Abstract
The molecular pathogenesis of myelodysplastic syndrome (MDS) and its progression to secondary acute myeloid leukemia (sAML) remain to be explored. Somatic C-CBL mutations were recently described in MDS. Our study aimed to determine the role of C-CBL mutations in the progression of MDS to sAML and sought to correlate with clinicohematological features and outcome. Bone marrow samples from 51 patients with high-risk MDS (13 with refractory cytopenia with multilineage dysplasia, 19 with refractory anemia with excess blast 1, and 19 with refractory anemia with excess blast 2) were analyzed for C-CBL mutations at both diagnosis and sAML in the same individuals. Mutational analysis was performed for exons 7 to 9 of C-CBL gene. Of the 51 paired samples, C-CBL mutations were identified in 6 patients at the sAML phase. One patient retained the identical C-CBL mutation (G415S) at sAML evolution and exhibited clonal expansion. The other five patients acquired C-CBL mutations (Y371S, F418S, L370_Y371 ins L, L399V, and C416W) during sAML evolution. Three of the six patients harboring C-CBL mutations at sAML had additional gene mutations including JAK2V617F, PTPN11, or N-RAS. There was no significant difference in clinicohematological features and overall survival with respect to C-CBL mutation status. Our results show that C-CBL mutation is very rare (0.6%) in MDS, but acquisition and/or expansion of C-CBL mutant clones occur in 11.8% of patients during sAML transformation. The findings suggest that C-CBL mutations play a role at least in part in a subset of MDS patients during sAML transformation.
Introduction
Myelodysplastic syndromes (MDSs) are hematological malignancies characterized by ineffective hematopoiesis and a high-risk transformation to secondary acute myeloid leukemia (sAML) [1]. MDSs are clonal hemopathies associated with acquired genetic aberrations. We and others have shown that genetic or epigenetic abnormalities might arise during MDS evolution or its progression to sAML [2,3]. Efforts have been made to determine the molecular pathogenesis of the progression of MDS to sAML as well as their clinical impact.
Human C-CBL gene locates on chromosome 11q23.3 and encodes a protein that contains several functional domains, including a tyrosine kinase (TK)-binding domain, a RING finger (RF) domain, a conserved Linker region between the TK-binding domain and RF in the N-terminal portion, and a C-terminal domain with ubiquitin ligase activity [4]. The C-CBL protein has E3 ubiquitin ligase activity and is responsible for the negative regulation of activated TKs [5,6]. The importance of C-CBL gene in hematopoiesis has been demonstrated by knockout mice that showed prolonged activation of TKs, enhanced sensitivity to hematopoietic growth factors, expanded hematopoietic stem cell pool, and myeloproliferative features [7–10].
Loss of heterozygosity could arise either by uniparental disomy (UPD), which represent the coexistence of duplication of an entire or partial chromosome from single parent and loss of the other allele, or by hemizygous deletion. Application of single-nucleotide polymorphism (SNP) microarrays has facilitated the identifications of novel mutated tumor suppressor genes or oncogenes with loss of normal alleles [11,12]. Recently, the detection of 11q acquired UPD (aUPD) has led to the identification of C-CBL mutations in various myeloproliferative neoplasm or MDS subtypes [9,13–16], particularly in chronic myelomonocytic leukemia (CMML) with a frequency of 5% to 25% [9,13,17–19]. We have further demonstrated that C-CBL mutations result in a gain-of-function mutation if a tumor suppressor associated with 11q aUPD, which is a novel leukemogenic mechanism in a subset of CMML [9]. Of the previous studies, C-CBL mutations were mostly analyzed on samples either at initial diagnosis or at the time of sAML transformation. Two studies had examined paired MDS and sAML samples; however, only one case each were included in their studies [14,15]. The impact of C-CBL mutations on outcome of patients with MDS and their role in the progression to sAML remain to be defined. In this study, we analyzed a large cohort of matched paired bone marrow (BM) samples from 51 patients with de novo high-risk MDS and its corresponding sAML to determine the frequency and characters of C-CBL mutations at both phases of disease. The mutation status of the C-CBL gene at either the diagnosis of MDS or sAML was correlated with the clinicohematological features and outcome to determine its clinical and prognostic relevance.
