Abstract
Objective
Despite pro-fibrotic effects, transforming growth factor (TGF)-β prevents arteriosclerosis by suppressing effector leukocytes and promoting smooth muscle differentiation. However, previous observations of increased TGF-β expression in arteriosclerotic plaques are not consistent with that of an effective protective factor. We investigated the expression, regulation, and responses of TGF-β in human arterial tissues and cells.
Methods and Results
The expression of TGF-β by intrinsic vascular cells was lower in arteriosclerotic than non-diseased coronary arteries. Activation of resident and infiltrating leukocytes did not elicit TGF-β production from coronary artery segments in organ culture. Instead, the basal expression of TGF-β by coronary arteries decreased after vessel procurement and ex vivo culture. Activation of cultured smooth muscle cells and endothelial cells with phorbol ester and ionophore also decreased TGF-β expression. Isolated cell types representing those found in the artery wall were all capable of signaling in response to TGF-β, however production of the cytoprotective molecule, interleukin-11 was cell type-dependent and restricted to smooth muscle cells and fibroblasts. Interleukin-11 reduced smooth muscle cell apoptosis to T cell effectors.
Conclusions
Inflammation and cellular activation diminish the basal expression of TGF-β by quiescent human vascular cells. Induction of interleukin-11 may contribute to the anti-arteriosclerotic actions of TGF-β.
Keywords: arteriosclerosis, smooth muscle cells, transforming growth factor-β, human
INTRODUCTION
The role of transforming growth factor (TGF)-β in inflammatory arterial diseases is complex. It has potent pro-fibrotic effects and results in the excessive accumulation of extracellular matrix (1). Consistent with a pathogenic function for neointimal expansion, many previous studies have described the expression of TGF-β mRNA and protein as low in non-diseased arteries and increased in vascular cells and infiltrating leukocytes after injury or arteriosclerosis (2-4). An alternative hypothesis proposes that TGF-β plays an anti-arteriosclerotic role (5). This interpretation is supported by serologic neutralization and genetic manipulation studies in hyperlipidemic mice in which a loss of TGF-β signaling increases the extent of arteriosclerosis (6-8). Thus, an increased expression of TGF-β in diseased arteries is viewed as a protective mechanism that maintains smooth muscle cells (SMC) in a differentiated state and limits the effector functions of pathogenic lymphocytes (9). This prevailing theory recognizes the local production of TGF-β by vascular cells and/or regulatory T cells during disease pathogenesis (10). Since many TGF-β functions are context- and cell type-specific, it is important to study the expression, regulation, and effects of TGF-β in coronary arteries, a key site for clinical manifestations of arteriosclerosis.
In the present study, we investigated if the expression of TGF-β by human vascular cells is modulated by arterial inflammation and vascular cell activation. We first examined the expression of TGF-β in clinical specimens of non-diseased and arteriosclerotic coronary arteries. We then extended our descriptive observations by investigating the effects of leukocyte and vascular cell activation on TGF-β expression in cultured artery segments and isolated cell populations. Finally, we compared TGF-β-mediated signaling and production of potentially anti-arteriosclerotic molecules between cell types representative of those found in the vessel wall. Surprisingly, we find that activation of human arteries and vascular cells decreases their expression of TGF-β, that effector artery-infiltrating leukocytes do not produce significant amounts of TGF-β, and that vascular cells differ markedly in their responses to TGF-β, such as the production of the cytoprotective molecule, interleukin (IL)-11.
MATERIALS AND METHODS
Arteries and Cells
Human research protocols were approved by Yale University and New England Organ Bank. Coronary arteries were obtained from explanted hearts of patients undergoing transplantation or of organ donors. The subject characteristics and the procurement techniques are described in the supplemental information. The specimens were immediately frozen in the operating room after macroscopic examination. Alternatively, epicardial coronary arteries were divided into 3 mm rings for organ culture. Vascular SMC and dermal fibroblasts were isolated by explant outgrowth. Venous endothelial cells (EC) were isolated from umbilical cords and we purchased arterial EC derived from coronary arteries (Lonza). Peripheral blood mononuclear cells (PBMC) were obtained by leukapheresis of healthy adult donors.
