Abstract
The mechanisms of acetaminophen (APAP)-mediated hepatic oncotic necrosis have been extensively characterized. However, it was recently demonstrated that fed CD-1 mice have a transient caspase activation which initiates apoptosis. To evaluate these findings in more detail, outbred (Swiss Webster, SW) and inbred (C57BL/6) mice were treated with APAP with or without pan-caspase inhibitor and compared to the apoptosis model of galactosamine (GalN)/endotoxin (ET). Fasted or fed APAP-treated C57BL/6 mice showed no evidence of caspase-3 processing or activity. Interestingly, a minor, temporary increase in caspase-3 processing and activity (150% above baseline) was observed after APAP treatment only in fed SW mice. The degree of caspase-3 activation in SW mice after APAP was minor compared to that observed in GalN/ET-treated mice (1600% above baseline). The pancaspase inhibitor attenuated caspase activation and resulted in increased APAP-induced injury (plasma ALT, necrosis scoring). The caspase inhibitor did not affect apoptosis because regardless of treatment only <0.5% of hepatocytes showed consistent apoptotic morphology after APAP. In contrast, >20% apoptotic cells were observed in GalN/ET-treated mice. Presence of the caspase inhibitor altered hepatic glutathione levels in SW mice, which could explain the exacerbation of injury. Additionally, the infiltration of hepatic neutrophils was not altered by the fed state of either mouse strain. Conclusion: Minor caspase-3 activation without apoptotic cell death can be observed only in fed mice of some outbred strains. These findings suggest that although the severity of APAP-induced liver injury varies between fed and fasted animals, the mechanism of cell death does not fundamentally change.
Keywords: acetaminophen, hepatotoxicity, DNA fragmentation, caspases, necrosis, apoptosis
INTRODUCTION
Acetaminophen (APAP) overdose is a major health concern in the clinic resulting in substantial morbidity and mortality each year (Larson et al. 2005). The first reports of APAP overdose resulting in liver cell necrosis were described nearly fifty years ago (Boyd and Bereczky, 1966; Davidson and Eastham, 1966) and still today APAP toxicity is considered a classical example of oncotic necrotic cell death (Gujral et al., 2002; Jaeschke et al., 2004, 2011; Schulte-Osthoff and Bantel, 2011). Hepatocytes within the centrilobular area swell, become highly vacuolated, lose the ability to maintain ion homeostasis and eventually the membrane integrity is compromised. This process is initiated by the formation of the reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which can covalently bind to cellular proteins (Cohen et al., 1997; Nelson, 1990). By comparing protein adducts of APAP and its nonhepatotoxic regioisomer 3′-hydroxyacetanilide (AMAP), it was recognized that toxicity correlates with binding to mitochondrial proteins (Tirmenstein and Nelson, 1989; Qiu et al., 2001). This initiates mitochondrial dysfunction resulting in increased oxidant stress, peroxynitrite formation and eventually opening of mitochondrial membrane permeability transition (MPT) pores (Jaeschke and Bajt, 2006). Downstream effects of the MPT include nuclear DNA damage mediated by two endonucleases, apoptosis-inducing factor (AIF) and endonuclease G (EndoG), which are released from the mitochondrial intermembrane space (Bajt et al., 2006). During this process, hepatocytes will display nuclear swelling and eventually caspase-independent DNA fragmentation. To assess the degree of DNA damage, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining is often used (Lawson et al., 1999). When evaluated histologically, DNA damage begins with punctate nuclear TUNEL staining but quickly becomes diffuse as the nucleus degenerates and DNA fragments spill into the cytoplasm and extracellular space (Jaeschke and Lemasters, 2003). The early punctate staining observed during APAP overdose can often be confused with apoptosis. Therefore, TUNEL-positive cells must also display the classic characteristics of apoptosis (cell shrinkage, chromatin margination, DNA condensation, formation of apoptotic bodies, etc.) to be verified as apoptotic (Kerr et al., 1972).
Consistent with the limited relevance of apoptotic cell death, no relevant activation of caspases can be found during APAP hepatotoxicity in fasted mice (Lawson et al., 1999; Adams et al., 2001; Gujral et al., 2002; El-Hassan et al., 2003; Jaeschke, et al., 2006). In addition, caspase inhibitors, which are highly effective against Fas- or TNF-induced apoptosis (Bajt et al., 2000; Jaeschke et al., 1998), did not protect against APAP toxicity in fasted mice (Lawson et al., 1999; Gujral et al., 2002; Jaeschke et al., 2006; Williams et al., 2010b, Antoine et al., 2009). Only a few papers have implied caspase inhibition has hepatoprotective effects after APAP overdose (El-Hassan et al., 2003; Hu and Colletti, 2010). However, the pretreatment regimen and the use of the solvent dimethyl sulfoxide (DMSO), which is a potent inhibitor of P450 (Park et al., 1988; Yoon et al., 2006), makes it almost certain that the protection was caused by the solvent not by the caspase inhibitior (Jaeschke et al., 2006; Schulze-Osthoff and Bantel, 2011; Jaeschke et al., 2011a). Together, these studies provide strong support for the hypothesis that APAP hepatotoxicity in mice involves oncotic necrotic cell death and not caspase-dependent apoptosis.
In contrast to these previous data, the recent findings by Antoine et al. (2009, 2010) provided for the first time more solid evidence for caspase activation based on evidence for caspase-3 processing, histological assessment of active caspase-3 staining and caspase-dependent cytokeratin-18 cleavage, and for apoptosis. Most importantly, this was only observed in fed mice with high ATP levels. Because most mechanistic studies in the past were done with fasted animals, the authors’ findings suggested that most previous toxicity studies missed a critical mechanistic aspect, i.e., an early caspase activation and apoptosis. Thus, Antoine et al. (2009, 2010) provided a mechanistic explanation why most other investigators never observed caspase-3 activation and apoptosis after APAP overdose. In addition, the authors suggested that the apoptosis in fed mice results in release of an oxidized form of high mobility group box 1 (HMGB1) protein, which is unable to trigger inflammatory mediator production through toll like receptor-4 activation (Antoine et al., 2010). This means that there might be less inflammation in fed compared to fasted mice, i.e., apoptotic cell death may limit the potential detrimental consequences of an excessive inflammatory response compared to necrosis (Antoine et al., 2010). If these findings are generally applicable, this would be at least in part a paradigm shift for the mechanisms of APAP toxicity and possibly for other hepatotoxic drugs. This would also mean that most of the published data generated with fasted mice would have to be re-evaluated. Therefore, the purpose of this study was to investigate the mechanisms of cell death during APAP hepatotoxicity in fed and fasted mice using both an inbred (C57BL/6) and an outbred (Swiss-Webster) strain to determine what role, if any, apoptosis and caspase activation plays in APAP-induced liver injury and inflammation.
