Abstract
Significant deficiencies in understanding of xenospecific immunity have impeded the success of preclinical trials in xenoislet transplantation. While galactose-α1,3-galactose, the gal epitope, has emerged as the principal target of rejection in pig-to-primate models of solid organ transplant, the importance of gal-specific immunity in islet xenotransplant models has yet to be clearly demonstrated. Here we directly compare the immunogenicity, survival and function of neonatal porcine islets (NPIs) from gal-expressing wild-type (WT) or gal-deficient galactosyl transferase knock-out (GTKO) donors. Paired diabetic rhesus macaques were transplanted with either WT (n=5) or GTKO (n=5) NPIs. Recipient blood glucose, transaminase, and serum xenoantibody levels were used to monitor response to transplant. Four of 5 GTKO versus 1 of 5 WT recipients achieved insulin-independent normoglycemia; transplantation of WT islets resulted in significantly greater transaminitis. WT NPIs were more susceptible to antibody and complement binding and destruction in vitro. Our results confirm that gal is an important variable in xenoislet transplantation. GTKO NPI recipients have improved rates of normoglycemia, likely due to decreased susceptibility of xenografts to innate immunity mediated by complement and preformed xenoantibody. Therefore, the use of GTKO donors is an important step towards improved consistency and interpretability of results in future xenoislet studies.
Keywords: Xenotransplantation, costimulation blockade, porcine islets, transgenic pigs, innate immunity, Gal knockout
Introduction
Donor availability continues to severely limit allotransplantation in all its forms. Xenogeneic donors represent a promising, readily available, and virtually unlimited alternative source of organs and tissues, making xenotransplantation a conceptually attractive therapy for use in humans. Many immunologic and practical obstacles impede clinical translation of this approach; however, the complexity of these obstacles differs significantly depending on the type of transplant and the clinical indication being addressed. Current evidence suggests that pancreatic islet transplantation may be the form of xenotransplantation most amenable to clinical translation in the near future (1), and indeed, previous investigators have established proof-of-concept that porcine islets can successfully reverse diabetes in nonhuman primates (2, 3); however, achieving the consistent preclinical success necessary for clinical translation of xenoislet transplantation will require careful investigation of the individual elements contributing to xenorecognition and development of novel strategies to overcome these factors.
The most important advance in dissecting xenospecific immunity has been identification of Galα(1,3)Galβ(1,4)GlcNAc-R, the Gal epitope, as the major target of naturally occurring xenoreactive antibodies in humans and the primary contributor to vascular incompatibility between pigs and primates (4, 5). Xenotransplantation of Gal-expressing solid organs results in rapid intravascular graft thromobosis and subsequent hyperacute rejection (HAR). In contrast, solid organs from galactosyl transferase knockout (GTKO), Gal-deficient porcine donors avoid anti-Gal antibody mediated HAR and achieve significantly improved survivals (6–8). Despite an obvious advantage in these models, two factors have made the importance of Gal-specific immunity in xenoislet transplant unclear. First, as cellular grafts, transplanted xenoislets are secondarily vascularized by recipient-type endothelium (9), and therefore HAR resulting from vascular incompatibility is neither observed nor expected. Secondly, porcine islet Gal expression is inversely related to age (10); as such, the low level of Gal expression on wild-type adult porcine islets may have obscured the benefit of donor Gal removal in previous comparisons (11–13). However, there remains a strong rationale that Gal-specific immunity may play an important, though different, role in islet xenograft injury.
Intraportally transplanted islets are subject to rapid destruction by innate inflammatory mechanisms known collectively as the instant blood-mediated inflammatory response (IBMIR) (14). The IBMIR involves activation of the complement and coagulation cascades as well as neutrophil infiltration, and can result in destruction of up to 75% of transplanted islets in non-immunosuppressed recipients (15). Preformed anti-Gal antibodies could play a major role in early islet destruction by binding to islet surfaces, initiating complement deposition and subsequent graft injury (16). In addition to the IBMIR, transplanted xenoislets are subject to delayed rejection mediated by humoral and cellular immune processes similar to those influencing allografts. Antibody-dependent cellular cytotoxicty (ADCC) mediated by either preformed or acquired anti-Gal antibodies could contribute to delayed xenograft rejection and affect length of graft survival. Therefore, Gal may be an important target in both early and delayed immunologic hazards.
Despite the potential for improved function, the relative efficacy of Gal-negative versus Gal-positive neonatal porcine islets (NPIs) has not been investigated. We set out to elucidate the role of Gal-specific immunity as a previously undescribed mediator of islet xenograft rejection. The goals of this study were 1) to compare the relative immunogenicity of GTKO and galactosyl transferase-hemizygous, phenotypic wild-type (WT) NPIs in vitro, 2) to compare the survival and function of transplanted GTKO versus WT NPIs using a diabetic nonhuman primate model, and 3) to evaluate the immediate and long-term immunologic response to transplantation of GTKO or WT islets in nonhuman primate recipients.
Materials and Methods
Neonatal Porcine Islet Procurement, Culture, and
One- to four-day-old large white/landrace neonatal pigs (1.5–2.0 kg, Fios Therapeutics, Rochester, MN) were used as pancreas donors. Pigs were either α1,3-galactosyl transferase nullizygous (GTKO) or hemizygous littermates (phenotypic WT). Pancreatectomies and islet isolations were carried out using a previously described modified Korbutt technique (17). In brief, harvested pancreas tissue was first digested with collagenase (Type XI, Sigma). Free islets were then filtered and cultured in supplemented Ham’s F10 medium (Gibco, Burlington, Ontario, Canada) for a period of 7 days.
Cultured neonatal porcine islet clusters were dissociated into single-cell suspensions in preparation for flow cytometry by gentle mechanical disruption (via pipet) in RPMI medium, followed by washing in phosphate-buffered saline (PBS), centrifugation, and resuspension.