Design and Methods
Patients and Materials
Between 1991 and 2010, 167 patients with the diagnosis of high-risk de novo MDS, including refractory cytopenia with multilineage dysplasia (RCMD), refractory anemia with excess blast 1 (RAEB-1), and refractory anemia with excess blast 2 (RAEB-2) were followed up to observe the evolution of sAML. The morphologic subtypes of MDS were classified according to the World Health Organization's classification [20]. Patients with CMML, refractory cytopenias with unilineage dysplasia, refractory anemia with ring sideroblasts, MDS-unclassified, MDS associated with isolated del(5q), and therapy-related MDS were excluded. The cytogenetic findings according to the International Prognostic Scoring System (IPSS) were available in 138 patients and were divided into three groups: 1) good = normal, -Y, del(5q), del(20q); 2) poor = complex or chromosome 7 abnormalities; and 3) intermediate = other abnormalities [21]. Eighty-six of 167 patients progressed to sAML, of which 51 patients (13 RCMD, 19 RAEB-1, and 19 RAEB-2) had matched paired BM samples at both MDS and sAML phases available for comparative analysis. They formed the basis of this study. Forty-two patients (82.4%) in MDS phase received supportive care only, five were treated with oral chemotherapy (hydroxyurea or melphalan), one with low-dose cytarabine, and three with standard AML protocol. Of the 51 patients at sAML phase, 15 were treated with AML protocol, 2 proceeded to allogeneic hematopoietic stem cell transplantation, 12 received low-dose cytarabine, 9 received oral chemotherapy, and 15 had supportive care only. The study was approved by the institutional review boards of Chang Gung Memorial Hospital, Taiwan, and the University of Tokyo, Japan.
Cell Fractionation
The mononuclear cells were obtained from BM samples by Ficoll-Hypaque density gradient centrifugation (1.077 g/ml; Amersham Pharmacia, Buckinghamshire, United Kingdom). The BM mononuclear cells were cryopreserved in medium containing 10% dimethylsulfoxide and 20% fetal bovine serum at -70°C or in liquid nitrogen until test.
DNA, RNA Extraction, and Complementary DNA Preparation
Genomic DNA (gDNA) and RNA were extracted from frozen BM mononuclear cells. RNA was reversely transcribed to complementary DNA (cDNA) with the SuperScript II RNase H2 Reverse Transcriptase Kit (Invitrogen Corporation, Carlsbad, CA) as described previously [22].
C-CBL Mutation Analysis
cDNA polymerase chain reaction (PCR) assay was performed as described previously [23]. For patients with available RNA samples, the cDNA PCR products were either subjected to direct sequencing and/or screened by denaturing high-performance liquid chromatography (DHPLC; WAVE Transgenomic, Omaha, NE) system [24]. In the DHPLC assay system, we always ran a control of the patient's sample mixed with 50% wild-type DNA to distinguish homozygous mutations from wild-type. The abnormal DHPLC profiles that suggested the presence of mutations were then sequenced. For patients without RNA samples available, mutations at exon 8 were all examined by direct sequencing of gDNA PCR products, whereas mutations at exons 7 and 9 were analyzed by either direct sequencing as previously described [9] or with DHPLC system followed by sequencing for abnormal profiles obtained. The primer sequences for cDNA PCR and DHPLC analysis are listed in Tables W1 and W2. All the mutations detected were confirmed by using alternative samples and/or primers and subjected to PCR assays with sequencing again.
Detection of Additional Gene Mutations
Mutational analysis of FLT3-ITD, FLT3-TKD, codons 12, 13, and 61 of N-RAS and K-RAS, C-KIT, and C-FMS (CSF1R) genes were performed as described previously [3,25–27]. JAK2V617F and PTPN11 were analyzed according to the methods described by Baxter et al. [28] and Tartaglia et al. [29], respectively.
SNP Microarray Analysis
High-density SNP array combined with CNAG (Copy Number Analyzed for Affymetrix GeneChip Mapping)/AsCNAR (allele-specific copy number analysis using anonymous references) software analysis was performed using Affymetrix GeneChip 50K Xbal, HindIII, or 250K Nspl as described before [9], in four patients at sAML phase in which C-CBL mutations were detected.
Statistical Analysis
Fisher exact test, χ2 analysis, and Wilcoxon rank sum test were used whenever appropriate to make comparisons between groups. Estimates of survival were calculated according to the Kaplan-Meier method. Comparisons of estimated survival curves were analyzed by the log-rank test. Statistical analyses were carried out by software SPSS 17.0 (SPSS, Inc, Chicago, IL). In all analyses, P values were two-sided and considered statistically significant when values lower than .05.