MATERIALS AND METHODS
Animals
Eight to twelve week old male C57BL/6J mice (Jackson Labs, Bar Harbor, ME) and Swiss Webster mice (Harlan Labs, Indianapolis, IN) with an average weight of 18 to 24 g were purchased and maintained at the University of Kansas Medical Center. All animals were housed in environmentally controlled rooms with 12 h light/dark cycle and allowed free access to food and water. Experiments followed the criteria of the National Research Council for the care and use of laboratory animals in research and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated.
Experimental design
Mice were intraperitoneally (i.p.) injected with 530 mg/kg APAP (dissolved in warm saline) or with an equivalent volume of saline; some mice were fasted overnight and others had free access to food (Antoine et al., 2009). Additionally, some mice were subsequently treated (i.p.) with 10 mg/kg Z-VD-fmk (pan-caspase inhibitor) dissolved in Tris-buffered saline or vehicle thirty minutes after APAP treatment (EP1013; a generous gift from Dr. S. X. Cai, Epicept Corp., San Diego, CA). Animals were sacrificed 3, 5 or 24 h after APAP. As a positive control for hepatocellular apoptosis some mice were treated (i.p.) with 700 mg/kg D-galactosamine (GalN) and 100 μg/kg endotoxin (ET) for six hours. Blood was drawn into heparinized syringes for measurement of plasma alanine aminotransferase (ALT) activity (Pointe Scientific, Canton, MI). The liver was removed and was rinsed in cold saline; liver sections were fixed in 10% phosphate buffered formalin for histological analyses. The remaining liver lobes were snap-frozen in liquid nitrogen and stored at −80°C. For ATP measurement, livers from anesthetized animals were freeze clamped in liquid nitrogen and immediately assayed.
Histology
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) for blinded evaluation of the areas of necrosis by the pathologist. The amount of necrosis is assessed by giving the percentage of microscopic field involved as compared to the number of fields occupied by the entire tissue section. In most cases this is done using low power fields (i.e., x2 or x4) (Gujral et al., 2002). In addition, liver sections were evaluated for apoptotic cells. Cells that showed morphological features of apoptosis (cell shrinkage, chromatin margination, apoptotic bodies) were counted in 15 high power fields per section (Gujral et al., 2002). Additional sections were stained for neutrophils using the anti-mouse neutrophil allotypic marker antibody (AbD Serotec, Raleigh, NC) as previously described (Williams et al., 2010a). Positively stained neutrophils consistent with cellular morphology were quantified in 15 high power fields (HPF). Some sections were also stained for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) in situ cell death assay (Roche, Indianapolis, IN) as previously described (Lawson et al., 1999).
Glutathione quantification
Glutathione (GSH) and glutathione disulfide (GSSG) were measured in liver homogenate using the modified Tietze method as previously described in detail (Jaeschke and Mitchell, 1990). Briefly, frozen tissue was homogenized in sulfosalicylic acid/EDTA. For total GSH determination samples were assayed using dithionitrobenzoic acid. Similarly, measurement of GSSG was performed using the same method after first trapping and removing GSH with N-ethylmaleimide.
Caspase-3 Activity Assay and Western Blotting
Liver caspase-3 activity was determined as previously described in detail (Lawson et al., 1999). Briefly, after homogenization of liver tissue in 25 mM HEPES buffer (containing 5 mM EDTA, 2 mM DTT and 0.1% CHAPS) centrifuged homogenate was added to a fluorogenic substrate (Ac-DEVD-AFC, Enzo Life Sciences, Plymouth Meeting, PA) with or without the presence of pan-caspase inhibitor (z-VAD-fmk, Enzo) as a sample background control and measured on a Gemini EM plate reader (Molecular Devices, Sunnyvale, CA). Results are expressed as relative light units (RFU) per unit time normalized to protein concentration (microBCA kit, Thermo Scientific, Rockford, IL). Tissue homogenate was also used for caspase-3 western blotting (Cell Signaling Technology, Beverly, MA) as described in detail (Bajt et al., 2000).
ATP quantification
Liver adenosine triphosphate (ATP) was quantified by homogenizing frozen tissue in 2N perchloric acid to deproteinate the sample and then the sample was neutralized with 5N potassium hydroxide, 3M potassium bicarbonate solution. Centrifuged homogenate was then assayed using the ATP Bioluminescent Assay Kit (FLAA-1KT; Sigma) and confirmed with 2D-NMR analysis as described (Zwingmann and Bilodeau, 2006).
Statistics
All results were expressed as mean ± SE. All comparisons were made between time points or at the same time point between the presence of caspase inhibitor; no comparisons were made between strains. Comparisons between multiple groups were performed with one-way or two-way ANOVA or, where appropriate, by Dunnett’s test as indicated in figure legends. If the data were not normally distributed, the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test was used. P < 0.05 was considered significant. Calculations were performed in SigmaStat (Systat Software, San Jose, CA).