Xenoantibody quantification
Total anti-pig and non-Gal IgG and IgM antibodies were measured in islet recipient serum using flow cytometry. Peripheral blood mononuclear cells (PBMCs) or pancreatic islets from either WT or GTKO piglets were isolated, washed and purified. Approximately 0.5 x 106 cells/well were blocked, incubated with sera (diluted 1:20 in PBS+2% fetal calf serum), and then incubated with secondary detection antibodies specific for either rhesus IgG (Nonhuman Primate Reagent Resource, Boston, MA) or monkey IgM (Southern Biotech, Birmingham, AL). Samples were analyzed using an LSRII flow cytometer (BD Biosciences, San Jose, CA).
Complement Assays
Binding of specific complement components to WT or GTKO islets was measured using a flow-based assay as previously described (16). After isolation, islets were incubated with plasma from naïve third-party monkey donors (1:1 with RPMI media). After incubation for 15 minutes at 37°C, cells were washed and stained with FITC-conjugated antibodies specific for the complement components C1q (US Biological, Swampscott, MA), or C3 (Lifespan Biosciences, Seattle, WA).
A complement-mediated cell lysis assay was performed in a manner similar to previously described methods (18). Islets from WT and GTKO donor piglets were incubated with heat-inactivated monkey serum (HIS), rabbit complement (Pel-Freez, Rogers, AR), HIS combined with rabbit complement or naïve third-party monkey serum for 15 minutes at 37°C. Heat inactivation of serum was achieved by placing samples in a warm water bath at 56°C for 30 minutes. Islet viability was determined by detection of 7-AAD dye (ViaProbe, BD Biosciences) uptake using flow cytometry.
Pre-Transplant Quantitative Assessment of Islet Preparations
On the day of transplantation, islets were assessed for quantity by dithizone (Sigma-Aldrich), for viability by SYTOX® Green Fluorescent Nucleic Acid Stain (Invitrogen, Eugene, OR), for bacterial contamination by Gram stain and culture and for in vitro function by static incubation assay and determination of glucose stimulation index (GSI) as previously described (2). Quantitative islet Gal expression was determined by flow cytometric analysis following incubation of islet preparations with an antibody specific for galactose alpha-1,3-galactose beta-1,4-GlcNAc-R epitope (clone 6D407, US Biological, Swampscott, MA).
Diabetes Induction and Recipient Care
Rhesus macaques (Macaca mulatta) were used as xenograft recipients. Diabetes was induced with streptozocin (STZ, 1250 mg/m2 IV; Zanosar, Teva, Irvine, CA) 4 weeks prior to transplant. After STZ administration, insulin (NPH, Ultralente; Eli Lilly, Indianapolis, IN) was administered twice daily to maintain fasting blood glucose (FBG) <130 mg/dL. Glycemic control was determined by intravenous glucose tolerance test (IVGTT) as previously described (2). Diabetes was confirmed by the failure of blood glucose to normalize 90 minutes after dextrose bolus in the absence of detectable primate c-peptide. All procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals (19),” and approved by our Institutional Animal Care and Use Committee (IACUC).
Islet Transplantation and Monitoring of Graft Function
Islet preparations were resuspended in 20 mL of transplant media supplemented with 200 units of heparin and etanercept 3mg/kg (Enbrel; Amgen & Wyeth, Philadelphia, PA). Following mini-laparotomy, ~50,000 islet equivalents (IEQ)/kg were transplanted intraportally into each of the NHP recipients via gravity drainage of the suspension into a mesocolic vein through a 22-gauge intravenous catheter.
Post-transplant, recipient fasting and postprandial blood glucose levels were monitored daily by ear-stick (Glucometer Elite; Bayer, Elkhart, IN). Insulin was administered twice daily to maintain FBG <200 mg/dL. IVGTTs were performed at monthly intervals during the post-transplant period, and continuing graft function confirmed by detection of porcine c-peptide (PCP) in recipient serum.
The experimental endpoints in this study were 1) functional rejection, the need for resumption of exogenous insulin (determined by FBG >200 for two consecutive days) following a period of normoglycemia and insulin independence, and 2) primary graft dysfunction (failure of engraftment), the failure to achieve insulin-independent normoglycemia for any period of time (four consecutive days after post-transplant day 36 with FBGs >300mg/dL that were not associated with events that can cause hyperglycemia, i.e. infection).
Rejection was confirmed by the absence of detectable porcine c-peptide, and by histologic examination of intrahepatic islets at the time of recipient necropsy.
Recipient Cohorts, Immunosuppressive Regimen, and Animal Treatment Protocol
Recipient pairs were matched by age and size, and housed in shared protective-contact quarters. One member of each pair was randomly selected to receive WT islets, and the other to receive GTKO islets. Five paired transplants (n = 10) were performed in this study.