Organ and Cell Culture
Artery rings and PBMC were cultured in Advanced RPMI 1640 medium (Invitrogen) without serum, SMC and fibroblasts were placed in serum-free M199 medium for 48 hr prior to treatment, and EC were only serum-deprived in ECGS-supplemented M199 medium at the time of treatment. Arteries and cells were treated with agonistic antibodies to CD3 at 1 μg/mL (for arteries) or on plates pre-coated with antibody at 10 μg/mL overnight (for cells) and to CD28 at 1 μg/mL (eBioscience), with phorbol myristate acetate (PMA) and ionomycin (Sigma-Aldrich) at 1 μg/mL and 1μM, respectively, and with recombinant human cytokines (R&D Systems) at various doses. Protein and mRNA expression were determined as previously described (11) and details of the assays are provided in the supplemental information.
TGF-β Reagents and Assays
Cultured arteries and cells were treated with recombinant human TGF-β1 (cat. # 240-B, R&D Systems). Immunolabeling was performed with mouse anti-human latency-associated peptide (LAP)-β1 (clone 27235, R&D Systems) and rabbit anti-pan TGF-β (mixture of human TGF-β1, porcine TGF-β1.2, porcine TGF-β2, and amphibian TGF-β5, cat. # AB-100-NA, R&D Systems). TGF-β1 levels in culture supernatants and artery/cell lysates were determined using a sandwich ELISA Duoset kit (cat. # DY240, R&D Systems) according to the manufacturer’s instructions (rndsystems.com/pdf/dy240.pdf). To activate latent TGF-β1 to an immunoreactive mature form, the samples were acidified with 1 N HCL for 10 min and then neutralized with 1.2 N NaOH/ 0.5 M HEPES for 10 min. Immunoblotting was performed with rabbit anti-human TGF-β1 (clone 56E4, Cell Signaling Technology). Real-time RT-PCR reactions were performed using predeveloped Applied Biosystems TaqMan probes to TGF-β1 (cat. # Hs00998122_m1), TGF-β2 (cat. # Hs00236092_m1), and TGF-β3 (cat. # Hs01086000_m1).
Statistical Analysis
Data were analyzed using Prism 4 software (GraphPad). Comparisons between two groups were by t-test and between more than two groups were by ANOVA. All P values were two-tailed and values <0.05 were considered to indicate statistical significance.
RESULTS
TGF-β expression is decreased in arteriosclerotic coronary arteries
To characterize the arterial expression of TGF-β in situ, we examined referent and diseased clinical specimens. Human coronary arteries from organ donors and recipients were classified as non-arteriosclerotic or arteriosclerotic based on macroscopic characteristics (translucent and pliable vs. opaque and rigid, respectively) and pathology was confirmed by histological appearances (Fig. 1A). The demographic data of individuals with normal vs. diseased arteries were similar (Supplemental Table I). TGF-β1 was the predominant form and TGF-β3 was the least abundant form of TGF-β mRNA within the arterial wall as detected by quantitative RT-PCR (Fig. 1B). The relative expression of transcripts for all three forms of TGF-β was diminished in diseased arteries. By immunohistochemistry, expression of the TGF-β precursor remnant, LAP and of mature TGF-β protein was greatest within the arterial media and endothelium and less intense in the neointima and adventitia (Fig. 1C). Similar to the transcript data, the expression of these proteins was diminished in arteriosclerotic vessels. By immunofluorescence, LAP expression co-localized to both EC and SMC which greatly outnumbered LAP-expressing infiltrating leukocytes, such as T cells (Supplemental Fig. 1). The results are consistent with basal expression of TGF-β by intrinsic cells of the healthy vessel wall that is downregulated in inflammatory arterial disease.
