Abstract
Myc, a pleiotropic transcription factor that is deregulated and/or overexpressed in most human cancers, instructs multiple extracellular programs that are required to sustain the complex microenvironment needed for tumor maintenance, including remodeling of tumor stroma, angiogenesis, and inflammation. We previously showed in a model of pancreatic β-cell tumorigenesis that acute Myc activation in vivo triggers rapid recruitment of mast cells to the tumor site and that this is absolutely required for angiogenesis and macroscopic tumor expansion. Moreover, systemic inhibition of mast cell degranulation with sodium cromoglycate induced death of tumor and endothelial cells in established tumors. Hence, mast cells are required both to establish and to maintain the tumors. Whereas this intimates that selective inhibition of mast cell function could be therapeutically efficacious, cromoglycate is not a practical drug for systemic delivery in humans, and no other systemic inhibitor of mast cell degranulation has hitherto been available. PCI-32765 is a novel inhibitor of Bruton tyrosine kinase (Btk) that blocks mast cell degranulation and is currently in clinical trial as a therapy for B-cell non-Hodgkin lymphoma. Here, we show that systemic treatment of insulinoma-bearing mice with PCI-32765 efficiently inhibits Btk, blocks mast cell degranulation, and triggers collapse of tumor vasculature and tumor regression. These data reinforce the notion that mast cell function is required for maintenance of certain tumor types and indicate that the Btk inhibitor PCI-32765 may be useful in treating such diseases.
Introduction
Many chronic diseases are associated with an aberrant inflammatory response (reviewed in Porta et al. [1]), although in the case of cancer, there is still debate as to whether inflammation is primarily a cause or a consequence of the pathological state. The simple notion that inflammation reflects a host defensive response to the tumor within its midst is consistent with reports that inflammation can be protective against certain cancers (for a review, see Grivennikov et al. [2]). However, this simple immune surveillance idea, although applicable in some instances, has been qualified by recent studies that indicate that “inflammation” is frequently a collateral consequence of the extensive tissue remodeling that tumor cells instruct within their immediate somatic microenvironment—remodeling that is mediated through the actions of heterotypic inflammatory cells such as macrophages, neutrophils, lymphocytes, and mast cells [3]. In this sense, inflammation is an integral part of the macroscopic tumorigenic process. Consistent with this idea, many studies have demonstrated that expansion and maintenance of macroscopic tumors is continuously dependent on the actions of inflammatory cells.
Using the well-described model of Myc-driven pancreatic insulinoma Tg(Ins-MYC/Er)1Gev;Tg(Ins-BCL2L1)2Ksp (designated henceforth Ins-MycERTAM;RIP7-Bcl-xL) [4,5], we previously demonstrated that activation of Myc in the β-cell compartment is, alone, sufficient to initiate and orchestrate a complex inflammatory and angiogenic response, characterized by the expeditious induction of a chemokine-encoding gene cluster and interleukin 1β [6,7], which trigger influx of mast cells, macrophages, and neutrophils into the tumors and their immediate milieu. Most notably, mast cells are rapidly recruited into the mesenchyme adjacent to islets, and the onset of islet angiogenesis and tumor expansion are observed immediately thereafter [7]. By a combined genetic and pharmacological approach, we further demonstrated that mast cell recruitment is absolutely required for tumor angiogenesis and macroscopic β-cell tumor expansion; moreover, systemic treatment of mice harboring established β-cell tumors with a mast cell inhibitor (sodium cromoglycate) triggered rapid onset of hypoxia and death of tumor and endothelial cells [7]. Whereas sodium cromoglycate is a very poor systemic drug with low bioavailability, our observations indicated that more systemically effective inhibitors of mast cell function might be therapeutically useful in treating pancreatic insulinoma and, possibly, other tumors. PCI-32765 is a novel, potent, and selective inhibitor of Bruton tyrosine kinase (Btk) already in clinical trials for B-cell non-Hodgkin lymphoma [8,9]. Btk is required for B-cell receptor signaling but is also critical for mast cell degranulation, acting downstream of the high-affinity IgE receptor (FcεRI) [10]. Indeed, Btk-deficient mice exhibit impaired degranulation after FcεRI cross-linking [11]. Here, we use PCI-32765 in an experimental model of pancreatic insulinoma to test the notion that inhibition of mast cell degranulation is a feasible therapeutic approach.