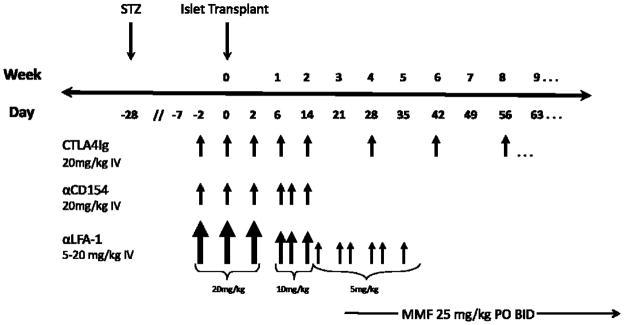
All islet recipients underwent induction immunosuppression with the chimeric anti-human CD154 monoclonal antibody 5C8 (Nonhuman Primate Reagent Resource, Boston, MA), and the mouse anti-human LFA-1 monoclonal antibody TS-1/22 (Biovest International, Minneapolis, MN). 5C8 was administered as an intravenous infusion of 20mg/kg on pre-transplant day 2, on day of transplant, and on post-transplant days 2, 6, 10, and 14. TS-1/22 was administered as a 20mg/kg infusion on pre-transplant days 2, day of transplant, and post transplant day 2, 10mg/kg on days 6, 10, and 14, and then 5mg/kg given twice weekly until post-transplant day 35. Maintenance immunosuppression included therapy with the chimeric protein CTLA-4Ig (BMS-188667, Bristol-Myers Squibb, Wallingford, CN) given in doses of 20mg/kg on days −2, 0, 2, 6 and 14, then once every other week for the duration of the experiment; mycophenolate mofetil (Cellcept; Roche Pharmaceuticals, Nutley, NJ) was given as a twice-daily PO dose of 25mg/kg starting 3 weeks after transplant until experimental endpoint.
Recipient transaminase levels were monitored from serum samples using a Liasys chemistry analyzer (Drew Scientific; Waterbury, CT).
Measurement of Anti-Gal Antibody by Enzyme-Linked Immunosorbent Assay (ELISA)
Anti-Gal IgG and IgM antibodies were measured in islet recipient sera using an indirect ELISA method as previously described (20). Plates were coated with Gal antigen (Galα1-3Gal-BSA, V-Labs, Covington, LA) and then blocked with 3% BSA. Recipient serum diluted at 1:10 with 1%BSA was added to the coated, washed ELISA plate in triplicate for each timepoint. Plates were then stained with either acid phosphatase-conjugated anti-rhesus IgG (Southern Biotech) or peroxidase conjugated anti-monkey IgM (KPL, Gaithersburg, MD) secondary antibodies, and developed with corresponding development buffers. ELISA plates were analyzed using a SpectraMax 340pc absorbance microplate reader (Molecular Devices, Sunnyvale, CA).
Nonhuman primate alloislet recipient sera were analyzed for comparison (unpublished cohort, manuscript in press). Immunosuppression for these recipients included anti-CD40 antibody (3A8) and basiliximab induction, in addition to rapamycin maintenance therapy. Diabetes induction and alloislet transplant were otherwise as described in our previously published alloislet cohorts (21).
Immunohistochemistry
Immunohistochemical analysis of transplanted intrahepatic islets was performed using standard hematoxylin and eosin as well as staining for insulin, IB4 lectin for Gal (Sigma, St. Louis, MO), CD3, CD68, CD20cy and neutrophil elastase (Dako; Carpenteria, Ca).
Statistical Analysis
Statistical calculations were performed using GraphPad Prism (GraphPad Software, La Jolla, CA) or SPSS (IBM, Somers, NY). Because of the small sample sizes being compared, non-parametric statistical tests were use to establish the significance of our results. Gal positivity and transaminase levels were all compared using a Mann-Whitney test. Preformed antibody levels, complement assays and anti-Gal ELISA results were compared using a paired Wilcoxon signed-rank test. Survival curves were analyzed using a stratified log-rank comparison. All tests were two-tailed, and a p-value ≤ 0.05 set as the threshold for significance in all comparisons.
Results
Islets from galactosyl transferase-hemizygous neonatal porcine donors express Gal
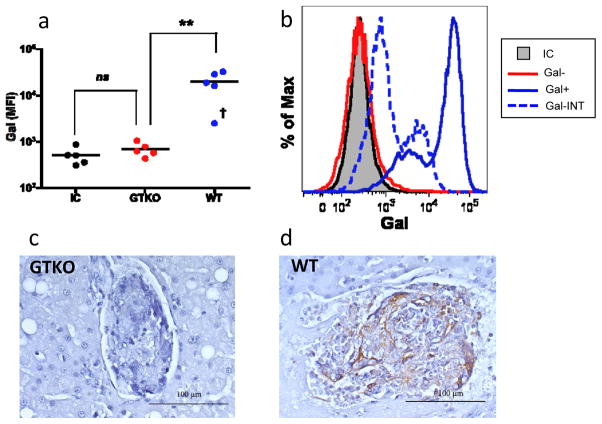
We first confirmed the difference in Gal expression between islets from the two neonatal porcine donor sources. After staining with a fluorophore-conjugated anti-Gal antibody, flow cytometric analysis was performed on islet preparations from GTKO donors and WT (galactosyl transferase-hemizygous) littermates. While GTKO islet preparations had a mean fluorescence intensity (MFI) of Gal staining virtually identical to islets stained with an isotype control, WT islet preparations demonstrated a high level of Gal staining (figure 1a), and were quantitatively and phenotypically indistinguishable in Gal expression from galactosyl transferase homozygous (+/+) NPIs (data not shown). One WT islet preparation demonstrated an intermediate Gal expression phenotype (figure 1b). To determine whether WT NPIs maintained Gal expression following transplant, intrahepatic islets underwent immunohistochemical analysis. WT islets demonstrated significant, diffuse Gal staining following transplant while GTKO islets were completely unstained (figure 1c,d).
Figure 1. Islets from galactosyl transferase-hemizygous neonatal porcine donors express Gal.
(a) Islet transplant preparations isolated from WT neonatal donors had significantly higher Gal expression as determined by flow cytometric analysis than GTKO counterparts (p<0.008, n=5); GTKO islet Gal expression was not significantly different than WT islets stained with an isotype control. One WT islet preparation had an MFI of Gal expression that was significantly lower than other WT preparations (denoted with†). (b) Histogram demonstrating Gal expression phenotype for islet preparations from representative GTKO and WT donors compared to an isotype control. One WT islet preparation demonstrated an intermediate Gal expression phenotype (represented with dashed line). (c,d) Immunohistochemical analysis of transplanted intrahepatic islets demonstrates presence of Gal epitope (brown stain) on WT (d) but not GTKO (c) islets. P-value determined using Mann-Whitney test. **p <0.01; MFI – mean fluorescence intensity; IC – isotype control; Gal-INT† – intermediate Gal expression phenotype.
