH, Kim MO. Nicotine tolerance to PC12 cell line: acute and chronic exposures modulate dopamine D2 receptor and tyrosine hydroxylase expression. Neurol Res. 2009;31:289–299. doi: 10.1179/174313209X382403. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Kitajima Y, Kai K, Hashiguchi K, Hiraki M, Noshiro H, Miyazaki K. Expression of hypoxic marker CA IX is regulated by site-specific DNA methylation and is associated with the histology of gastric cancer. Am J Pathol. 2011;178:515–524. doi: 10.1016/j.ajpath.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K. Adrenal function of low-birthweight children. Endocr Dev. 2005;8:34–53. doi: 10.1159/000084092. [DOI] [PubMed] [Google Scholar]
- Papaioannou KA, Markopoulou CE, Gioni V, Mamalis AA, Vayouraki HN, Kletsas D, Vrotsos IA. Attachment and proliferation of human osteoblast-like cells on guided bone regeneration (GBR) membranes in the absence or presence of nicotine: an in vitro study. Int J Oral Maxillofac Implants. 2011;26:509–519. [PubMed] [Google Scholar]
- Pellanda LC, Duncan BB, Vigo A, Rose K, Folsom AR, Erlinger TP. Low birth weight and markers of inflammation and endothelial activation in adulthood: the ARIC study. Int J Cardiol. 2009;134:371–377. doi: 10.1016/j.ijcard.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre MA, Petrik J, Ellis R, Inman MD, Holloway AC, Labiris NR. Fetal and Neonatal Exposure to Nicotine Disrupts Postnatal Lung Development in Rats: Role of VEGF and its Receptors. Int J Toxicol. 2011;30:244–252. doi: 10.1177/1091581810395332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanjaneya M, Conner AC, Brown JE, Chen J, Digby JE, Barber TM, Randeva HS. Adiponectin (15–36) stimulates steroidogenic acute regulatory (StAR) protein expression and cortisol production in human adrenocortical cells: Role of AMPK and MAPK kinase pathways. Biochim Biophys Acta. 2011;1813:802–809. doi: 10.1016/j.bbamcr.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Russell MA, Wilson C, Patel UA, Feyerabend C, Cole PV. Plasma nicotine levels after smoking cigarettes with high, medium, and low nicotine yields. Br Med J. 1975;2:414–416. doi: 10.1136/bmj.2.5968.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci U S A. 2008;105:16356–16361. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres F, Carney SL. Nicotine regulates SH-SY5Y neuroblastoma cell proliferation through the release of brain-derived neurotrophic factor. Brain Res. 2006;1101:36–42. doi: 10.1016/j.brainres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Simard M, Cote M, Provost PR, Tremblay Y. Expression of genes related to the hypothalamic-pituitary-adrenal axis in murine fetal lungs in late gestation. Reprod Biol Endocrinol. 2010;8:134. doi: 10.1186/1477-7827-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Umeda T, Takashima N, Nakagawa R, Maekawa M, Ikegami S, Yoshikawa T, Kobayashi K, Okanoya K, Inokuchi K, Osumi N. Evaluation of Pax6 mutant rat as a model for autism. PLoS One. 2010;5:e15500. doi: 10.1371/journal.pone.0015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang M, Peng RX, Le J. Influences of 3-methylcholanthrene, phenobarbital and dexamethasone on xenobiotic metabolizing-related cytochrome P450 enzymes and steroidogenesis in human fetal adrenal cortical cells. Acta Pharmacol Sin. 2006;27:1093–1096. doi: 10.1111/j.1745-7254.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Ping J, Peng RX, Yue J, Xia XY, Li QX, Kong R, Hong JY. Changes of multiple biotransformation phase I and phase II enzyme activities in human fetal adrenals during fetal development. Acta Pharmacol Sin. 2008;29:231–238. doi: 10.1111/j.1745-7254.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- Xita N, Tsatsoulis A. Fetal origins of the metabolic syndrome. Ann N Y Acad Sci. 2010;1205:148–155. doi: 10.1111/j.1749-6632.2010.05658.x. [DOI] [PubMed] [Google Scholar]
- Yildiz D. Nicotine, its metabolism and an overview of its biological effects. Toxicon. 2004;43:619–632. doi: 10.1016/j.toxicon.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lu J, Zhao X, Wu W, Wang H, Wu Q, Chen X, Fan W, Chen H, Wang F, Hu Z, Jin L, Wei Q, Shen H, Huang W, Lu D. A variant in the CHEK2 promoter at a methylation site relieves transcriptional repression and confers reduced risk of lung cancer. Carcinogenesis. 2010a;31:1251–1258. doi: 10.1093/carcin/bgq089. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu M, Xiao H, Lee MT, Levin L, Leung YK, Ho SM. Methylation of a single intronic CpG mediates expression silencing of the PMP24 gene in prostate cancer. Prostate. 2010b;70:765–776. doi: 10.1002/pros.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Xue H, Artemenko I, Jefcoate C. Novel signaling stimulated by arsenite increases cholesterol metabolism through increases in unphosphorylated steroidogenic acute regulatory (StAR) protein. Mol Cell Endocrinol. 2005;231:95–107. doi: 10.1016/j.mce.2004.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.