Materials and Methods
Generation and Maintenance of Mice
Tg(Ins-MYC/Er)1Gev;Tg(Ins-BCL2L1)2Ksp mice were generated as described in Pelengaris et al. [4]. Jh Targeted Mutation Mice (Taconic, Hudson, NY) were a generous gift from Lisa Coussens. MycERTAM was activated in pancreatic islets by daily intraperitoneal (i.p.) injection of 1 mg of tamoxifen dissolved in peanut oil vehicle. PCI-32765 was administered either daily by i.p. injection (5 mg/kg) dissolved in saline or in drinking water (0.16 mg/ml in 1% HP-beta-CD). Mice were housed and treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco. At least five mice were present in each experimental cohort with the exception of the glucose tolerance assay, where cohorts comprised three mice.
Hexaminidase Release Assay
RBL-2H3 cells were obtained from ATCC, Manassas, VA in December 2007 (ATCC CRL-2256). The cell line was authenticated at ATCC according to the company's standard procedures (including cell morphology, karyotyping, and cytochrome C oxidase I testing). After a single passage, the aliquots of the cells were frozen in liquid N2 and then thawed in 2010 for the experiments used in this paper. Cells were plated at 5 x 105 cells/ml in minimum essential medium + 10% fetal calf serum in black-walled 96-well plates. Twenty-four hours later, anti-DNP-BSA IgE (Calbiochem, Billerica, MA) was added to 1 µg/ml followed by increasing doses (0.01–3 µM) of PCI-32765. Cells were incubated for 1 hour and then washed three times in degranulation buffer (as described in Sainte-Laudy et al. [12]).Degranulation was then induced by cross-linking with DNP-BSA for 1 hour. Hexaminidase released into cytosol or culture medium was quantitated using a fluorescent substrate cleavage hexosaminidase assay. No cytotoxicity (Alamar Blue assay) was evident after PCI-32765 treatment alone. Each experiment was run in duplicate and repeated at least three times with similar results.
Tissue Preparation and Histology
Mice were killed and cardiac perfused with phosphate-buffered saline (PBS) followed by zinc-buffered formalin. Pancreata were removed and either frozen in OCT or fixed overnight in zinc-buffered formalin and processed for paraffin embedding. For histologic analysis, tissue sections (5 µm) were stained with hematoxylin and eosin.
Immunohistochemistry
For immunohistochemical analysis, sections were deparaffinized, rehydrated, and subjected to high-temperature antigen retrieval in 10 mM citrate buffer (pH 6.0). The primary antibodies used were as follows: rabbit monoclonal anti-Ki67 (clone SP6) (Lab Vision, Fremont, CA), rat monoclonal anti-MECA32 (BD PharMingen, San Diego, CA), polyclonal guinea pig anti human insulin antibody (Biogenex, Fremont, CA), mouse monoclonal anti-human chromogranin A (clone 5H7; DAKO, Carpinteria, CA), and rabbit polyclonal anti-human c-Kit (DAKO). These primary antibodies were applied for 2 hours in blocking buffer (2.5% BSA, 5% goat serum, 0.3% Triton X-100 in PBS), followed by species-appropriate secondary Alexa Fluor 488 dye-conjugated antibodies (Amersham, Piscataway, NJ) or Vectastain ABC kit and DAB reagents (Vector Laboratories, Burlingame, CA). Fluorescence antibody-labeled slides were mounted in DAKO fluorescent mounting medium containing 1 µg/ml Hoechst counterstain. HRPconjugated secondary antibodies were visualized by DAB staining (Vector Laboratories). Apoptotic cells were detected with ApopTag Detection kit (Chemicon International, Billerica, MA). Images were obtained with an Axiovert S100 TV inverted fluorescence microscope (Zeiss, Thornwood, NY) and Open Lab 3.5.1 software or with an Axiovert 100 inverted microscope (Zeiss) equipped with a Hamamatsu Orca digital camera (Bridgewater, NJ). At least 12 islets were analyzed for each experimental condition.
To quantitate islet size, pancreata from five mice per cohort were analyzed, and at least 12 islets per mouse were measured. For proliferation and apoptosis quantification, six islets per mouse and five mice per cohort were analyzed.