Gal is a major target of preformed xenoantibody
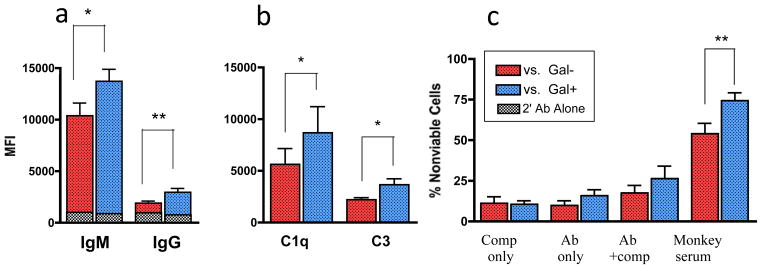
To investigate the role of preformed Gal-specific antibody in early islet destruction, we compared the relative susceptibility of GTKO and WT NPIs to binding of anti-pig IgG and IgM. When incubated with sera obtained from naïve nonhuman primates (NHPs), WT islets bound significantly more xenoantibody than GTKO NPIs (figure 2a), confirming that Gal is a major target of preformed, circulating antibody in untreated rhesus macaques.
Figure 2. Gal-expressing neonatal porcine islets demonstrate increased immunogenicity in vitro.
WT NPIs were more susceptible to binding of (a) IgG (p=0.002, n=10) and IgM xenoantibody (p<0.05, n=10) and (b) complement components C1 (p=0.03, n=7) and C3 (p<0.02, n=7) than GTKO NPIs when incubated with naïve recipient sera in a flow cytometric assay. (c) More WT NPIs showed evidence of injury as determined by uptake of 7-AAD viability dye following incubation with naïve monkey sera (p<0.008, n=8). This effect was not noted following incubation with heat-inactivated serum or complement alone. P-values determined using paired wilcoxon signed rank test. *p <0.05; **p <0.01; HIS – heat-inactivated serum; comp – rabbit complement.
WT islets are more susceptible to complement binding and complement-dependent destruction
Complement has been implicated as a major mediator of early xenoislet destruction as a component of the IBMIR (14); therefore, we evaluated the relative susceptibility of WT and GTKO NPIs to complement activity in vitro. WT NPIs incubated with naïve recipient plasma initiated significantly greater binding of the individual complement components C1q and C3 compared to GTKO NPIs (figure 2b). To determine if this predisposition to complement binding resulted in increased cytotoxicity by complement-dependent mechanisms, we performed a cell viability assay. There was significantly greater damage to WT NPIs following incubation with monkey serum (figure 2c). Greater cytotoxicty was achieved with addition of antibody and complement together (in the form of fresh serum) than with either alone, suggesting that GTKO NPIs are less vulnerable to killing by antibody-dependent, complement-mediated mechanisms.
Pretransplant Islet Characterization
Prior to pancreas procurement, individual donor piglets were identified as either Gal-positive or -negative by phenotypic analysis of donor red blood cells and donor genotyping (supplemental figures 1 and 2, supplemental methods). For each paired transplant, islets isolated from the pancreata of phenotypic gal-positive piglets were pooled to make the WT transplant preparation, while islets from the gal-negative piglets were pooled to make the GTKO transplant preparation. Approximately 4–6 piglet pancreata were harvested to obtain the necessary number of islets for a single WT or GTKO transplant. GTKO and WT NPI preparations were quantitatively and functionally similar in vitro (table 1).
Table 1.
Islet / Recipient characteristics*
| WT (n = 5) | KO (n = 5) | P -value** | |
|---|---|---|---|
| IEQs (1×104)/kg | 5.72 (5.00 – 6.00) | 5.52 (5.33 – 5.80) | 0.8125 |
| GSI | 2.10(1.72 – 2.86) | 1.49 (1.32 – 2.06) | 0.1875 |
| % Viability | 79.0 (77.2 – 81.0) | 81.6 (78.0 – 82.2) | 0.0625 |
| Recipient weight (kg) | 4.4 (3.9 – 4.7) | 4.9 (3.6 – 5.2) | 0.3125 |
Median values followed by range in parentheses.
Wilcoxon signed rank test.
IEQs – Islet equivalents. GSI – Glucose stimulation index. NS – not significant.
Transplantation of Gal-deficient islets improves rates of insulin independence in diabetic nonhuman primate recipients
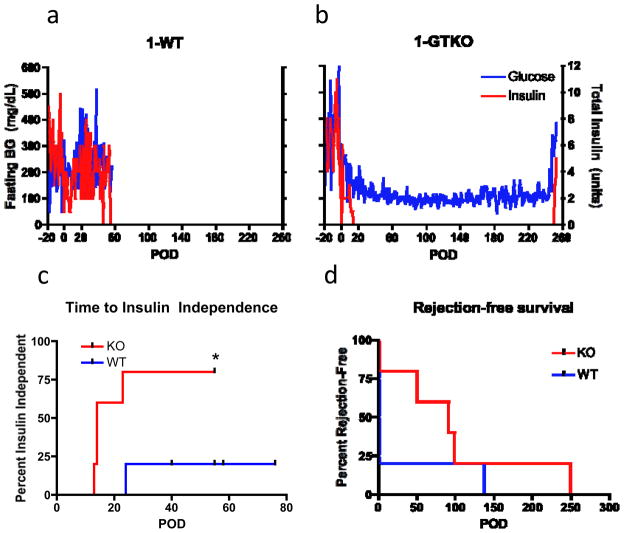
The ability of WT and GTKO NPIs to reverse diabetes was compared using an established nonhuman primate islet xenotransplantation model. Diabetic rhesus macaques (n=10) received an intraportal infusion of isolated, cultured NPIs. Paired subjects received either WT (n=5) or GTKO (n=5) NPIs. Immunosuppression was identical for all xenoislet recipients and consisted of induction therapy with anti-CD154 and anti-LFA-1 antibodies, as well as maintenance therapy with CTLA-4Ig and mycophenolate mofetil (figure 3). Primary outcomes were insulin independence and rejection-free graft survival. In all, 4 of 5 GTKO recipients achieved prolonged insulin independent normoglycemia, compared to only 1 of 5 WT recipients (figure 4a,b); time to insulin independence was also significantly shorter for GTKO NPI recipients (p=0.03, logrank test; figure 4c). Rejection-free survivals were similar among engrafting GTKO and WT recipients (p=0.3, logrank test; figure 4d), and are listed in table 2. Recipients failing to achieve euglycemia by 40 days post-transplant were considered to exhibit primary graft dysfunction, or “nonengraftment.”