RESULTS
Liver injury in fed and fasted C57BL/6 and Swiss Webster mice
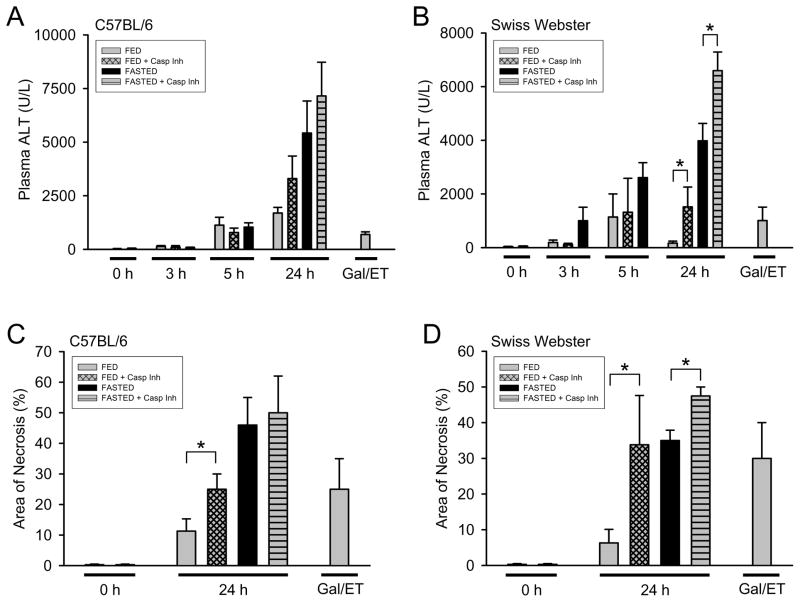
To assess the differences in liver injury in fed and fasted mice following APAP administration, ALT levels were measured in plasma of mice at 0, 3, 5 and 24h (Fig. 1A and 1B). Additionally, blinded histological scoring of liver injury was performed at 24h (Fig. 1C and 1D). In both C57BL/6 (inbred) and Swiss Webster (SW, outbred) strains, the severity of liver injury was dependent on the fed/fasted state of the animals, with fasted mice having greater liver injury by 24h. Early in the injury phase of APAP overdose (3h and 5h), no significant differences in APAP-induced injury could be seen between fed and fasted animals. Because Antoine et al. (2009) reported an increase in liver injury in fed animals after treatment with a caspase- inhibitor, fed and fasted (C57BL/6 and SW) mice were treated with the pan-caspase inhibitor Z-VD-fmk. The caspase inhibitor significantly enhanced APAP-induced liver injury in both fed and in fasted SW mice (Fig 1B and 1D). Similarly, a trend toward increased liver injury was seen in fed C57BL/6 mice treated with the caspase inhibitor (Fig. 1A and 1C).
Figure 1. Acetaminophen-induced liver injury in fed and fasted C57BL/6 and Swiss Webster mice with and without caspase inhibitor.
Fed and fasted mice were treated with 530 mg/kg APAP ± pan-caspase inhibitor (10mg/kg Z-VD-fmk or vehicle) and plasma ALT was measured at 0h, 3h, 5h and 24h in C57BL/6 (A) and Swiss Webster (B) mice. The area of necrosis was quantified by blinded evaluation of H&E stained liver sections in C57BL/6 (C) and Swiss Webster (D) mice (vehicle control, 24h APAP-treated and 6h GalN/ET-treated mice). Data represent means ± SE of n = 4–6 animals per group. *P < 0.05 (comparison as indicated by the brackets were done by one-way ANOVA dependent on the presence of caspase inhibitor comparing the 0h and 24h time points)
Caspase-3 processing and activity
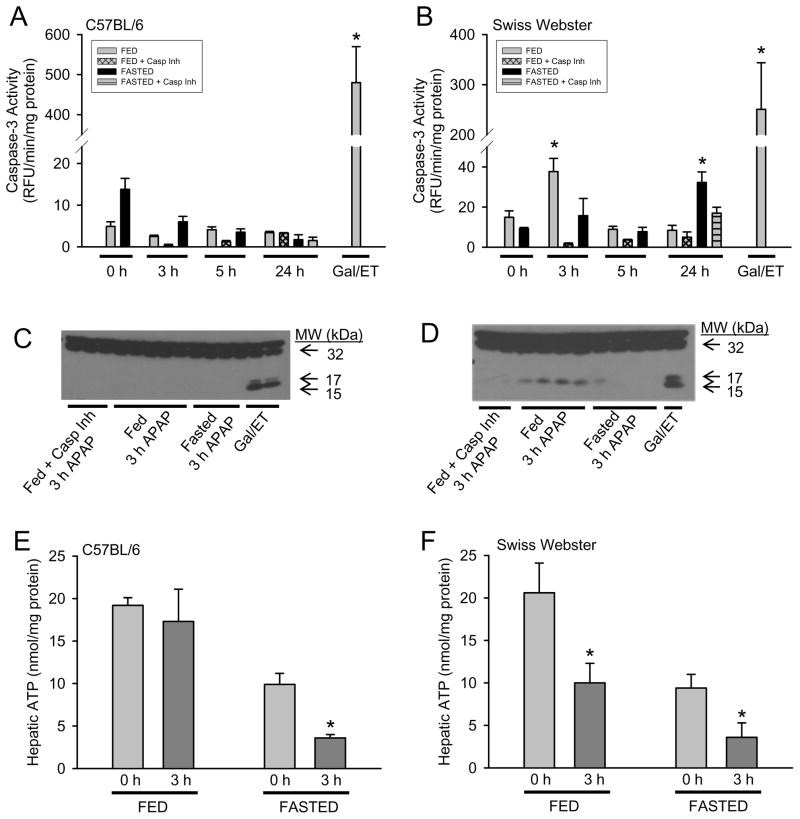
It has been well established in several inbred strains of mice that during APAP overdose there is no caspase-3 processing or increased enzyme activity. In addition, caspase inhibition does not modulate injury in fasted animals (Gujral et al., 2002; Jaeschke et al., 2006; Williams et al., 2010b). Antoine et al. (2009, 2010) showed a temporary caspase processing in fed CD-1 mice, which is an outbred strain. Thus, we assessed evidence for caspase activation in both fed and fasted C57BL/6 (inbred) and SW (outbred) mice. In confirmation of what was observed in CD-1 mice, fed SW mice also showed a transient increase in caspase-3 activity (Fig. 2B) and processing by western blot (Fig. 2D) at 3h that was no longer detectable at 5h by activity (Fig 2B) or processing (data not shown). No evidence of caspase-3 activation was observed in fasted SW mice with the exception of a minor increase at 24h (Fig. 2B), which was not supported by processing (data not shown). C57BL/6 mice showed no increase in caspase-3 activity or processing following APAP at any time evaluated (Fig. 2A and 2C). The treatment of mice with GalN/ET is a positive control for hepatocellular caspase-dependent apoptosis. In comparison to the GalN/ET positive control for both strains of mice, which reflects apoptosis in 20–30% of hepatocytes (Jaeschke et al., 1998), the transient caspase-3 activity and processing observed in APAP-treated SW mice is minor.