Glucose Tolerance Assay
Mice were fasted for 24 hours of fasting and then challenged with an i.p. bolus of glucose (10 µl/g of 1-M stock solution), and their blood glucose levels were then determined every 20 minutes for a further 2 hours.
Btk Occupancy Assay
For analysis of Btk occupancy after oral dosing of PCI-32765, spleens were disaggregated into isolated splenocytes, which were then incubated for 5 minutes in red blood cell-lysing buffer (Sigma-Aldrich, St Louis, MO). Cells were then treated with PCI-33380, and lysates were analyzed as described in Honigberg et al. [8].
Results
Systemic PCI-32765 Efficiently Inhibits Mast Cell Degranulation
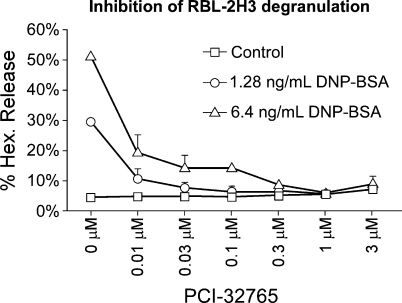
PCI-32765 has previously been shown to bind covalently to, and to be selective for, Cys481 in the Btk active site, inhibiting its enzymatic activity [8,9]. To assess the capacity of PCI-32765 to suppress murine mast cell function in vitro, we used rat RBL-2H3 cells, which are established analogs of rat mucosal mast cells. RBL-2H3 cell surface IgE receptors were cross-linked in the absence or presence of increasing amounts (0.01–3 µM) of PCI-32765 and the impact on degranulation assayed by the release of the granule-derived enzyme hexosaminidase in cells and culture supernatants (Figure 1). PCI-32765 efficiently inhibited hexosaminidase release, indicating efficacious blockade of mast cell degranulation.
Figure 1.
PCI-32765 inhibits mast cell degranulation. In vitro quantitation of mast cell degranulation by hexaminidase release assay. Rat RBL-2H3 mast cells were incubated with PCI-32765 and degranulation then triggered by IgE receptor cross-linking. The extent of degranulation was quantitated by assaying release of hexosaminidase. Numbers represent means of duplicate measurements.
PCI-32765 Blocks Expansion of Myc-Driven Insulinomas
To exclude a possible role of B cells in our insulinoma model that would confound interpretation of any potential PCI-32765 effect, we used J H gene-deficient mice that lack mature B lymphocytes [13] and crossed them into our Ins-MycERTAM;RIP7-Bcl-xL model. Myc was then activated in the pancreatic β cells by daily injection of tamoxifen for 2 weeks to elicit tumorigenesis. Such sustained Myc activation drives progressive and synchronous tumorigenic expansion of the β-cell compartment, accompanied by remodeling of the tumor stroma and proliferation of the endothelial compartment, resulting in multifocal insulinoma [4,6,7]. Even in the complete absence of B-cell function, Myc activation led to expansion of the islets into insulinomas (Figure W1), excluding B-cell requirement for β-cell tumorigenesis, allowing us to focus on other potential targets of PCI-32765.