Results
C-CBL Mutations in Paired Samples of MDS and sAML
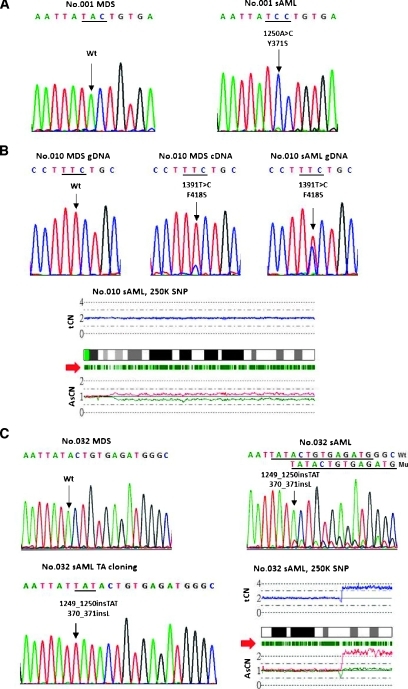
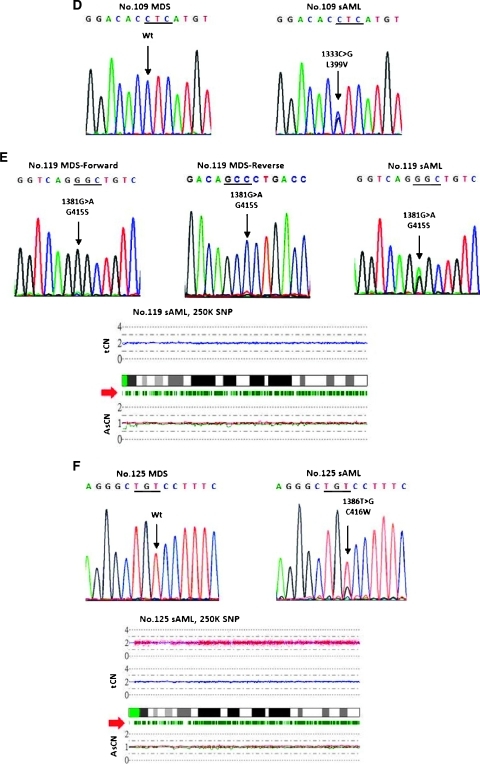
C-CBL mutation was detected in only 1 of 167 de novo high-risk MDS at the initial diagnosis. Eighty-six patients progressed to sAML with a median time of 9.8 months (range = 1.0–143.1 months). Of the 51 paired MDS/sAML samples, 1 patient (no. 119) with RCMD had a C-CBL mutation located at C-terminal of the RF domain (G415S) at initial diagnosis; she retained the same C-CBL mutation at sAML evolution. The other five patients acquired C-CBL mutations during sAML transformation. Patient no. 109 with sAML transformed from RCMD gained a missense mutation at the linker region (L399V). Two sAML patients (nos. 001 and 125) transformed from RAEB-1 gained a mutation at the linker region (Y371S) and the RF domain (C416W), respectively. Of the two RAEB-2 patients who acquired C-CBL mutations during sAML transformation, one (no. 010) had a mutation at the RF domain (F418S) and the other (no. 032) had an insertion mutation (L370_Y371 ins L) at the linker region. The frequency of C-CBL mutations increased from 0.6% (1/167) in the MDS phase to 11.8% (6/51) at sAML transformation. The clinicohematological features and the C-CBL mutation status of the six paired BM samples at both MDS and sAML phases are shown in Table 1.
Table 1.
MDS/sAML Patients with C-CBL Mutations.
| Patient No. | Age (Year)/Sex | WHO Subtype | Marrow Blasts (%) | Cytogenetics at MDS | C-CBL Amino Acid Change | IPSS | Time to AML (Months) | Survival from MDS (Months) | |
| MDS/sAML | MDS | sAML | |||||||
| 001 | 70/F | RAEB-1/sAML | 5.5/71.7 | NA | - | Y371S | >1.0 | 3.5 | 3.9 |
| 010 | 48/F | RAEB-2/sAML | 17.0/32.2 | 46,XX,dup(1)(q21q32)[28/28] | - | F418S | 2.5 | 5.3 | 28.1 |
| 032 | 72/M | RAEB-2/sAML | 16.6/79.0 | 46,XY,-5,-8,-9,add(11)(q25), t(12;18)(p11;p11),-17,+4mar[6/20] | - | L370_Y371 ins L | 3.0 | 2.2 | 3.3 |
| 109 | 22/M | RCMD/sAML | 1.4/35.2 | NA | - | L399V | ≥0.5 | 7.0 | 7.6 |
| 119 | 54/F | RCMD/sAML | 5.0/59.0 | NA | G415S | G415S | 1.0 | 22.3 | 25.2 |
| 125 | 64/F | RAEB-1/sAML | 8.5/45.8 | 45,XX,-7[22/26]/46,XX[4/26] | - | C416W | 1.5 | 14.9 | 23.8+ |
F indicates female; M, male; NA, not available; WHO, World Health Organization.