Figure 3. Experimental timeline and immunosuppressive protocol.
All nonhuman primate xenoislet recipients in this study received identical immunosuppression. Arrows indicate individual infusions, with arrow size indicating relative dose. This regimen was similar to our previously described xenoislet immunosuppressive protocols (2), with several important differences: in the current protocol CTLA4Ig was used instead of belatacept to achieve CD28/B7 costimulatory pathway blockade, oral mycophenolate mofeltil was given instead of IM rapamycin and begun 3 weeks after transplant, TS-1/22 replaced basiliximab, and the anti-CD154 clone 5C8 was used instead of H106. In addition, recipients in the current study were rendered diabetic with streptozocin instead of pancreatectomy. STZ – streptozocin. MMF – mycophenolate mofetil.
Figure 4. Transplantation of Gal-deficient islets results in superior graft function and more rapid return of euglycemia.
(a-b) WT islet recipients (a) most often experienced primary graft dysfunction, while transplantation of GTKO NPIs (b) resulted in prolonged insulin-independent normoglycemia, as shown in this representative pair. (c) Time to insulin independence was significantly shorter following transplantation of GTKO NPIs (p=0.03); however, duration of rejection-free survival (d) was similar for the two treatment groups (p=0.3). In (c), censored events represent recipient sacrifice. P-values calculated using logrank test.
Table 2.
Time to engraftment and rejection-free graft survival.
| Recipient ID | Time to Engraftment (days) | Rejection-free Survival (days) |
|---|---|---|
| 1-WT | DNE | n/a |
| 1-KO | 13 | 249 |
| 2-WT | DNE | n/a |
| 2-KO | 14 | 91 |
| 3-WT | DNE | n/a |
| 3-KO | 14 | 50 |
| 4-WT | 24 | 137 |
| 4-KO | 23 | 99 |
| 5-WT | DNE | n/a |
| 5-KO | DNE | n/a |
DNE – did not engraft. WT – wild type (Gal +).
GTKO – galactosyl transferase knock-out (Gal −).
Transplantation of Gal-deficient islets induces less intrahepatic inflammation
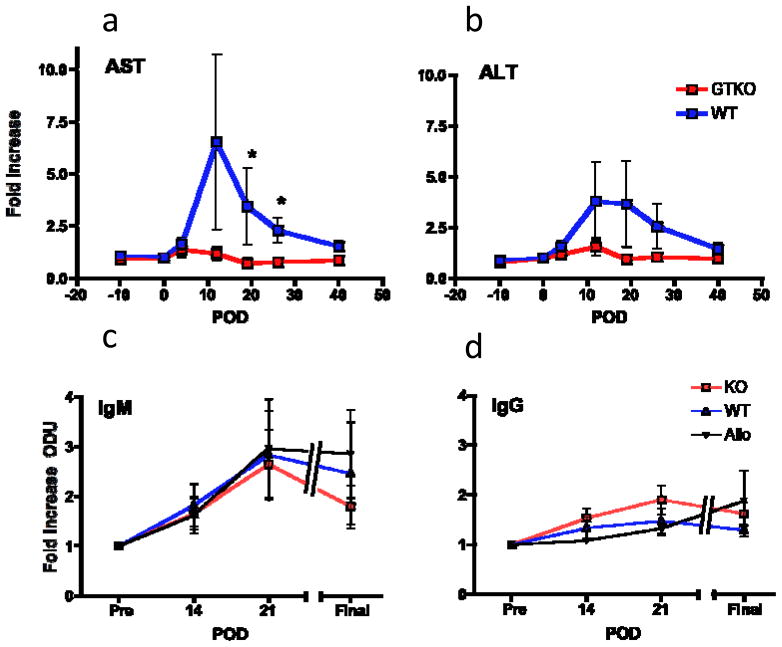
As an estimate of the inflammatory response to intraportal islet transplantation, we measured transaminases in the early post transplant period. The liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are released from injured hepatocytes in response to a variety of insults, and are used in clinical practice as nonspecific measures of liver inflammation. Transplantation of WT islets resulted in significantly greater elevation of AST and ALT compared to GTKO islets (figure 5a,b).
Figure 5. Gal-deficient islets induce less liver inflammation but similar xenoantibody levels as Gal-positive islets.
(a-b) WT islet recipients experienced a greater increase from baseline in the liver enzymes AST (c) and ALT (d); this difference was significant for AST on days 21 (p=0.03) and 28 (p=0.03) post-transplant. (e-f) All WT and GTKO islet recipients experienced a transient increase in anti-Gal IgM and a smaller increase in anti-Gal IgG antibodies, which peaked ~21 days post-transplant. There was no significant difference in antibody levels between groups at any timepoint. Alloislet recipients (n=4), shown for comparison, demonstrated a similar pattern of increase in anti-Gal IgM and IgG following transplant. P-values calculated using Mann-Whitney Test. *p <0.05; BG – blood glucose; POD – post-op day; AST – aspartate aminotransferase; ALT – alanine aminotransferase; ODU – optical density units.