Figure 2. Role of fed/fasted state on caspase-3 activity/processing and hepatic ATP levels.
Fed or fasted C57BL/6 and SW mice were treated with 530 mg/kg APAP ± pan-caspase inhibitor (10mg/kg Z-VD-fmk or vehicle) and liver tissue was homogenized. Hepatic caspase-3 activity was determined by AC-DEVD-AMC cleavage in C57BL/6 (A) and SW (B) mice. To confirm activity, caspase-3 western blot analysis was performed on liver homogenate from 3h APAP-treated C57BL/6 (C) and SW (D) mice. Hepatic ATP levels were determined by chemiluminescence assay in control and 3h APAP-treated C57BL/6 (E) and SW (F) mice and normalized to protein concentration. Data represent means ± SE of n = 4–6 mice per group (panels A–D) or n = 3 mice per group (panels E–F). *P < 0.05 (compared to respective fed/fasted 0 h control as determined by Dunnett’s test)
ATP content in fed and fasted mice during APAP overdose
Antoine et al. (2010) hypothesized that caspase-3 activation occurs only in fed mice at 3 h after APAP treatment because in fasted mice there is insufficient ATP to initiate caspase processing. We confirmed the finding that there is a temporary capase-3 activity increase and processing in fed mice of an outbred strain. However, this was not observed in C57BL/6 mice. Therefore, we determined if this difference in caspase activity was due to altered hepatic ATP between mouse strains. Fasting mice overnight resulted in an approximately 50% decrease in hepatic ATP levels in both mouse strains (Fig. 2E and 2F), and fasted mice after APAP had an additional 67–69% decrease in hepatic ATP levels in both mouse strains. Interestingly, 3 hours after APAP treatment fed C57BL/6 mice showed no significant difference in ATP levels from fed controls, but the ATP levels of fed SW mice were reduced by 49% (Fig. 2E and 2F). Thus, the temporary caspase activation was observed in animals with lower ATP levels. Additionally, fasted mice of both strains treated with GalN/ET showed substantial caspase activation and apoptotic cell death (data not shown). Together these findings are inconsistent with the hypothesis that ATP levels are the mitigating factor for caspase activity during APAP overdose.
No morphological evidence of APAP-induced apoptosis in both strains of mice
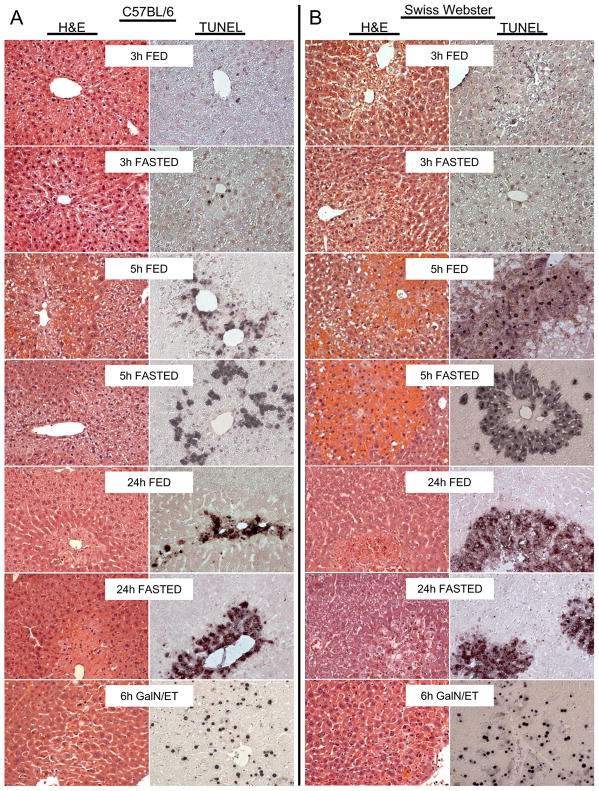
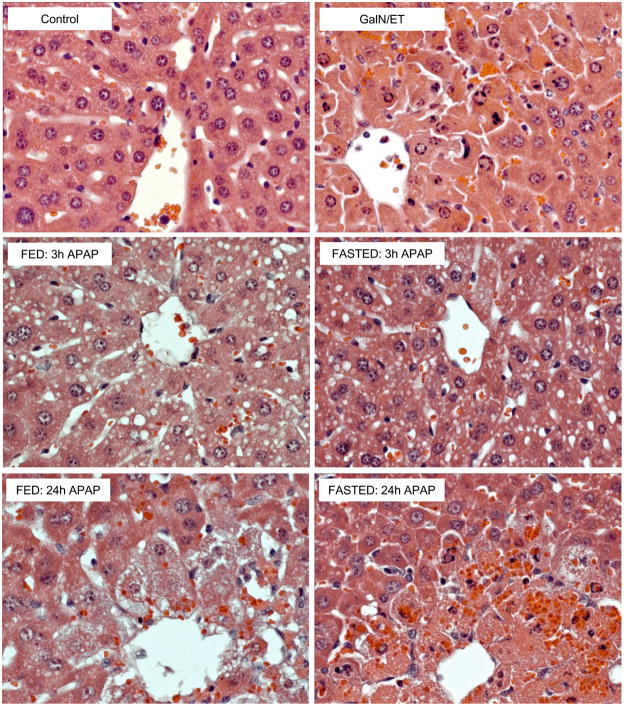
Despite caspase-3 activity and processing in the liver 3h after APAP in SW mice no evidence of apoptosis over control (<0.5% of hepatocytes undergoing apoptosis) could be observed at this time or subsequent times by histology. This was determined by blinded evaluation of H&E sections by the pathologist (Fig. 3A, 3B, 4). In contrast, the GalN/ET treated mice showed >20% of hepatocytes consistent with apoptosis as distinguished by cell shrinkage, chromatin margination, formation of apoptotic bodies, and nuclear condensation (Fig. 3A, 3B, 4). Additionally, TUNEL stained liver sections showed injury consistent with oncotic necrosis in APAP-treated mice (Fig. 3A, 3B). APAP-induced necrosis resulted in an initial punctuate nuclear staining, but soon diffuse staining could be seen in the cytosol and over time the nucleus could no longer be observed; in addition, injured hepatocytes became swollen and highly vacuolated. This pattern of oncotic necrosis can be observed in fed and fasted mice for both C57BL/6 and SW mice. To demonstrate these morphological features in more detail, centrilobular regions of untreated controls, GalN/ET and APAP-treated SW mice are shown (Figure 4). The control section shows no evidence of apoptosis or oncotic necrosis, but >20% of hepatocytes in the GalN/ET-treated mice show loss of cellular contact (cell shrinkage), membrane blebbing, and condensation of nuclear chromatin all of which are indicative of apoptosis. Three hours after APAP no evidence of apoptosis is observed, however, centrilobular hepatocytes become vacuolated indicating the early stages of oncosis in both fed and fasted mice (Figure 4). By 24h the later stages of oncotic necrosis are apparent including the loss of membrane integrity and karyolysis in both fed and fasted APAP-treated mice. These histological findings demonstrate that despite transient caspase-3 activity no increased apoptotic cell death occurs during APAP overdose.