Figure 1. TGF-β expression is decreased in arteriosclerotic coronary arteries.
(A) Representative photomicrographs of EVG (bar=1 mm) and H&E (bar=400 μm) stains of non-arteriosclerotic and arteriosclerotic human coronary arteries. (B) Quantitative RT-PCR analysis of TGF-β1, TGF-β2, and TGF-β3 transcripts; *P<0.05, Arteriosclerotic (n=9 donors) vs. Non-arteriosclerotic (n=4 donors), t-test. (C) Immunohistochemical detection (brown color) of LAP and mature TGF-β; lumen oriented above, internal elastic lamina marked by arrow, and external elastic lamina marked by arrowhead (bar=100 μm). Insets (at the same magnification as the larger panels) show staining with an isotype-matched irrelevant antibody.
Arterial expression of TGF-β is not modulated by leukocyte activation
To assess the role of artery-infiltrating leukocytes to vascular expression of TGF-β, we used an organ culture system with human coronary artery segments in serum-free medium (since both plasma and platelet releasate contain TGF-β) that enables selective activation of leukocytes within the vessel wall. Total TGF-β1 was measured by ELISA after acidification of the samples to release TGF-β non-covalently bound to LAP and allow for serologic recognition of total cytokine. Levels of active TGF-β (without acidification of the samples) were very low around the limit of detection (data not shown). Stimulation of resident T cells in non-arteriosclerotic and infiltrating T cells in arteriosclerotic coronary arteries by agonistic antibodies to the T cell receptor component, CD3 and to the T cell costimulator, CD28 did not augment TGF-β1 secretion after 24-96 hr (Fig. 2A), although the levels of another regulatory cytokine, IL-10 were modestly increased (Fig. 2B). Since TGF-β may be cell membrane-associated or secreted but bound to the extracellular matrix and thus not detectable in the culture supernatant, we also assessed protein expression in lysates of the cultured arteries. Indeed, the levels of TGF-β were far higher within the arterial tissues than in the supernatant. However, TGF-β1 expression within the cells and extracellular matrix remained unchanged at 96 hr after T cell activation, although there was greater production of IL-10 as well as the pro-inflammatory cytokine, IL-6 (Fig. 2C). Similar results were obtained when another activator of T cells (and of macrophages too), PMA/ionomycin was used with accelerated effects on IL-10 and IL-6 production detectable by 6 hr (Fig. 2D). These experiments using polyclonal activators suggest that artery-infiltrating leukocytes do not synthesize significant amounts of TGF-β upon activation.
Figure 2. Arterial expression of TGF-β is not modulated by leukocyte activation.
Human coronary arteries were divided into 3 mm rings and cultured in serum-free medium. (A) Total TGF-β1 and (B) IL-10 supernatant levels were measured after treatment of non-arteriosclerotic (n=16 from 5 donors) and arteriosclerotic rings (n=13-16 from 5 donors) with agonistic antibodies to CD3 and CD28 for 24-96 hr; *P<0.05, CD3/CD28 vs. Untreated, ANOVA. Additionally, supernatant and arterial lysate levels of total TGF-β1, IL-10, and IL-6 were measured at (C) 96 hr after CD3/CD28 treatment (n=9-10 from 3 donors) or (D) 6 hr after PMA/ionomycin treatment (n=8-16 from 6 donors); *P<0.05, Treated vs. Untreated, ANOVA.