Anti-Gal antibody is increased following transplantation of both Gal-postive and Gal-deficient islets
To evaluate the humoral response to transplantation of WT versus GTKO NPIs, we measured levels of anti-Gal antibody in our recipients following transplant using anti-Gal ELISA. Interestingly, all WT and GTKO islet recipients demonstrated an increase in anti-Gal IgM antibody levels that peaked at 3 weeks post-transplant (figure 5c). There was not a significant difference in degree of increase between the two groups. To confirm that upregulation of anti-Gal antibody was not solely dependent on exposure to Gal (or any other xenoantigen), we performed anti-Gal ELISA using sera from immunosuppressed alloislet recipients. Again, anti-Gal IgM levels were significantly increased following transplant, to a similar extent as both WT and GTKO xenoislet recipients. We noted a much smaller increase in anti-Gal IgG in all groups tested (figure 5d); no one group had a significantly larger increase in IgG levels.
Gal-deficient islets are not protected from eventual cellular rejection
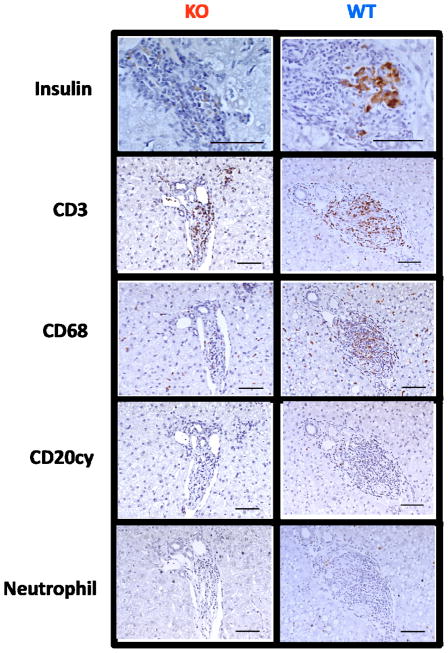
Liver sections obtained from each recipient at necropsy underwent immunohistochemical evaluation in order to characterize any inflammatory infiltrates affecting xenografts. Intrahepatic islets from recipients experiencing functional rejection demonstrated minimal insulin positivity and dense lymphocytic infiltrates consisting mainly of CD3+ T cells (figure 6). There was no difference in the degree or character of the cellular infiltrate between the two donor groups.
Figure 6. Gal-deficient islets are not protected from eventual cellular rejection.
Immunohistochemical analysis of intrahepatic islets from recipients experiencing functional xenograft rejection demonstrated dense lymphocytic infiltrates with loss of insulin positivity. This infiltrate stained heavily for CD3+ T cells, and moderately for CD68+ macrophages. CD20+ B cells and neutrophils did not appear to comprise a significant portion of the infiltrating cells.
Discussion
The complement cascade is one of the major mediators of innate immunity, and an important component of the IBMIR. As such, strategies that inhibit complement activity or prevent complement deposition have been effective in decreasing early islet destruction (13, 16, 18). The classical pathway of complement deposition is initiated through binding of antibody to cell surfaces. Because naturally occurring anti-Gal antibodies can make up as much as 2% of a recipient primate’s preformed antibody repertoire (22), tissues from Gal-deficient donors could be more resistant to complement-mediated injury. Indeed, the results of the in vitro experiments described in this study show that GTKO NPIs are less susceptible to binding of preformed antibody, binding of the individual complement components C1q and C3, and destruction by antibody- and complement-dependent mechanisms than their WT NPI counterparts. Together these results suggest that GTKO NPIs may be less vulnerable to early destruction mediated by the IBMIR.
Decreased susceptibility to early destruction could also explain the improvement in graft function observed following transplant of GTKO NPIs. Primary graft dysfunction, also known as “nonengraftment,” has been ascribed to rapid loss of a significant percentage of the islet xenograft following intraportal infusion (15, 23) resulting in inadequate islet mass to produce insulin independence. In this study, GTKO NPI recipients had lower rates of primary graft dysfunction than paired WT recipients, suggesting that a larger percentage of Gal-deficient islets may survive the early post-transplant period and achieve full insulin production capability. In contrast, WT and GTKO NPI recipients achieving insulin independence experienced similar duration of rejection-free survival, suggesting that differences in the Gal-specific adaptive response to the two donor types may be relatively less important in influencing experimental outcome.
Innate inflammatory processes may also be implicated as a cause of the transaminits observed following transplant in this study. Previous authors have reported that elevations in the liver transaminases ALT and AST occur routinely following intraportal allo- as well as autoislet transplant in humans (24–26). Bystander hepatocyte injury mediated by complement/platelet deposition and proinflammatory cytokine release has been implicated in this process (25). In the current study, we observed an elevation of AST and ALT following transplant that was greater for recipients of WT islets. In the absence of external confounding factors, this observed difference could be attributable to the increased immunogenicity of WT NPIs; the Gal epitope’s ability to bind preformed antibody and complement and subsequently trigger a more vigorous inflammatory response may cause increased intrahepatic inflammation.
Previous investigators have determined that substantial heterogeneity of Gal expression exists among individual donors (18), but it remains unclear whether lower islet Gal expression predicts improved recipient glycemic control following transplant. In this study, the single WT recipient experiencing insulin independence received islets with significantly lower Gal expression than all other WT NPI preparations (see figure 1a,b). Though only a single case, this finding lends additional weight to the importance of Gal as a target of xenograft destruction.