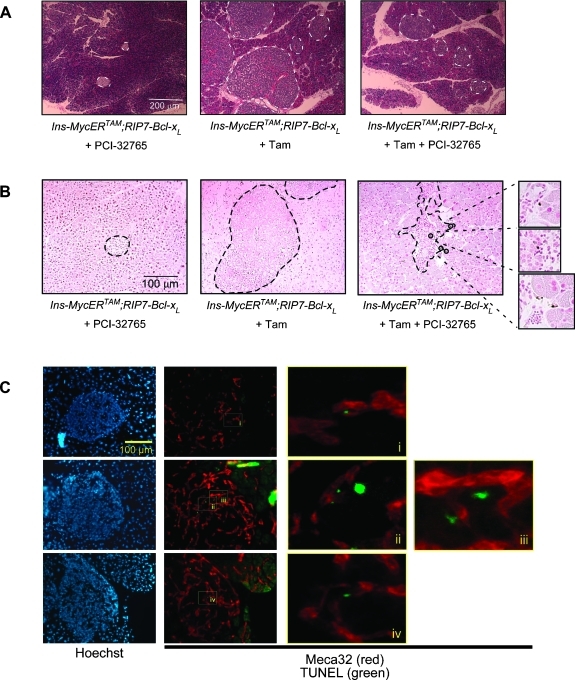
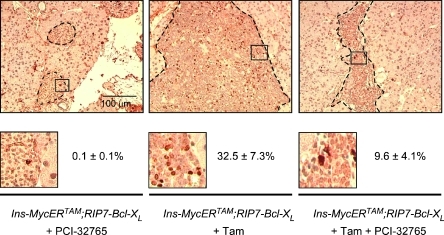
To assess the impact of PCI-32765 on insulinoma growth, we treated Ins-MycERTAM;RIP7-Bcl-xL mice for 2 weeks with tamoxifen. At this point, when all the mice presented overt and angiogenic β-cell tumors, either 5 mg/kg PCI-32765 or control vehicle was administered for a further 7 days, together with daily tamoxifen to maintain Myc activity. Mice were then sacrificed, and pancreata were harvested for immunohistochemical analysis. At this stage, as a consequence of Myc activation, in each section, we observed an average 4.6 ± 0.4-fold increase in the islet area over controls. However, in animals treated with PCI-32765, the increase was reduced to 1.84 ± 0.35 (Figure 2A). This difference in tumor size coincided with the appearance of apoptotic (positive for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)) cells within each tumor mass (3.2% ± 1.1%), whereas no apoptotic cells were evident in the vehicle-treated controls (Figure 2B). Of note, however, almost half (48.7%± 5.1%) of the apoptotic cells were not β endocrine but endothelial, as evidenced by double staining for the endothelial cell marker Meca32 (Figure 2C). These data are consistent with our previous studies showing that inhibition of mast cell degranulation rapidly triggers collapse of tumor vasculature, tumor hypoxia, and regression [7]. Intriguingly, PCI-32765 treatment also induced a significant reduction in the proportion of proliferating tumor cells (9.6% ± 4.1% in PCI-32765-treated vs 32.5%± 7.3% in vehicle-treated tumors; Figure 3). This was unexpected because pharmacological inhibition of mast cell degranulation with cromoglycate does not elicit measurable inhibition of tumor cell proliferation in the same tumor model [7].
Figure 2.
Short-term (1 week) treatment with PCI-32765 induces death of both tumor and adjacent endothelial cells. (A) Hematoxylin and eosin (H&E) staining of pancreata harvested from Ins-MycERTAM;RIP7-Bcl-xL mice treated with PCI-32765 only (left panel), with tamoxifen only (middle panel), or with both tamoxifen and PCI-32765 (right panel). (B) TUNEL staining of sections described above. TUNEL-positive apoptotic cells are indicated by black circles. (C) Meca32 (red) and TUNEL (green) double staining showing the presence of dying endothelial cells in tumors treated with PCI-32765. Hoechst nuclear counterstaining (blue) is shown in the left panels. i, ii, iii, and iv indicate higher magnification of TUNEL-positive apoptotic endothelial cells.
Figure 3.
PCI-32765 inhibits the proliferation of tumor cells. Ki67 (proliferative marker) staining of sections of pancreata harvested from Ins-MycERTAM;RIP7-Bcl-xL mice treated with PCI-32765 only (left panel), with tamoxifen only (middle panel), or both (right panel). Lower inserts show higher-magnification images of Ki67-positive cells. Quantification of Ki67-positive cells is also provided for of each genotype and treatment condition.
PCI-32765 Does Not Compromise Glucose Tolerance
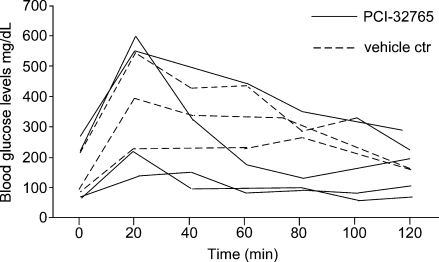
To ascertain whether the impact of PCI-32765 on β-cell tumors is a trivial consequence of general toxicity to pancreatic β cells, we measured physiological β-cell function in the presence and absence of compound. Mice were fasted for 24 hours, challenged with a single i.p. bolus of glucose, and blood glucose was then monitored thereafter every 20 minutes for 2 hours. Neither the efficiency nor the kinetics of response to glucose challenge was adversely affected by treatment with PCI-32765 (Figure 4). Moreover, sustained treatment (4 weeks) of normal mice with PCI-32765 led to no discernible loss of pancreatic β-cell function over time (data not shown). Hence, PCI-32765 has no deleterious impact on normal pancreatic β-cell function or viability.