Figure 3. Time course of histology in fed and fasted mice.
Fed or fasted mice were treated with 530 mg/kg APAP (3h, 5h and 24h) or GalN/ET (6h). C57BL/6 (A) and SW (B) liver sections were stained with hematoxylin and eosin (H&E) or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). All panels 200x magnification.
Figure 4. High power magnification of centrilobular regions in fed and fasted mice.
Fed or fasted SW mice were treated with 530 mg/kg APAP (3h and 24h), saline vehicle or GalN/ET (6h). Liver sections were stained with hematoxylin and eosin (H&E). All panels 400x magnification.
Glutathione recovery after APAP
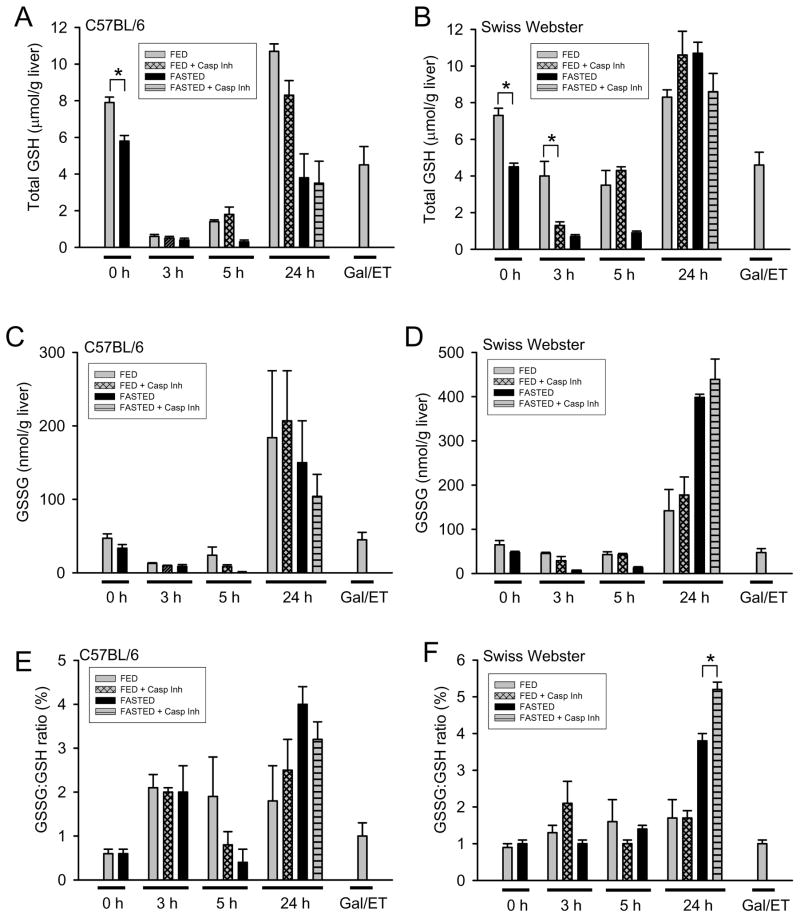
Most in vivo models of APAP overdose use fasted mice because depleted GSH allows the use of lower doses of APAP resulting in more consistent liver injury. Both C57BL/6 and SW mice showed decreased hepatic GSH levels after fasting (Fig. 5A and 5B). At 3h after APAP, C57BL/6 mice still had nearly complete GSH depletion regardless of the fed/fasting state, which began to recover in fed mice but not fasted mice by 5h (Fig. 5A). At 3h post-APAP, caspase inhibitor treated fed SW mice had substantially lower GSH levels than fed SW mice; by 5h this difference in hepatic GSH was no longer seen with the caspase inhibitor (Fig. 5B). SW mice by 24h had full GSH recovery regardless of fed or fasted state. Fed C57BL/6 mice had GSH recovery equivalent to SW mice at 24h, however fasted C57BL/6 mice had yet to fully recover their hepatic GSH content. GSSG and GSSG-to-GSH ratio are indicators of oxidant stress. During early phases of injury (3h and 5h) total GSH is depleted and therefore the ability to generate GSSG is impaired (Fig. 5C–5F). At 24h, all C57BL/6 groups show increased oxidant stress as measured by GSSG (Fig. 5C) and GSSG-to-GSH ratio (Fig. 5E). SW mice at 24h showed elevated GSSG (Fig. 5D) and GSSG-to-GSH ratio (Fig. 5F) with the greatest increases observed in fasted mice, which showed more severe liver injury.
Figure 5. Total hepatic glutathione (GSH) and glutathione disulfide (GSSG).