Arterial expression of TGF-β is decreased after artery procurement and culture
Since PMA induces TGF-β synthesis in several non-immune cell types (12), we also investigated their effects on TGF-β transcript expression by intrinsic vascular cells in non-arteriosclerotic coronary arteries with relatively few artery-infiltrating leukocytes. Surprisingly, treatment with PMA and ionomycin markedly diminished TGF-β1 and TGF-β3 mRNA within 6 hr (Fig. 3A). However, a similar, albeit lesser, decline in transcript abundance was also noted in response to vessel procurement and ex vivo culture alone compared to control specimens frozen immediately after explanting the heart. The artery rings were capable of transcription under these conditions as evidenced by a great increase of IL-6 mRNA. The decline in TGF-β expression and induction of IL-6 production after organ culture was confirmed at the protein level (Supplemental Fig. 2). In view of the fact that PMA and ionomycin are not physiological stimuli, we also assessed the effects of several pro-inflammatory cytokines on cultured arteries. IL-1α, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-17 treatment of coronary artery segments for 24 hr did not modulate TGF-β1 production, although its levels were again lower in cultured than snap frozen arteries and IL-6 production was induced under the same conditions (Supplemental Fig. 3). Besides PMA, TGF-β has been described to positively regulate its own production in certain non-immune cells (13). However, we were unable to detect such an autocrine effect in cultured arteries, although treatment with TGF-β manifested biologic effects of IL-6 synthesis (Fig. 3B). We confirmed that TGF-β1 and TGF-β3 transcripts diminished in untreated arteries after organ culture and similar results were found for TGF-β2 by 18 hr. Notably, exogenous TGF-β did not prevent an associated decline in smooth muscle contractile markers (Supplemental Fig. 4). Together, these data suggest that the vascular activation and phenotypic modulation associated with artery procurement and ex vivo culture (14) decreases TGF-β expression within the vessel wall and that this loss of TGF-β is not the cause of the observed SMC de-differentiation.
Figure 3. Arterial expression of TGF-β is decreased after vessel procurement.
Human coronary arteries were divided into 3 mm rings and either immediately frozen (0 hr) for which treatment was not applicable (NA) or cultured in serum-free medium. (A) TGF-β1, TGF-β2, TGF-β3, and IL-6 transcript expression in artery rings (n=11 from 4 donors) stimulated with PMA/ionomycin for 6 hr; +P<0.05, Cultured vs. Not cultured and *P<0.05, PMA/ionomycin vs. Untreated, ANOVA. (B) TGF-β1, TGF-β2, TGF-β3, and IL-6 transcript expression in artery rings (n=9-15 from 4 donors) stimulated with TGF-β at 10 ng/mL for 6-18 hr; +P<0.05, Cultured vs. Not cultured, ANOVA.
Vascular cell expression of TGF-β is decreased by phorbol ester activation
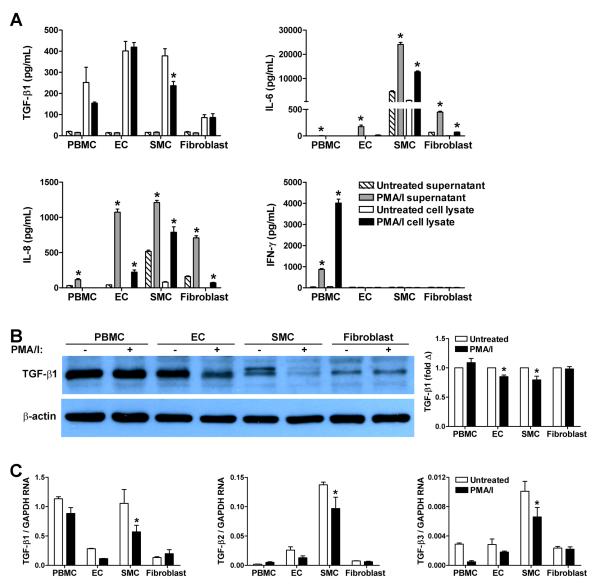
We further investigated the regulation of TGF-β expression by isolated cell populations representative of those found in the artery wall, viz. EC, SMC, fibroblasts, and PBMC. As for the organ culture experiments, the supernatants of untreated cells in serum-free medium contained minimal TGF-β1 close to the limit of detection and far higher levels of cytokine protein were cell-associated. Cellular activation by treatment with PMA/ionomycin for 6 hr did not result in TGF-β1 release or production, although there was robust secretion and accumulation of IL-6 by SMC in particular, IL-8 by all intrinsic vascular cell types, and IFN-γ by leukocytes (Fig. 4A). On the contrary, the basal expression of TGF-β1 by SMC decreased after PMA/ionomycin activation. Decreased TGF-β expression by PMA/ionomycin-activated SMC was verified by immunoblotting (Fig. 4B). A lesser decline in TGF-β expression was seen in venous EC, as well as in arterial EC (Supplemental Fig. 5). Similar results were obtained by quantitative RT-PCR in that levels of TGF-β1, TGF-β2, and TGF-β3 transcripts were decreased in SMC after PMA/ionomycin treatment (Fig. 4C). These cell culture experiments show that TGF-β expression is dynamic and down-regulated after cellular activation of vascular cells, particularly in SMC, and may explain the consequences of arterial inflammation and procurement-associated stresses on arterial TGF-β expression.