Figure 1 shows the sequencing electropherograms of the six paired BM MDS/sAML samples carrying C-CBL mutations and the CNAG output for the four sAML samples available for SNP array analysis. Patient no. 001 acquired a homozygous mutation (Y371S) during sAML evolution (Figure 1A). Patient no. 010 was negative for C-CBL mutation at the initial diagnosis of MDS. She gained a small C-CBL mutant clone in the follow-up sample 3.5 months later when her disease was still in the MDS phase, and then she had an expansion of C-CBL mutant clone at the sAML phase 6 months after the diagnosis of MDS. The SNP array analysis showed the presence of 11q-UPD at the sAML phase; the UPD-positive cells were 33% calculated by the observed difference in allele-specific copy number (ASCN) divided by the expected value (Figure 1B), implying that the presence of a homozygous mutation in a fraction of cells in patient no. 010. C-CBL mutation was not detected in patient no. 032 at diagnosis of MDS but a small mutant clone was identified at the sAML phase (Figure 1C, right upper panel). Because the allelic burden of the mutant clone was very low, the PCR product was then cloned into the PCRII-TO PO vector (Invitrogen). Twenty-six clones were subsequently sequenced and six mutant clones were obtained, of which the sequence confirmed the presence of L370_Y371L shown in Figure 1C, left lower panel. The SNP array analysis revealed a small deletion and an amplification at 11q23.3 in the sAML sample. Patient no. 109 acquired a missense mutation (L399V) only after sAML transformation (Figure 1D). The only one patient (no. 119) harboring the identical C-CBL mutations at both MDS and sAML phases carried a small subclone of mutant at the MDS phase and progressed to a higher level, which slightly exceeded the wild-type allele. SNP array analysis for the sAML sample did not reveal any abnormality in 11q23.3 (Figure 1E). Patient no. 125, negative for C-CBL mutation at MDS phase, acquired a missense mutation (C416W) after sAML transformation (Figure 1F). SNP array analysis did not show an abnormal finding in 11q23.3 at the sAML phase.
Figure 1.
Chromatograms of C-CBL mutations in six paired MDS/sAML samples (Wt, wild-type; Mu, mutant) and CNAG output for total copy number (tCN) and allele-specific copy number (AsCNs) in the long arm of chromosome 11 in four patients at the sAML phase. The green bars below each ideogram of 11q indicate the position of heterozygous SNP calls (red arrowhead). Dissociation of AsCN plot indicates the presence of UPD in 11q. (A) Patient no. 001 acquired a homozygous missense mutation (Y371S) after sAML transformation. (B) Patient no. 010 had wild-type C-CBL gene at the initial diagnosis of RAEB-1, acquired a small missense mutant clone (C418S) later, and the level of the mutant increased further at sAML phase. SNP array analysis at sAML revealed an 11q-UPD. (C) Patient no. 032, negative for C-CBL mutation at the MDS phase, acquired L370_Y371 ins L at sAML phase. The small C-CBL mutant clone was confirmed by TA cloning. SNP array analysis showed a small deletion and an amplification at 11q23.3 at the sAML phase. (D) Patient no. 109 acquired a missense mutation (L399V) only after sAML transformation. (E) Patient no. 119 had a small subclone of G415S mutant at the MDS phase, which was more clearly shown in reverse complement and expanded during sAML transformation. SNP array analysis for the sAML sample did not reveal any abnormality at 11q23.3. (F) Patient no. 125, negative for C-CBL mutation at MDS phase, acquired a missense mutation (C416W) after sAML transformation. SNP array analysis did not show abnormal findings in 11q23.3 at sAML phase.