Figure 4.
PCI-32765 does not compromise glucose tolerance. Mice previously subjected to 24-hour fasting were challenged by i.p. injection of a bolus of glucose (10 µl/g of 1 M stock solution), and their blood glucose levels were measured every 20 minutes during the ensuing 2 hours. Continuous lines represent mice pretreated for 3 days with PCI-32765, whereas broken lines depict vehicle-treated controls.
Sustained PCI-32765 Treatment Elicits Regression of Multifocal Insulinomas
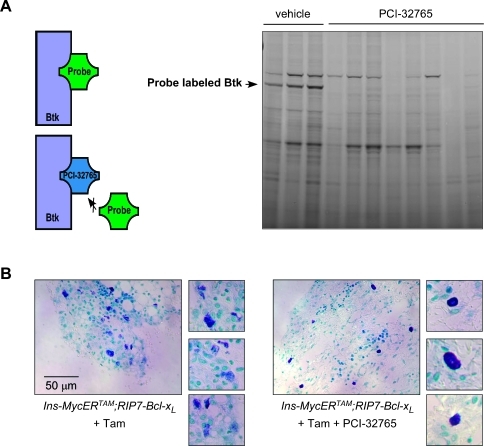
To model the impact of PCI-32765-based therapy on established β-cell tumors, we mimicked the gastrointestinal route of PCI-32765 administration adopted in ongoing PCI-32765 clinical trials. PCI-32765 was introduced into the drinking water to deliver a dose of 30 mg/kg per day. Ins-MycERTAM;RIP7-Bcl-xL mice were pretreated for 2 weeks with tamoxifen to induce β-cell tumors, and PCI-32765 (or vehicle control) was then administered orally (together with daily tamoxifen to maintain Myc activity) for a further 4 weeks. Using a fluorescently tagged PCI-32765 derivative (PCI-33380 [8]), we ascertained that this dose achieves complete Btk occupancy (Figure 5A). As we expected, although systemic inhibition of Btk had no effect on recruitment of mast cells to tumor stroma, it efficiently blocked their degranulation (13% ± 2.1% degranulating cells in PCI-32765-treated vs 87.2% ± 3.2% in vehicle-treated tumors; Figure 5B).
Figure 5.
Oral administration of PCI-32765 achieves complete Btk occupancy and systemically blockades mast cell degranulation. (A) Btk occupancy assay on splenocyte lysates derived from Ins-MycERTAM;RIP7-Bcl-xL mice treated with tamoxifen and either PCI-32765 or vehicle control. The black arrow indicates band relative to Btk. A schematic of the assay is shown on the left. (B) Toluidine blue staining of pancreatic stroma of Ins-MycERTAM;RIP7-Bcl-XL mice treated with tamoxifen only (left panels) or with both tamoxifen and PCI-32765 (right panels). The smaller panels on both sides show higher magnification of single mast cells.