Porcine islets surviving early destruction are still subject to delayed xenograft rejection mediated by humoral immunity (2, 3, 27). We anticipated that Gal might be a significant target of acquired as well as persistent preformed xenoantibody well after the initial peritransplant period. In this study, we observed that anti-Gal IgM increases significantly in the weeks following transplant of WT NPIs, and surprisingly, following transplant of GTKO NPIs and alloislets, suggesting that upregulation of anti-Gal is not solely dependent on exposure to Gal antigen. Previous investigators have demonstrated that naturally-occurring antibodies may upregulate following insults such as ischemia/reperfusion (28), and in addition, that transplantation of xenografts from GTKO donors can elicit a significant (albeit transient) increase in anti-Gal antibody (29). The results observed in our experiment suggest that anti-Gal antibody may increase nonspecifically as a reaction to injury, in this case intraportal islet infusion, as a component of the innate immune response.
In contrast to IgM, anti-Gal IgG levels remained relatively unchanged in all recipients over the course of the experiment, possibly due to the immunosuppression used in this study. Blockade of the CD40/CD154 interaction has been shown to inhibit T-dependent antibody responses and B cell isotype switching (30); accordingly, the significant anti-Gal IgG response previously observed in recipients transplanted with WT islets under cover of conventional immunosuppressive agents (31) appeared to be blunted following treatment with CD154-specific antibodies (2, 3, 32). Therefore, CD154-specific therapy in this study may have prevented bridging of the innate anti-Gal IgM response to an adaptive anti-Gal IgG response.
Evaluation of the importance of Gal-specific immunity has been complicated by differences in donor tissue sources amongst investigators. Whereas fetal and neonatal porcine islets (NPIs) express the Gal epitope to a significant extent on endocrine and non-endocrine cellular components, adult islet preparations may express Gal only on vascular endothelial contaminants as well as a small percentage of endocrine cells (18, 33, 34). The model described in the current study differs from previous trials comparing WT and GTKO islets (11–13) in that we used neonatal instead of adult porcine donors. Despite differences in Gal expression, our group feels that neonatal donors possess significant immunologic (10, 35, 36) and logistical (17, 37) advantages, and likely represent the most useful and practical donor source for future large-scale clinical production models. While the benefits of genetically modified, Gal-deficient donors may be most evident in the neonatal model, preformed antibodies may still target the small percentage of Gal-positive endocrine cells and Gal-expressing contaminants in adult islet preparations, resulting in an incremental increase in inflammation and graft injury. Therefore, the functional advantages of GTKO neonatal islets demonstrated in this study are pertinent to all xenoislet transplant models.
Clinical application of islet xenotransplantation in humans has been impeded by the vigorous xenospecific immune response and by the lack of a consistently effective yet translatable immunosuppressive regimen. While the data presented in this study characterize an integral component of xenospecific immunity, our immunosuppressive regimen lacks translational potential due to the use of clinically unavailable CD154- and LFA-1-specific antibodies. Our primary aim was to establish proof-of-concept that Gal-specific immunity affects islet xenografts; additional preclinical studies will be necessary to determine the optimal immunosuppressive regimen and islet mass for use in human trials.
In conclusion, we have directly compared the immunogenicity, function, and survival of WT and GTKO neonatal porcine islets. Our results represent the first evidence that Gal-deficient donors can improve the outcome of islet transplantation. Gal-specific immunity may result in increased early xenoislet destruction, which is manifested as improved rates of insulin independence following transplantation of Gal-deficient islets. The use of GTKO donors is therefore an important step towards improved consistency and interpretability of results in future xenoislet trials.
Supplementary Material
Supplemental Figure 1: Whole blood phenotyping identifies donors as WT or GTKO.
Supplemental Figure 2: Genotyping confirms donor Gal status.
Acknowledgments
The authors would like to acknowledge the Juvenile Diabetes Research Foundation (Grant # 21-2006-882), the National Institute of Health (Grant # 1U01AI090956-01), and the Yerkes National Primate Center (Grant # P51RR-00065) for their funding of this project, and Sebastian Perez for assistance with statistical analysis.