Other Genetic Abnormalities in MDS/sAML Patients Harboring C-CBL Mutations
Coexistence of additional gene mutations in C-CBL mutated patients was found in four of six patients (Table 2). Patient nos. 010, 119, and 125 acquired N-RAS mutation, JAK2V617F, and PTPN11 mutation, respectively, during sAML transformation. We did not find any cooperating mutation involving receptor TKs (RTKs) or the RAS pathway with C-CBL gene in patient nos. 001, 032, and 109. In addition, patient no. 125 had evidence of cytogenetic clonal evolution, 45,XX,-7[22/26]/46,XX[4/26] at MDS and 45,XX,-7[20/25]/45, XX,-7,del(16)(q12.1)[2/25]/46,XX[3/25] at sAML.
Table 2.
Additional Gene Mutations in MDS/sAML Patients Harboring C-CBL Mutations.
| Patient No. | Mutation Status at MDS/sAML Phase | ||||||||
| C-CBL Amino Acid Change | N-RAS | K-RAS | FLT3-ITD | FLT3-TKD (D835) | JAK2V617F | PTPN11 | C-KIT | C-FMS | |
| 001 | -/+ (Y371S) | -/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- |
| 010 | -/+/+ (F418S)* | -/+ (Q61H) | -/- | -/- | -/- | -/- | -/- | -/- | -/- |
| 032 | -/+ (L370_Y371 ins L) | -/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- |
| 109 | -/+ (L399V) | -/- | -/- | -/- | -/- | -/- | -/- | -/- | -/- |
| 119 | +/+ (G415S) | -/- | -/- | -/- | -/- | -/++ | ND/- | -/- | ND/- |
| 125 | -/+ (C416W) | -/- | -/- | -/- | -/- | -/- | -/+ (Q510L) | -/- | ND/ND |
++ indicates homozygous mutation; ND, not done.
Mutation status at MDS/MDS/sAML phases.
Clinicohematological Features and Outcome of MDS/sAML with C-CBL Mutations
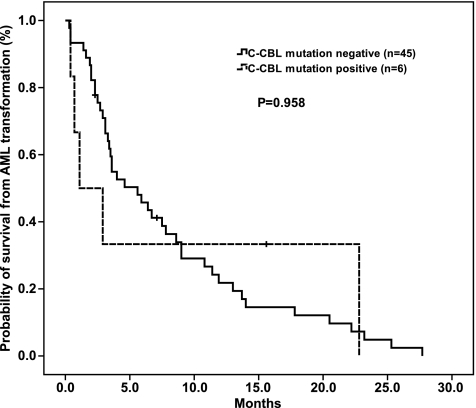
Of the 51 patients, there was no difference in age, sex, hemoglobin level, platelet counts, white blood cell counts, percentage of blasts in BM or peripheral blood, cytogenetics, or IPSS (≤1.5 vs ≥2.0) between C-CBL mutation-positive and -negative groups at both MDS and sAML. The time to sAML transformation and the survival from the diagnosis of MDS in the six patients who harbored C-CBL mutations at sAML phase are shown in Table 1. Because only one MDS patient harbored C-CBL mutation at the initial diagnosis in the whole cohort of MDS patients, it precluded a meaningful analysis of the mutation status on the risk and time to sAML transformation or overall survival. No significant difference in overall survival from the diagnosis of sAML was observed regarding C-CBL mutation status (n = 51, estimated overall survival = 1.1 [95% confidence interval {CI} = 0–3.7] vs 5.6 [95% CI = 2.9–8.3] months; P = .958; Figure 2) in patients with sAML evolved from MDS.
Figure 2.
Kaplan-Meier estimates of overall survival in sAML patients according to C-CBL mutation status. The survival from the diagnosis of sAML was 1.1 months (95% CI = 0–3.7) for the patient with C-CBL mutation compared with the estimated median survival of 5.6 months (95% CI = 2.9–8.3) in C-CBL mutation-negative patients (P = .958).
Discussion
In the present study, an analysis of C-CBL mutations in the matched paired BM samples from 51 patients at both phases of MDS and sAML was performed. We found that only one MDS patient had C-CBL mutation at initial presentation, and additional five patients acquired C-CBL mutations during the disease progression to sAML. The frequency of C-CBL mutations at MDS was very low and markedly increased at sAML transformation (1/167 vs 6/51). To the best of our knowledge, the present series is the first longitudinal and systematical study that demonstrated the acquisition and/or clonal expansion of C-CBL mutations in the progression of MDS to sAML.