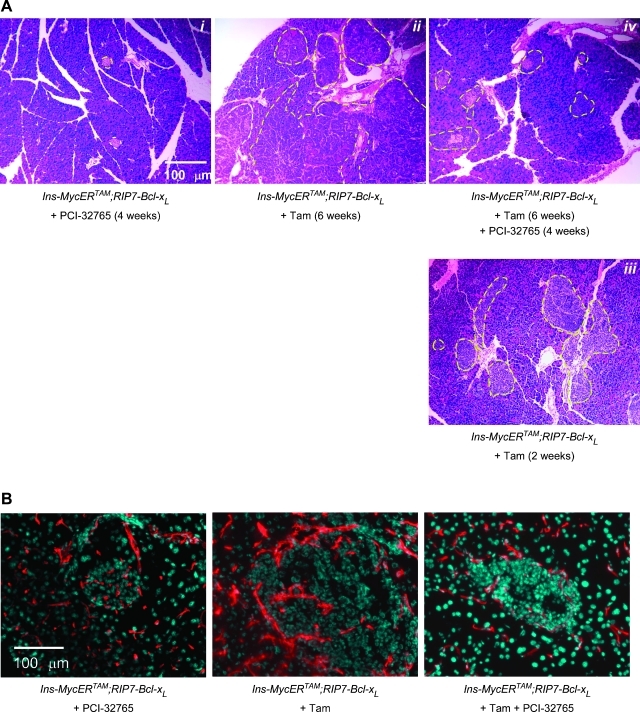
After 6 weeks of sustained Myc activation in non-PCI-32765-treated Ins-MycERTAM;RIP7-Bcl-xL mice, β-cell tumors attain a size (measured as area per section) that is 16.97 ± 4.3-fold that in normal islets (Figure 6A). By contrast, 4 weeks of cotreatment with PCI-32765 elicited a dramatic regression of all, shrinking the tumors to a mere 2.08 ± 0.4-fold relative to untreated islets (Figure 6A). Importantly, such regressed islet tumors remain phenotypically distinct from their normal counterparts, exhibiting ragged margins indicative of extensive cell turnover and remodeling. The regressed β-cell masses also exhibited a degenerate and rudimentary vasculature compared with control tumors not treated with PCI-32765 (Figure 6B). Blood vessels in the regressed PCI-32765-treated tumors seldom reached the inner parts of the islets, which frequently (∼18% of the total islets) exhibited voids and hemorrhage (Figure W2).
Figure 6.
Long-term treatment with PCI-32765 causes tumor regression. Tumor bearing mice (already treated with tamoxifen for 2 weeks) were treated with PCI-32765 or vehicle control in combination with a daily tamoxifen injection for 4 weeks. (A) H&E staining of sections of pancreata harvested from Ins-MycERTAM;RIP7-Bcl-xL mice treated with PCI-32765 only (i), with tamoxifen only (6 weeks in ii and 2 weeks in iii) or both (iv). (B) Meca32 staining (red) and Hoechst nuclear counterstaining (blue) of the same sections as described in A.
Discussion
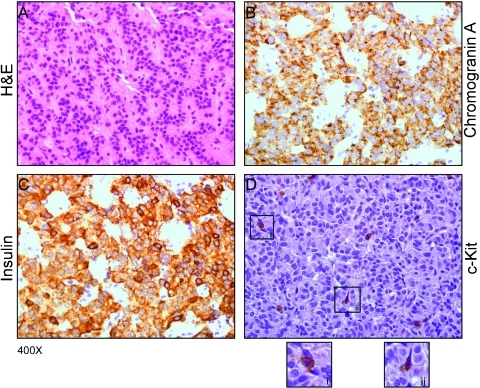
We previously reported in a mouse model of Myc-driven pancreatic β-cell tumorigenesis that tumor progression is causally linked, and continuously dependent on, infiltration of mast cells [7]. In their absence, Myc-driven β cells exhibit a transformed phenotype and markedly elevated proliferation rates yet are unable to expand into macroscopic tumors because of their inability to establish and maintain a microenvironment conducive to angiogenesis and macroscopic tumor growth. Moreover, systemic inhibition of mast cells with sodium cromoglycate, an inhibitor of mast cell degranulation, triggered degeneration of tumor vasculature, tumor hypoxia, and partial tumor regression [7]. Cromoglycate has very poor systemic bioavailability and is impractical for systemic use. Nonetheless, the partial tumor regression it induced suggested that efficient pharmacological inhibition of mast cell degranulation might be a therapeutically beneficial strategy in treating insulinoma and, potentially, other pancreatic tumors. Indeed, immunohistochemical analysis of human biopsies from patients affected by insulinoma shows that mast cells significantly infiltrate both tumors and tumor stromas (Figure 7). Intriguingly, recent observations that elevated mast cell infiltration in human pancreatic ductal adenocarcinoma correlates tightly with higher tumor grade and worse prognosis [14] intimate that mast cells may play an analogous causal role in exocrine pancreatic neoplasia.
Figure 7.
Human insulinomas exhibit significant mast cells infiltration. Biopsies from human tumors were analyzed by H&E (A) and immunohistochemistry using chromogranin A antibody (B), insulin antibody (C), and c-Kit antibody (D) to identify mast cells. Inserts (i) and (ii) show high-magnification images of degranulating mast cells.