Hepatic content of total GSH (GSH + GSSG) was quantified in C57BL/6 (A) and SW (B) mice at various times after APAP. Similarly, GSSG was measured in C57BL/6 (C) and SW (D) mice. The GSSG-to-GSH ratio was calculated from each mouse and data for the groups are presented for C57BL/6 (E) and SW (F) mice. Data represent means ± SE of n = 4–6 mice per group. *P < 0.05 (comparison as indicated by the brackets were determined by two-way ANOVA dependent on fed/fasted state and presence of caspase inhibitor)
Hepatic neutrophil recruitment in fed and fasted mice
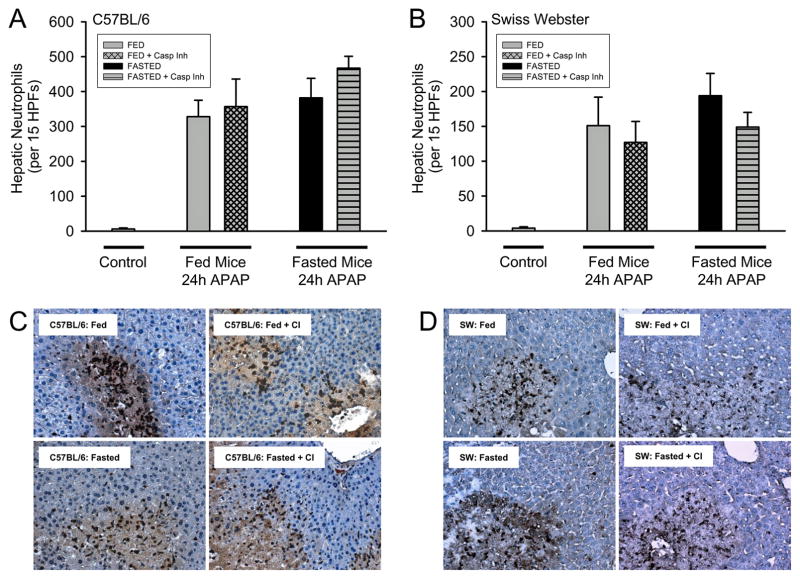
It was previously reported that fed mice showed attenuated inflammation and hepatic leukocyte recruitment (Antoine et al., 2010). To determine if this effect was also a strain specific phenomenon, hepatic neutrophils were quantified in C57BL/6 and SW mice at 24h via immunohistochemistry (Fig. 6). Interestingly, hepatic neutrophil recruitment was not altered by fed or fasting state or by the presence of caspase inhibitor in C57BL/6 (Fig. 6A) or SW mice (Fig. 6B). Despite reduced necrotic injury in fed mice (Fig. 1C and 1D) a higher density of neutrophils could be observed within necrotic lesions (Fig. 6C and 6D) thereby resulting in equivalent hepatic neutrophil recruitment.
Figure 6. Hepatic neutrophil accumulation after APAP treatment in C57BL/6 and SW mice.
Neutrophil numbers were quantified in 15 randomly selected high power fields (HPF; x400) in C57BL/6 (A) and SW (B) mice. Representative immunohistochemistry are shown for C57BL/6 (C) and SW (D) mice. Data represent means ± SE of n = 4–6 mice per group.
DISCUSSION
Role of caspase-3 activation in APAP hepatotoxicity
The main objective of this investigation was to reconcile the discrepant results involving the activation of caspase-3 during APAP overdose and determine if caspase activation can modulate the mechanisms of cell death. Antoine et al. (2009, 2010) demonstrated that CD-1 mice experience a transient caspase-3 activation (based on caspase-3 processing, immunohistochemistry of activated caspase-3 and detection of the caspase-dependent cleavage product of cytokeratin-18) after APAP overdose in fed mice. Our data indicate similar caspase-3 processing and enzyme activity in another outbred mouse strain (SW), but consistent with previously published data (Williams et al. 2010b), no processing or caspase-3 enzyme activity could be detected in the inbred mouse strain C57BL/6 at any time after APAP treatment regardless whether the animal was fed or fasted. Similarly, when we previously evaluated the role of apoptosis during APAP overdose, no caspase-3 processing or enzyme activity was detected in a different inbred strain, C3Heb/FeJ (Gujral et al., 2002; Lawson et al., 1999). In these investigations, fed and fasted animals were used (Gujral et al., 2002). Thus, based on the evidence provided by all studies together (Antoine et al., 2009, 2010; Gujral et al., 2002, Lawson et al., 1999; Williams et al., 2010b), the transient increase of caspase-3 activation appears to be a strain-dependent phenomenon that can occur in fed mice of certain outbred strains.
Does the hepatic ATP content determine the mode of cell death?
It is well known that apoptosis is an ATP-dependent cell death mechanism (Nicotera et al., 1998). Activation of caspase-9 by the apoptosome requires not only cytochrome c release from mitochondria but also ATP (Bratton and Salveson, 2010). This applies to receptor-mediated apoptosis in hepatocytes, which requires amplification through mitochondria (Yin et al., 1999; Bajt et al., 2000), as well as for the mitochondria-initiated apoptosis pathway (Green and Kroemer, 2004). If sufficient levels of ATP are not maintained, the process deteriorates to secondary necrosis (Jaeschke and Lemasters, 2003). However, the exact levels of ATP that are necessary to support apoptosis are unknown. Antoine et al. (2010) concluded that the availability of ATP may be the determining factor whether or not the cells are able to initiate caspase activation during APAP hepatotoxicity. This conclusion was based on the correlation of caspase activation with higher ATP levels in fed animals compared to the lack of caspase activation in fasted animals with lower ATP content (Antoine et al., 2010). However, our data do not support this conclusion. Although fasting mice overnight depleted hepatic ATP by 50%, it did not prevent caspase activation or apoptotic cell death in the GalN/ET model. This demonstrates that the reduced hepatic ATP levels as a result of fasting are still sufficient to allow caspase activation and apoptotic cell death. In addition, only fed SW mice showed minor caspase activation despite having lower ATP levels than fed C57BL/6 mice at 3 h after APAP treatment. These data are not consistent with the conclusion of Antoine et al. (2010) that the reduced hepatic ATP levels due to fasting the animals is the reason for the absence of caspase activation. Our data suggest that hepatic ATP levels of >1 μmol/g liver are enough to support caspase activation and therefore the difference in caspase activation appears to be a strain-dependent effect.