Figure 4. TGF-β expression in cultured cells.
PBMC, EC, SMC, and fibroblasts were cultured in serum-free medium and treated or not with PMA/ionomycin for 6 hr. (A) Total TGF-β1, IL-6, IL-8, and IFN-γ levels in the supernatants and cell lysates were measured by ELISA (n=3); *P<0.05, PMA/ionomycin vs. Untreated, ANOVA. (B) Total TGF-β1 and β-actin of cell lysates was determined by western blotting (representative of 3 experiments) and the relative protein expression was assessed by densitometry (n=3); *P<0.05, PMA/ionomycin vs. Untreated, ANOVA. (C) TGF-β1, TGF-β2, TGF-β3 transcript expression was measured by quantitative RT-PCR (n=3); *P<0.05, PMA/ionomycin vs. Untreated, ANOVA.
TGF-β induces IL-11 production by vascular cells in a cell type-dependent manner
Finally, we assessed if the various cell types present within arteries can respond to TGF-β under serum-free medium conditions. Isolated cell populations were all responsive as assessed by SMAD2 phosphorylation, with signaling detected in PBMC at 10-fold lower doses of TGF-β1 than in venous EC and SMC (Fig. 5A) as well as in arterial EC and fibroblasts (Supplemental Fig. 6). However, TGF-β-mediated signaling resulted in cell type-specific transcriptional responses, in that SMC and fibroblasts, but not PBMC or EC, produced IL-6 (Fig. 5B). The latter cell types were capable of producing IL-6 in response to another cytokine, IL-1α. Since the production of pro-inflammatory factors, such as IL-6, is not consistent with the proposed anti-arteriosclerotic role for TGF-β, we examined for additional TGF-β-inducible molecules by microarray analysis. TGF-β treatment of cultured SMC for 6 hr resulted in significant and >2-fold changes in the expression of 52 genes (Supplemental Table II), and one of the most highly induced genes was IL-11 which has a cytoprotective function in immune-mediated and inflammatory injury (15). We confirmed that TGF-β elicited IL-11 secretion in cultured SMC and fibroblasts, but not in EC or PBMC though the latter cell types did not produce IL-11 in response to IL-1α either (Fig. 5C). IL-11 protected IFN-γ-pretreated SMC from TRAIL-mediated apoptosis that illustrates the potential importance of this TGF-β-dependent interplay in SMC-T cell interactions and in the pathogenesis of arteriosclerosis (Fig. 5D). In addition to the cell culture experiments, we confirmed that TGF-β induced the production of IL-11 in cultured arteries (Supplemental Fig. 7). In parallel with the decreased expression of TGF-β, there was also a trend to lesser IL-11 mRNA in arteriosclerotic compared to non-diseased arteries. These data demonstrate ubiquitous, but cell type-specific, responses to TGF-β by the major cell types found in arteries.
Figure 5. TGF-β responses in cultured cells.