Because signaling of RTK-activating mutations, RAS pathways, and C-CBL mutations are similar in cell models, we also performed the mutational analyses for these genes. Coexistence of C-CBL mutations with other gene mutations involving the RTKs and RAS pathways was common in our patients at the sAML phase. Three patients had additional mutations of N-RAS, JAK2, or PTPN11 genes during sAML evolution. The only one MDS patient who retained the identical C-CBL mutant clones at sAML transformation acquired JAK2V617F during disease progression. These observations suggested that acquisition of C-CBL mutation collaborating with other gene mutations played a role in the transformation of sAML from MDS. Furthermore, a clonal cytogenetic evolution was also detected in one patient (no. 125) during sAML progression. For those who did not harbor C-CBL mutations at sAML phase, 10 patients acquired the activating mutations in RTKs and/or RAS pathways (data not shown). Our result showed that C-CBL mutations constituted one of the accumulated genetic alternations associated with the progression of MDS to sAML. Patient nos. 001, 032, and 109, who acquired C-CBL mutations at sAML transformation, had only C-CBL mutations detected among the genetic lesions we analyzed, suggesting that C-CBL mutations might play a major role in the disease progression or cooperate with other genetic abnormalities not examined in the present study for these patients.
Patient no. 010 with wild-type C-CBL gene at initial diagnosis of MDS acquired C-CBL mutation later when her disease was still in the MDS phase and the mutant level increased further in the sAML phase. The presence of UPD-positive cells in a subfraction of cells (33%) calculated by a signal ratio attributed to the presence of 11qUPD at the sAML phase in this case without accompanying a homozygous sequencing electropherogram. In patient no. 119 carrying C-CBL mutations in both MDS and sAML phases, the very small C-CBL mutant clone expanded to a slightly predominant clone during sAML evolution in the absence of UPD in the CNAG output. The observed discrepancy might be explained as an allele measurement on sequencing electropherogram probably not accurately enough to conclude the predominance of the mutant allele based on such a subtype difference of signals. Barresi et al. [14] also described an expansion of C-CBL mutated subclone occurred in a case during MDS progression to sAML. This finding indicated that the C-CBL mutated subclone conferred a growth advantage when MDS progressed to sAML. Acquisition with expansion of C-CBL mutated clones was also reported in one patient during the progression of primary myelofibrosis to sAML [30].
The sequencing analysis showed that five of the six C-CBL mutations in MDS or sAML were missense mutations; the remaining one was an insertion mutation. All of the C-CBL mutations found in our patients involved the linker region or RF domain that is central to the E3 ubiquitin ligase activity [5,6]. Because we analyzed mutations specifically at exons 7, 8, and 9, C-CBL mutations located outside exons 7 to 9 would not be detected in the present study. It is of note that most of the C-CBL mutations reported previously was missense mutation. Insertion mutations were only described in one AML patient with ins(SK366) at intron7/exon8 splice site [31]. The mutation character of three-base insertion that leads to L370_Y371 ins L without frameshift at the linker region of C-CBL, which was verified by cloning analysis, had not been described before. L370_Y371 ins L might cause conformational change of the Linker region and result in decreased E3 activity. It has been found that homozygous C-CBL mutations were frequently observed in patients with CMML and strongly associated with 11q aUPD [9,13,19]. In the present study, we found that UPD seemed to be less frequent in patients with MDS/sAML.
Whether C-CBL mutations confer unique clinical characteristics is not clearly defined. We did not find any association between C-CBL mutations and specific clinicohematological features at the initial presentation or at sAML. In the literature review along with the present result, except for one with refractory anemia [19], MDS patients harboring C-CBL mutations were mostly of RAEB or RCMD subtypes [9,13–15]. Current evidences suggested that C-CBL mutations were associated with more aggressive types of MDS. The impact of C-CBL mutations on clinical outcome in MDS is not known because of the rare occurrence of the mutation at the initial diagnosis. The clinical and prognostic relevance of C-CBL mutation on MDS remains to be determined by a larger cohort of patients. We did not observe a survival impact of C-CBL mutation in sAML patients. Our patients received different treatment options both at MDS and sAML stages; this might have influences on survival analysis. Nevertheless, sAML has a very poor prognosis; the cohort of patients that carrying C-CBL mutations in this series would have a poor outcome. It is the fact that C-CBL mutation in sAML evolution is of biologic relevance but not necessary of prognostic importance.