DNA ladders and apoptosis
Antoine et al. (2009, 2010) provided evidence for caspase-dependent internucleosomal DNA fragmentation by showing DNA ladders in livers of fed but not from fasted animals between 3 and 5 h after APAP treatment. However, DNA laddering has been demonstrated in fasted C3Heb/FeJ (Cover et al., 2005) and C57BL/6 (unpublished data) mice in the absence of caspase activation or morphological evidence of apoptosis. Furthermore, DNA ladders are detectable well beyond 5 h after APAP exposure (Ray et al., 1993). Thus, DNA ladders are evidence for endonuclease-mediated DNA fragmentation but are not specific for activation of caspase-activated DNase and apoptotic cell death (Corcoran et al., 1994; Schulze-Osthoff and Bantel, 2011). In APAP hepatotoxicity, nuclear DNA fragmentation is initially dependent on mitochondrial bax translocation (Bajt et al., 2008) and later on mitochondrial damage (Cover et al., 2005) due to the translocation of endonuclease G and apoptosis-inducing factor from the mitochondria to the nucleus (Bajt et al., 2006, 2011). Although small molecular weight DNA fragments are generated during APAP hepatotoxicity, there are clearly also larger DNA fragments produced (Jahr et al., 2001). As DNA fragmentation starts around 5 h after APAP, the observed difference between fed and fasted animals could have been simply a delayed mitochondrial dysfunction and release of endonucleases in the fasted animals. Certainly, DNA fragmentation as indicated by DNA ladder, TUNEL staining and anti-histone ELISA is a hallmark of APAP-induced necrotic cell death.
Antoine et al. (2010) also used injection of glucose and glycine to maintain hepatic glycogen and ATP in fasted mice. In this study they showed this supplementation allows for caspase activation after APAP as indicated by DNA ladders and caspase processing. These experiments were similar to previous cell culture studies where APAP-induced necrotic cell death was prevented by fructose and glycine resulting in later apoptotic cell death (Kon et al., 2004). However, fructose alone, which is sufficient to prevent the decline of ATP levels, did not protect or cause apoptosis (Kon et al., 2004). This is consistent with cell culture experiments where animals are fed a sucrose diet before cell isolation to maintain glycogen levels. Primary mouse hepatocytes isolated from these animals have high glycogen and ATP levels but still die by oncotic necrosis and not apoptosis (Bajt et al., 2004). In addition, delayed injection of glycine and glutamic acid provided energy substrates for mitochondria and increased hepatic ATP levels but did not protect against APAP-induced necrosis (Saito et al., 2010). Together, these data support the conclusion that simply manipulating hepatic ATP levels can not promote apoptosis and prevent necrosis in APAP-induced liver injury. Moreover, pretreatment with glycine as done by Antoine et al. (2010) is known to have multitude of beneficial effects in numerous models making the mechanistic interpretation of such experiments very difficult (Zhong et al., 2003).
How many cells must be apoptotic to be physiologically relevant?
In the study by Antoine et al. (2010) apoptotic hepatocytes were characterized as “numerous” at early time points, however, numerical quantification was never shown. In our study, based on the blinded evaluation of all tissue sections by the pathologist, both C57BL/6 (inbred) and SW (outbred) mice never had apoptotic hepatocytes exceeding 0.5% of total cells following APAP overdose. These findings are consistent with previously published results obtained from C3Heb/FeJ mice (Gujral et al. 2002) in which both fed and fasted mice were evaluated. The very small percentage of hepatocytes undergoing apoptosis (<0.5%) relative to hepatocytes undergoing necrosis (~50%) seems insignificant even if one considers that necrotic cells remain visible for several days and apoptotic cells can be removed in less than 9 h (Corcoran et al., 1994). Modulation of such a small percentage of damaged cells most likely would not have an impact on overall injury. This conclusion is directly supported by the fact that caspase inhibitors do not protect against APAP-induced liver injury (Lawson et al., 1999; Gujral et al., 2002; Jaeschke et al., 2006; Antoine et al., 2009; Williams et al., 2010b).
The inflammatory response in fed and fasted mice
APAP-induced liver injury triggers a substantial inflammatory response characterized by activation of macrophages and neutrophils (Jaeschke et al., 2011b). Damage-associated molecular patterns (DAMPs), which include HMGB1 protein, are released by necrotic cells and can induce formation of pro-inflammatory mediators. The release of HMGB1 during APAP-induced liver injury is well documented (Scaffidi et al., 2002; Antoine et al., 2009; Martin-Murphy et al., 2010; Williams et al., 2011). However, in order to bind to toll-like receptors on macrophages, HMGB1 requires the reduction of a critical cysteine residue (Yang et al., 2010). It was hypothesized that caspase-dependent oxidation of this cysteine group in apoptotic cells may eliminate the capacity of HMGB1 to bind to macrophages and induce an inflammatory response (Kazama et al., 2008). Based on these data, Antoine et al. (2010) characterized the oxidation status of HMGB1 released after APAP treatment and found that in fed mice HMGB1 was mainly in the oxidized form whereas HMGB1 was reduced in fasted mice. Thus, the authors concluded that the inflammatory response is more pronounced in fasted animals than in fed mice (Antoine et al., 2010). However, inflammatory cytokine levels such as TNF-α showed no relevant difference at 5 h and neutrophils were only judged qualitatively in H&E sections. In our study, we did not observe significant differences in hepatic neutrophil recruitment between fed and fasted mice at 24 h in either strain of mice. However, C57BL/6 mice had overall about twice as many neutrophils in the tissue compared to SW mice. It has to be kept in mind that during a sterile inflammatory response necrotic cells release many DAMPs, which can to various degrees promote cytokine formation and activation of inflammatory cells. Even if there is a temporary difference in the oxidation status of HMGB1 due to temporary caspase activation, given the various DAMPs it is not surprising that the difference in neutrophil recruitment is minimal.
Why does the caspase inhibitor increase injury with high doses of APAP?