(A) PBMC, EC, and SMC were treated with TGF-β1 at the concentrations indicated for 30 min under serum-free conditions and phospho-SMAD2 and total SMAD2 were assessed by western blotting (representative of 3 experiments). Additionally, PBMC at 1×106 cells/well or EC, SMC, and fibroblasts at 3×105 cells/well were cultured in serum-free medium in 24-well plates and treated with TGF-β1 at the doses and times indicated or with IL-1α to document cell responsiveness. (B) IL-6 and (C) IL-11 levels in the culture supernatant were measured by ELISA (n=4); *P<0.05, TGF-β1-treated vs. Untreated, ANOVA. (D) IFN-γ-pretreated SMC were incubated with TRAIL at 40 ng/mL in the absence or presence of IL-11 at 5 ng/mL. Annexin V binding to adherent and floating cells was determined by flow cytometry after 24 hr (n=4), *P<0.05, IL-11 vs. Vehicle, t-test.
DISCUSSION
Our work reveals several new findings regarding the expression, regulation, and responses of TGF-β in human arterial tissues and cells. The basal expression of TGF-β by quiescent vascular cells decreases in inflammatory arterial disease, after artery procurement and ex vivo culture, and upon PMA/ionomycin-mediated activation of cultured vascular cells. Besides its known immunosuppressive effects and the promotion of SMC differentiation, TGF-β may also prevent arterial immune-mediated injury via induction of the protective cytokine, IL-11 by SMC and fibroblasts, but not EC or leukocytes.
Many previous studies have described increased expression of TGF-β in atherosclerotic arteries (2-4). However, this has not been a consistent finding and our results using several techniques and reagents supports the minority point of view that TGF-β expression decreases in diseased arteries. Others have found high levels of TGF-β throughout the normal murine aortic wall and the expression of active TGF-β by vascular cells was diminished by pro-atherogenic defects in lipid metabolism (16). In hyperlipidemic compared to wild-type mice, aortic TGF-β mRNA and protein decreased significantly during atheroma formation (17) and analyses of clinical specimens observed lower TGF-β expression at atheroprone sites of the aorta (18). The parallel changes in all three forms of TGF-β transcripts with that of both LAP and mature cytokine in our clinical specimens imply that the deficiency of TGF-β is due to decreased production rather than loss of sequestered protein from the extracellular matrix. This suggests that TGF-β synthesis is down-regulated after arterial inflammation and immune injury. We similarly observed a reduction in TGF-β mRNA and protein levels in parallel studies of human coronary artery segments interposed into immunodeficient mouse recipients after the introduction of allogeneic human PBMC (19). The discrepancy in the literature regarding arterial TGF-β expression may be due to the complexity of arteriosclerotic lesions. An informative report compared TGF-β expression at different stages of human atherosclerosis and found decreased mRNA and protein in arteries with early signs of disease compared to those without lesions, but increased levels in arteries with advanced disease (20). The diseased arteries we studied were mostly of the former category without complex lesions. Besides a differing focus on plaque-infiltrating leukocytes and neointimal cells rather than intrinsic vessel wall cells, other confounding issues in the field may include the use of TGF-β antibodies that do not recognize latent cytokine and the analysis of autopsy specimens in which the phenotype of arterial SMC is rapidly modulated after death.
Surprisingly, the activation of leukocytes within the arterial wall did not result in detectable TGF-β production given the increasing evidence for an important role of regulatory T cells in the pathogenesis of arteriosclerosis (10). One reason may be that the natural frequency of regulatory T cells infiltrating the artery wall may be small compared to that of effector memory T cells or that the amount of TGF-β produced by artery-infiltrating regulatory T cells is small compared to that produced by intrinsic vascular cells. Notably, TGF-β production in response to antigen recognition is not readily detectable unless particular regulatory T cell populations are selected and expanded (21). Our findings regarding the paucity of TGF-β-producing T cells within the artery wall suggest that it may be challenging to prevent arteriosclerosis by increasing local production of TGF-β through the expansion of regulatory T cell populations (22). However, circulating TGF-β and TGF-β expressed on the surface of circulating T cells represent alternative sources of cytokine that may act on vascular cells, in particular EC that are in direct contact with the bloodstream.