To sum up, we analyzed C-CBL mutations in matched paired BM samples of patients with high-risk de novo MDS at initial presentation and sAML. Our results showed that C-CBL mutations were rare in MDS at the presentation, but acquisition and/or expansion of C-CBL mutated clones occurred not infrequently during its progression to sAML. The higher occurrence of C-CBL mutations at sAML transformation in patients with MDS suggested that C-CBL mutation might play a role, either dominantly or cooperatively with other genetic abnormalities, in a subset of MDS patients during sAML evolution.
Supplementary Material
Acknowledgments
The authors thank Yu-Feng Wang for her secretarial assistance.
Footnotes
This work was supported by grants from the National Health Research Institute (NHRI-EX99-9711SI), the Department of Health (DOH99-TD-C-111-006), Chang Gung Memorial Hospital (CMRPG380611), and Mackay Memorial Hospital (MMH-E-99009), Taiwan. The authors reported no potential conflicts of interest.
This article refers to supplementary materials, which are designated by Tables W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Tefferi A, Vardiman JW. Mechanisms of disease: myelodysplastic syndromes. N Engl J Med. 2009;361:1872–1885. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 2.Quesnel B, Guillerm G, Vereecque R, Wattel E, Preudhomme C, Bauters F, Vanrumbeke M, Fenaux P. Methylation of the p15INK4b gene in myelodysplastic syndromes is frequent and acquired during disease progression. Blood. 1998;91:2985–2990. [PubMed] [Google Scholar]
- 3.Shih LY, Huang CF, Wang PN, Wu JH, Lin TL, Dunn P, Kuo MC. Acquisition of FLT3 or N-ras mutations is frequently associated with progression of myelodysplastic syndrome to acute myeloid leukemia. Leukemia. 2004;18:466–475. doi: 10.1038/sj.leu.2403274. [DOI] [PubMed] [Google Scholar]
- 4.Nau MM, Lipkowitz S. Comparative genomic organization of the cbl genes. Gene. 2003;308:103–113. doi: 10.1016/s0378-1119(03)00471-2. [DOI] [PubMed] [Google Scholar]
- 5.Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt MH, Dikic I. The Cbl interactome and its functions. Nat Rev Mol Cell Biol. 2005;6:907–918. doi: 10.1038/nrm1762. [DOI] [PubMed] [Google Scholar]
- 7.Lee PS, Wang Y, Dominguez MG, Yeung YG, Murphy MA, Bowtell DD, Stanley ER. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, Thien CB, Langdon WY, Bowtell DD. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, Tamura A, Honda H, Sakata-Yanagimoto M, Kumano K, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460:904–908. doi: 10.1038/nature08240. [DOI] [PubMed] [Google Scholar]
- 10.Zeng S, Xu Z, Lipkowitz S, Longley JB. Regulation of stem cell factor receptor signaling by Cbl family proteins (Cbl-b/c-Cbl) Blood. 2005;105:226–232. doi: 10.1182/blood-2004-05-1768. [DOI] [PubMed] [Google Scholar]
- 11.Mohamedali A, Gäken J, Twine NA, Ingram W, Westwood N, Lea NC, Hayden J, Donaldson N, Aul C, Gattermann N, et al. Prevalence and prognostic significance of allelic imbalance by single-nucleotide polymorphism analysis in low-risk myelodysplastic syndromes. Blood. 2007;110:3365–3373. doi: 10.1182/blood-2007-03-079673. [DOI] [PubMed] [Google Scholar]
- 12.Raghavan M, Lillington DM, Skoulakis S, Debernardi S, Chaplin T, Foot NJ, Lister TA, Young BD. Genome-wide single nucleotide polymorphism analysis reveals frequent partial uniparental disomy due to somatic recombination in acute myeloid leukemias. Cancer Res. 2005;65:375–378. [PubMed] [Google Scholar]
- 13.Dunbar AJ, Gondek LP, O'Keefe CL, Makishima H, Rataul MS, Szpurka H, Sekeres MA, Wang XF, McDevitt MA, Maciejewski JP. 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res. 2008;68:10349–10357. doi: 10.1158/0008-5472.CAN-08-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barresi V, Palumbo GA, Musso N, Consoli C, Capizzi C, Meli CR, Romano A, Di Raimondo F, Condorelli DF. Clonal selection of 11q CN-LOH and CBL gene mutation in a serially studied patient during MDS progression to AML. Leuk Res. 2010;34:1539–1542. doi: 10.1016/j.leukres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Rocquain J, Carbuccia N, Trouplin V, Raynaud S, Murati A, Nezri M, Tadrist Z, Olschwang S, Vey N, Birnbaum D, et al. Combined mutations of ASXL1, CBL, FLT3, IDH1, IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, TET2 and WT1 genes in myelodysplastic syndromes and acute myeloid leukemias. BMC Cancer. 