In the study by Antoine et al. (2010), the caspase inhibitor was used only in fed mice, which resulted in an enhanced injury similar to what was observed in fasted mice. In our study, the caspase inhibitor increased hepatic injury (ALT and area of necrosis) in both fed and fasted mice. Interestingly at a more moderate APAP overdose (300 mg/kg) the caspase inhibitor did not cause any modulation of injury at 6h or 24h (Lawson et al., 1999; Williams et al. 2010b). The degree of injury is highly dependent on the levels of hepatic GSH. Initially, GSH is responsible for the detoxification of NAPQI (Corcoran et al., 1985). Thus, the higher GSH levels in fed mice represents a higher detoxification capacity for NAPQI and therefore results in less injury. However, at later times GSH is critical for the scavenging of ROS generated by mitochondrial injury (Knight et al., 2002). Any delay in GSH recovery, especially in the mitochondria, will have a profound impact on injury at later times (Saito et al., 2010). Caspase inhibitor-treated, fed SW had 70% less GSH than fed, vehicle-treated mice 3 h after APAP. This difference in hepatic GSH at the onset of injury can explain the enhanced injury observed later in fed SW mice treated with the caspase inhibitor. The molecular mechanism of this effect requires further investigations.
Summary and Conclusions
Although our data confirm the temporary caspase activation in fed animals of a different outbred strain of mice, we also report the important new finding that this does not occur in inbred strains such as C57BL/6 mice. In addition, higher hepatic ATP levels do not correlate with APAP-induced caspase activation and apoptosis. Furthermore, using a positive control for apoptosis, fasting does not inhibit caspase activation or apoptosis in the GalN/ET model. Most importantly, the limited caspase activation after APAP overdose is insufficient to actually cause apoptotic cell death. Moreover, when the inflammatory response was quantified, it is obvious that the number of neutrophils in the liver, which are the potentially cytotoxic innate immune cells, did not differ between fed and fasted animals in both strains of mice. Thus, the conclusions by Antoine et al. (2010) that “the inhibition of caspase-driven apoptosis and HMGB1 oxidation by ATP depletion from fasting promotes an inflammatory response during drug-induced hepatotoxicity/liver pathology” could not be confirmed in our study. Overall, our data do not support the hypothesis that fasting causes a major shift in the mode of cell death, mechanism of injury and/or extent of the inflammatory response after APAP overdose between fed and fasted animals due to lower ATP levels. Thus, results obtained with both fed and fasted mice can yield important new insight into the pathophysiology of APAP-induced liver injury.
Supplementary Material
HIGHLIGHTS.
During acetaminophen overdose caspase-3 can be activated in fed mice of certain outbred strains
Hepatic ATP levels are not the determining factor for caspase activity
Caspase-3 activity does not result in increased hepatocellular apoptotic cell death
Neutrophil recruitment during acetaminophen occurs independently of nutritional status
Fed or fasted state does not alter the mechanisms of acetaminophen-induced cell death
Acknowledgments
The authors thank Sarah Hawley, Canary Foundation, Palo Alto, CA for help with the statistical analysis. This investigation was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916 to H.J., and by grants P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. C.D. Williams was supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors do not have any conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol. 2001;60:907–915. doi: 10.1124/mol.60.5.907. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd EM, Bereczky GM. Liver necrosis from paracetamol. Br J Pharmacol Chemother. 1966;26:606–614. doi: 10.1111/j.1476-5381.1966.tb01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–14. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Fix L, Jones DP, Moslen MT, Nicotera P, Oberhammer FA, Buttyan R. Apoptosis: molecular control point in toxicity. Toxicol Appl Pharmacol. 1994;128:169–81. doi: 10.1006/taap.1994.1195. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Racz WJ, Smith CV, Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther. 1985;232:864–72. [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Davidson DG, Eastham WN. Acute liver necrosis following overdose of paracetamol. Br Med J. 1966;2:497–499. doi: 10.1136/bmj.2.5512.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191:118–129. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Harrill AH, Watkins PB, Su S, Ross PK, Harbourt DE, Stylianou IM, Boorman GA, Russo MW, Sackler RS, Harris SC, Smith PC, Tennant R, Bogue M, Paigen K, Harris C, Contractor T, Wiltshire T, Rusyn I, Threadgill DW. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 2009;19:1507–1515. doi: 10.1101/gr.090241.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Colletti LM. CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology. 2010;52:691–702. doi: 10.1002/hep.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85–9. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Farhood A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity (letter) Hepatology. 2011a;53:718–719. doi: 10.1002/hep.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2011b doi: 10.1111/j.1478–3231.2011.02501.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65. [PubMed] [Google Scholar]
- Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–75. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192:387–94. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Leist M, Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett. 1998;102–103:139–42. doi: 10.1016/s0378-4274(98)00298-7. [DOI] [PubMed] [Google Scholar]
- Park Y, Smith RD, Combs AB, Kehrer JP. Prevention of acetaminophen-induced hepatotoxicity by dimethyl sulfoxide. Toxicology. 1988;52:165–175. doi: 10.1016/0300-483x(88)90202-8. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3′-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv Exp Med Biol. 2001;500:663–673. doi: 10.1007/978-1-4615-0667-6_99. [DOI] [PubMed] [Google Scholar]
- Ray SD, Kamendulis LM, Gurule MW, Yorkin RD, Corcoran GB. Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. FASEB J. 1993;7:453–63. doi: 10.1096/fasebj.7.5.8462787. [DOI] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–54. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Bantel H. Necrosis versus apoptosis in acetaminophen-induced hepatotoxicity (letter) Hepatology. 2011;53:1070. doi: 10.1002/hep.24027. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol. 2011;252:289–97. doi: 10.1016/j.taap.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010a;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol. 2010b;247:169–78. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–91. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Yoon MY, Kim SJ, Lee BH, Chung JH, Kim YC. Effects of dimethylsulfoxide on metabolism and toxicity of acetaminophen in mice. Biol Pharm Bull. 2006;29:1618–1624. doi: 10.1248/bpb.29.1618. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–40. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Bilodeau M. Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology. 2006;43:454–463. doi: 10.1002/hep.21075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.