Our observation that TGF-β expression decreases after procurement injury and ex vivo culture stresses supports a link to vascular activation. The phenotypic modulation of arterial SMC is known to occur soon after cessation of perfusion (14). We show that the production of TGF-β is reduced, in addition to that of SMC differentiation markers, within hours of removing the vessels from the donor. These manifestations may be of importance in clinical transplantation as loss of TGF-β in the arteries of organ grafts may predispose to vascular dysfunction and immunologic injury on re-establishing perfusion in the recipient. Exogenous TGF-β treatment did not prevent the decline in smooth muscle contractile proteins, despite the presence of TGF-β control elements in their promoter regions (23). The reduction in TGF-β expression by PMA-treated SMC and EC is consistent with that of PMA-activated arteries, but is unexpected given that PMA is a potent inducer of TGF-β in other cell types (12). However, the regulation and effects of TGF-β are commonly cell type-dependent, including that of autocrine induction (13).
Our microarray analysis identifies IL-11 as one of the most highly induced molecules in vascular SMC by TGF-β. Both IL-11 and IL-6 have previously been identified as TGF-β targets in fibroblasts (24). We show that SMC in addition to fibroblasts, but not EC or PBMC, secrete IL-11 and IL-6 in response to TGF-β in a time- and dose-dependent fashion. The reason for this cell type-dependent regulation of cytokine production is unknown and is of interest for further study. IL-11 confers resistance to EC against cellular- and humoral-mediated cytolysis (15) and inhibits the proliferation of SMC in response to growth factors (25). We have previously shown that IFN-γ alone induces a modest degree of SMC apoptosis in donor-dependent fashion that is consistently enhanced by the activated T cell product, TRAIL (26). Our current data demonstrates that IL-11 is cytoprotective for SMC against cytolytic T cell effectors and may serve similar functions in quiescent TGF-β-expressing arteries.
In conclusion, our data shows that TGF-β expression is diminished in activated arteries and vascular cells which limits a protective role for this cytokine in the pathogenesis of arteriosclerosis. Strategies to maintain or increase the expression of TGF-β by intrinsic vascular cells may be useful in preventing arteriosclerosis.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by NIH grant PO1 HL70295.
Footnotes
Disclosures The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roberts AB, McCune BK, Sporn MB. TGF-beta: regulation of extracellular matrix. Kidney Int. 1992;41:557–559. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- 2.Nikol S, Isner JM, Pickering JG, Kearney M, Leclerc G, Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992;90:1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahadori L, Milder J, Gold L, Botney M. Active macrophage-associated TGF-beta co-localizes with type I procollagen gene expression in atherosclerotic human pulmonary arteries. Am J Pathol. 1995;146:1140–1149. [PMC free article] [PubMed] [Google Scholar]
- 4.Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, Tararak E, Condron M, Kostolias G. Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation. 1999;99:2883–2891. doi: 10.1161/01.cir.99.22.2883. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe JC, Grainger DJ. Transforming growth factor-beta and the protection from cardiovascular injury hypothesis. Biochem Soc Trans. 1995;23:403–406. doi: 10.1042/bst0230403. [DOI] [PubMed] [Google Scholar]
- 6.Grainger DJ, Mosedale DE, Metcalfe JC, Böttinger EP. Dietary fat and reduced levels of TGFbeta1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J Cell Sci. 2000;113:2355–2361. doi: 10.1242/jcs.113.13.2355. [DOI] [PubMed] [Google Scholar]