Mast cell degranulation is critically dependent on the activity of Btk [15,16] for which a specific inhibitor, PCI-32765, has been optimized for oral availability, potency, selectivity, low toxicity, and capacity to sustain Btk inhibition in vivo [8,9]. PCI-32765 has been also recently shown to be efficacious in animal models of arthritis, lupus, and B-cell lymphoma [8] and is currently in clinical development for the treatment of B-cell non-Hodgkin lymphoma owing to the critical role Btk plays in B-cell receptor signal pathway. Given its ability to interfere also with mast cell function and its excellent drug-like properties, we tested the impact of PCI-32765 on growth and maintenance of insulinomas in our validated Ins-MycERTAM;RIP7-Bcl-xL preclinical mouse model [4,6,7,17]. PCI-32765 triggered a dramatic regression of tumors without affecting normal β-cell function or having any discernible adverse impact on overall health of mice. As predicted for an inhibitor of mast cell degranulation, PCI-32765 did not prevent recruitment of mast cells to tumors but, instead, blocked their degranulation once there. Intriguingly, PCI-32765 also inhibited proliferation of neoplastic β cells, although the mechanism underlying this additional therapeutic benefit is presently unclear.
Our data further support the emerging notion that mast cell inhibition is a novel and practical strategy for the treatment of insulinoma and potentially other pancreatic neoplastic diseases and suggests that PCI-32765 is as an effective systemic mast cell inhibitor in clinical assessment of this principle.
Supplementary Material
Acknowledgments
The authors thank Lisa Coussens for precious advice and reagents. The authors are indebted to Fanya Rostker for her technical assistance and to colleagues in the Evan Laboratory for their invaluable criticism and advice.
Abbreviations
- Btk
Bruton tyrosine kinase
- H&E
hematoxylin and eosin
Footnotes
This work was supported by grants from the National Cancer Institute (2R01 CA98018) and the Bear Necessities Pediatric Cancer Foundation.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. 2006;13:118–137. doi: 10.1159/000092969. [DOI] [PubMed] [Google Scholar]
- 4.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhou YP, Pena JC, Roe MW, Mittal A, Levisetti M, Baldwin AC, Pugh W, Ostrega D, Ahmed N, Bindokas VP, et al. Overexpression of Bcl-x(L) in β-cells prevents cell death but impairs mitochondrial signal for insulin secretion. Am J Physiol Endocrinol Metab. 2000;278:E340–E351. doi: 10.1152/ajpendo.2000.278.2.E340. [DOI] [PubMed] [Google Scholar]
- 6.Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1β. Genes Dev. 2006;20:2527–2538. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Mycinduced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 8.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengler PA, Burril LC, Mendonca RV, Sweeney MD, Scott KC, Grothaus PG, et al. Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem. 2007;2:58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 10.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 11.Setoguchi R, Kinashi T, Sagara H, Hirosawa K, Takatsu K. Defective degranulation and calcium mobilization of bone-marrow derived mast cells from Xid and Btk-deficient mice. Immunol Lett. 1998;64:109–118. doi: 10.1016/s0165-2478(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 12.Sainte-Laudy J, Boumediene A, Touraine F, Orsel I, Brianchon C, Bonnaud F, Cogne M. Use of both CD63 up regulation and IgE down regulation for the flow cytometric analysis of allergen induced basophil activation. Definition of an activation index. Inflamm Res. 2007;56:291–296. doi: 10.1007/s00011-007-7014-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 14.Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG, Dangi-Garimella S, Wang E, Munshi HG, Khazaie K, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res. 2006;16:2257–2265. doi: 10.1158/1078-0432.CCR-09-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, Maeda-Yamamoto M, Miura T, Han W, Hartman SE, et al. Involvement of Bruton's tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. Btk plays a crucial role in the amplification of Fc εRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor ER, Soucek L, Brown-Swigart L, Shchors K, Bialucha CU, Evan GI. Reversible kinetic analysis of Myc targets in vivo provides novel insights into Myc-mediated tumorigenesis. Cancer Res. 2006;66:4591–4601. doi: 10.1158/0008-5472.CAN-05-3826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.