2010;10:401. doi: 10.1186/1471-2407-10-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reindl C, Quentmeier H, Petropoulos K, Greif PA, Benthaus T, Argiropoulos B, Mellert G, Vempati S, Duyster J, Buske C, et al. CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subtypes. Clin Cancer Res. 2009;15:2238–2247. doi: 10.1158/1078-0432.CCR-08-1325. [DOI] [PubMed] [Google Scholar]
- 17.Grand FH, Hidalgo-Curtis CE, Ernst T, Zoi K, Zoi C, McGuire C, Kreil S, Jones A, Score J, Metzgeroth G, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113:6182–6192. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- 18.Kohlmann A, Grossmann V, Klein HU, Schindela S, Weiss T, Kazak B, Dicker F, Schnittger S, Dugas M, Kern W, et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol. 2010;28:3858–3865. doi: 10.1200/JCO.2009.27.1361. [DOI] [PubMed] [Google Scholar]
- 19.Makishima H, Cazzolli H, Szpurka H, Dunbar A, Tiu R, Huh J, Muramatsu H, O'Keefe C, Hsi E, Paquette RL, et al. Mutations of E3 ubiquitin ligase Cbl family members constitute a novel common pathogenic lesion in myeloid malignancies. J Clin Oncol. 2009;27:6109–6116. doi: 10.1200/JCO.2009.23.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 21.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 22.Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN, Kuo MC, Lai CL, Hsu HC. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 23.Kuo MC, Liang DC, Huang CF, Shih YS, Wu JH, Lin TL, Shih LY. RUNX1 mutations are frequent in chronic myelomonocytic leukemia and mutations at the C-terminal region might predict acute myeloid leukemia transformation. Leukemia. 2009;23:1426–1431. doi: 10.1038/leu.2009.48. [DOI] [PubMed] [Google Scholar]
- 24.Xiao W, Oefner PJ. Denaturing high-performance liquid chromatography: a review. Hum Mutat. 2001;17:439–474. doi: 10.1002/humu.1130. [DOI] [PubMed] [Google Scholar]
- 25.Liang DC, Shih LY, Fu JF, Li HY, Wang HI, Hung IJ, Yang CP, Jaing TH, Chen SH, Liu HC. K-ras mutations and N-ras mutations in childhood acute leukemias with or without mixed-lineage leukemia gene rearrangements. Cancer. 2006;106:950–956. doi: 10.1002/cncr.21687. [DOI] [PubMed] [Google Scholar]
- 26.Shih LY, Huang CF, Wu JH, Wang PN, Lin TL, Dunn P, Chou MC, Kuo MC, Tang CC. Heterogeneous patterns of FLT3 ASP(835) mutations in relapsed de novo acute myeloid leukemia: a comparative analysis of 120 paired diagnostic and relapse bone marrow samples. Clin Cancer Res. 2004;10:1326–1332. doi: 10.1158/1078-0432.ccr-0835-03. [DOI] [PubMed] [Google Scholar]
- 27.Shih LY, Liang DC, Huang CF, Chang YT, Lai CL, Lin TH, Yang CP, Hung IJ, Liu HC, Jaing TH, et al. Cooperating mutations of receptor tyrosine kinases and Ras genes in childhood core-binding factor acute myeloid leukemia and a comparative analysis on paired diagnosis and relapse samples. Leukemia. 2008;22:303–307. doi: 10.1038/sj.leu.2404995. [DOI] [PubMed] [Google Scholar]
- 28.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 29.Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hählen K, Hasle H, Licht JD, Gelb BD. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34:148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 30.Beer PA, Delhommeau F, LeCouédic JP, Dawson MA, Chen E, Bareford D, Kusec R, McMullin MF, Harrison CN, Vannucchi AM, et al. Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood. 2010;115:2891–2900. doi: 10.1182/blood-2009-08-236596. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes MS, Reddy MM, Croteau NJ, Walz C, Weisbach H, Podar K, Band H, Carroll M, Reiter A, Larson RA, et al. Novel oncogenic mutations of CBL in human acute myeloid leukemia that activate growth and survival pathways depend on increased metabolism. J Biol Chem. 2010;285:32596–32605. doi: 10.1074/jbc.M110.106161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.