- 7.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamaté C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 8.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 10.Taleb S, Tedgui A, Mallat Z. Regulatory T-cell immunity and its relevance to atherosclerosis. J Intern Med. 2008;263:489–499. doi: 10.1111/j.1365-2796.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 11.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akhurst RJ, Fee F, Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988;331:363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- 13.O’Reilly MA, Danielpour D, Roberts AB, Sporn MB. Regulation of expression of transforming growth factor-beta 2 by transforming growth factor-beta isoforms is dependent upon cell type. Growth Factors. 1992;6:193–201. doi: 10.3109/08977199209026926. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Makarova N, Cheng Y, E S, Ji RR, Zhang C, Farrar P, Tigyi G. The early- and late stages in phenotypic modulation of vascular smooth muscle cells: differential roles for lysophosphatidic acid. Biochim Biophys Acta. 2008;1781:571–581. doi: 10.1016/j.bbalip.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahboubi K, Biedermann BC, Carroll JM, Pober JS. IL-11 activates human endothelial cells to resist immune-mediated injury. J Immunol. 2000;164:3837–3846. doi: 10.4049/jimmunol.164.7.3837. [DOI] [PubMed] [Google Scholar]
- 16.Grainger DJ, Kemp PR, Liu AC, Lawn RM, Metcalfe JC. Activation of transforming growth factor-beta is inhibited in transgenic apolipoprotein(a) mice. Nature. 1994;370:460–462. doi: 10.1038/370460a0. [DOI] [PubMed] [Google Scholar]
- 17.Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, Zhou ZH, Liao MY, Yao R, Yu X, Wang D, Cheng Y, Liao YH, Cheng X. The Th17/Treg functional imbalance during atherogenesis in ApoE(-/-) mice. Cytokine. 2010;49:185–193. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Borkowski P, Robinson MJ, Kusiak JW, Borkowski A, Brathwaite C, Mergner WJ. Studies on TGF-beta 1 gene expression in the intima of the human aorta in regions with high and low probability of developing atherosclerotic lesions. Mod Pathol. 1995;8:478–482. [PubMed] [Google Scholar]
- 19.Lebastchi AH, Khan SF, Qin L, Li W, Zhou J, Hibino N, Yi T, Rao DA, Pober JS, Tellides G. TGF-beta expression by human vascular cells inhibits IFN-gamma production and arterial media injury by alloreactive memory T cells. Am J Transplant. doi: 10.1111/j.1600-6143.2011.03676.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panutsopulos D, Papalambros E, Sigala F, Zafiropoulos A, Arvanitis DL, Spandidos DA. Protein and mRNA expression levels of VEGF-A and TGF-beta1 in different types of human coronary atherosclerotic lesions. Int J Mol Med. 2005;15:603–10. [PubMed] [Google Scholar]
- 21.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, Schiopu A, Taggart DP, Wood KJ. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948–10956. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- 24.Pelaia G, Gallelli L, D’Agostino B, Vatrella A, Cuda G, Fratto D, Renda T, Galderisi U, Piegari E, Crimi N, Rossi F, Caputi M, Costanzo FS, Vancheri C, Maselli R, Marsico SA. Effects of TGF-beta and glucocorticoids on map kinase phosphorylation, IL-6/IL-11 secretion and cell proliferation in primary cultures of human lung fibroblasts. J Cell Physiol. 2007;210:489–497. doi: 10.1002/jcp.20884. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman MA, Selzman CH, Reznikov LL, Raeburn CD, Barsness K, McIntyre RC, Jr, Hamiel CR, Harken AH. Interleukin-11 attenuates human vascular smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol. 2002;283:H175–180. doi: 10.1152/ajpheart.00987.2001. [DOI] [PubMed] [Google Scholar]
- 26.Bai Y, Ahmad U, Wang Y, Li JH, Choy JC, Kim RW, Kirkiles-Smith N, Maher SE, Karras JG, Bennett CF, Bothwell AL, Pober JS, Tellides G. Interferon-gamma induces X-linked inhibitor of apoptosis-associated factor-1 and Noxa expression and potentiates human vascular smooth muscle cell apoptosis by STAT3 activation. J Biol Chem. 2008;283:6832–6842. doi: 10.1074/jbc.M706021200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.