Abbreviations
- HAR
hyperacute rejection
- GTKO
galactosyl-transferase knockout
- WT
wild-type
- IBMIR
instant blood-mediated inflammatory reaction
- ADCC
antibody-dependent cellular cytotoxicity
- NPIs
neonatal porcine islets
- HIS
heat-inactivated serum
- GSI
glucose stimulation index
- STZ
streptozocin
- FBG
fasting blood glucose
- IVGTT
intravenous glucose tolerance test
- IACUC
institutional animal care and use committee
- PCP
porcine c-peptide
- MFI
mean fluorescence intensity
- NHPs
nonhuman primates
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ekser B, Cooper DK. Overcoming the barriers to xenotransplantation: prospects for the future. Expert Rev Clin Immunol. 2010;6(2):219–230. doi: 10.1586/eci.09.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 3.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 4.Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14(10):480–482. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- 5.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1–3)Gal epitopes. Proc Natl Acad Sci U S A. 1993;90(23):11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierson RN, 3rd, Dorling A, Ayares D, Rees MA, Seebach JD, Fishman JA, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16(5):263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 8.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 9.Vajkoczy P, Olofsson AM, Lehr HA, Leiderer R, Hammersen F, Arfors KE, et al. Histogenesis and ultrastructure of pancreatic islet graft microvasculature. Evidence for graft revascularization by endothelial cells of host origin Am J Pathol. 1995;146(6):1397–1405. [PMC free article] [PubMed] [Google Scholar]
- 10.Rayat GR, Rajotte RV, Hering BJ, Binette TM, Korbutt GS. In vitro and in vivo expression of Galalpha-(1,3)Gal on porcine islet cells is age dependent. J Endocrinol. 2003;177(1):127–135. doi: 10.1677/joe.0.1770127. [DOI] [PubMed] [Google Scholar]
- 11.Rood PP, Bottino R, Balamurugan AN, Smetanka C, Ayares D, Groth CG, et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;83(2):202–210. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 12.Casu A, Bottino R, Balamurugan AN, Hara H, van der Windt DJ, Campanile N, et al. Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: implications for translation into clinical practice. Diabetologia. 2008;51(1):120–129. doi: 10.1007/s00125-007-0844-4. [DOI] [PubMed] [Google Scholar]
- 13.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 14.Bennet W, Sundberg B, Lundgren T, Tibell A, Groth CG, Richards A, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69(5):711–719. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof N, Shibata S, Wijkstrom M, Kulick DM, Salerno CT, Clemmings SM, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11(5):396–407. doi: 10.1111/j.1399-3089.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 16.Goto M, Tjernberg J, Dufrane D, Elgue G, Brandhorst D, Ekdahl KN, et al. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15(4):225–234. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97(9):2119–2129. doi: 10.1172/JCI118649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rayat GR, Rajotte RV, Elliott JF, Korbutt GS. Expression of Gal alpha(1,3)gal on neonatal porcine islet beta-cells and susceptibility to human antibody/complement lysis. Diabetes. 1998;47(9):1406–1411. doi: 10.2337/diabetes.47.9.1406. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Laboratory Animal Resources (U.S.). Committee on Care and Use of Laboratory Animals. NIH publication. Bethesda, Md: U.S. Dept. of Health and Human Services, Public Health Service; Guide for the care and use of laboratory animals; p. v. [Google Scholar]
- 20.Richards AC, Davies HF, McLaughlin ML, Copeman LS, Holmes BJ, Dos Santos Cruz G, et al. Serum anti-pig antibodies as potential indicators of acute humoral xenograft rejection in pig-to-cynomolgus monkey kidney transplantation. Transplantation. 2002;73(6):881–889. doi: 10.1097/00007890-200203270-00009. [DOI] [PubMed] [Google Scholar]
- 21.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120(12):4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780(2):75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14(4):288–297. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 24.Wahoff DC, Papalois BE, Najarian JS, Kendall DM, Farney AC, Leone JP, et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg. 1995;222(4):562–575. doi: 10.1097/00000658-199522240-00013. discussion 575–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafael E, Ryan EA, Paty BW, Oberholzer J, Imes S, Senior P, et al. Changes in liver enzymes after clinical islet transplantation. Transplantation. 2003;76(9):1280–1284. doi: 10.1097/01.TP.0000098822.85924.4C. [DOI] [PubMed] [Google Scholar]
- 26.Barshes NR, Lee TC, Goodpastor SE, Balkrishnan R, Schock AP, Mote A, et al. Transaminitis after pancreatic islet transplantation. J Am Coll Surg. 2005;200(3):353–361. doi: 10.1016/j.jamcollsurg.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155(11):5249–5256. [PubMed] [Google Scholar]
- 28.Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44(1–3):103–110. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Ezzelarab M, Hara H, Busch J, Rood PP, Zhu X, Ibrahim Z, et al. Antibodies directed to pig non-Gal antigens in naïve and sensitized baboons. Xenotransplantation. 2006;13(5):400–407. doi: 10.1111/j.1399-3089.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 30.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229(1):294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantarovich D, Blancho G, Potiron N, Jugeau N, Fiche M, Chagneau C, et al. Rapid failure of pig islet transplantation in non human primates. Xenotransplantation. 2002;9(1):25–35. doi: 10.1034/j.1399-3089.2002.0o144.x. [DOI] [PubMed] [Google Scholar]
- 32.Buhler L, Deng S, O’Neil J, Kitamura H, Koulmanda M, Baldi A, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9(1):3–13. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 33.Bennet W, Bjorkland A, Sundberg B, Davies H, Liu J, Holgersson J, et al. A comparison of fetal and adult porcine islets with regard to Gal alpha (1,3)Gal expression and the role of human immunoglobulins and complement in islet cell cytotoxicity. Transplantation. 2000;69(8):1711–1717. doi: 10.1097/00007890-200004270-00030. [DOI] [PubMed] [Google Scholar]
- 34.Kin T, Nakajima Y, Aomatsu Y, Kanehiro H, Hisanaga M, Ko S, et al. Humoral human xenoreactivity against isolated pig pancreatic islets. Surg Today. 2000;30(9):821–826. doi: 10.1007/s005950070065. [DOI] [PubMed] [Google Scholar]
- 35.Bloch K, Assa S, Lazard D, Abramov N, Shalitin S, Weintrob N, et al. Neonatal pig islets induce a lower T-cell response than adult pig islets in IDDM patients. Transplantation. 1999;67(5):748–752. doi: 10.1097/00007890-199903150-00018. [DOI] [PubMed] [Google Scholar]
- 36.Murray AG, Nelson RC, Rayat GR, Elliott JF, Korbutt GS. Neonatal porcine islet cells induce human CD4+, but not CD8+, lymphocyte proliferation and resist cell-mediated cytolytic injury in vitro. Diabetes. 1999;48(9):1713–1719. doi: 10.2337/diabetes.48.9.1713. [DOI] [PubMed] [Google Scholar]
- 37.Emamaullee JA, Shapiro AM, Rajotte RV, Korbutt G, Elliott JF. Neonatal porcine islets exhibit natural resistance to hypoxia-induced apoptosis. Transplantation. 2006;82(7):945–952. doi: 10.1097/01.tp.0000238677.00750.32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Whole blood phenotyping identifies donors as WT or GTKO.
Supplemental Figure 2: Genotyping confirms donor Gal status.