Abstract
Background
Activated vitamin D analog, paricalcitol, has been shown to attenuate the development of cardiac hypertrophy in Dahl salt sensitive (DSS) rats. To determine whether an anti-hypertrophic effect is class specific, we tested if doxercalciferol (a pro-hormone vitamin D2 analog) could also attenuate the development of cardiac hypertrophy in DSS rats.
Methods and Results
Male DSS rats were fed a high salt (HS) diet for 6 weeks beginning at 6 weeks of age. Doxercalciferol was administered intraperitoneally (i.p.) at 150ng, 3 times a week (Mon, Wed, Fri) for six weeks. Pathological and echocardiographic findings demonstrated that rats on HS diet with doxercalciferol administration had significant decrease in cardiac hypertrophy and improved cardiac function compared to the HS + vehicle. In addition, there was a significant decrease in plasma brain natriuretic peptide (BNP) level and tissue atrial natriuretic factor (ANF) mRNA level with doxercalciferol treatment. Doxercalciferol also significantly reduced the level of protein kinase C-α (PKCα) suggesting that PKC-mediated cardiac hypertrophy may be associated with vitamin D deficiency.
Conclusions
Administration of doxercalciferol attenuated the development of HS diet induced cardiac hypertrophy and cardiac dysfunction in DSS rats.
Keywords: heart failure, high salt, Dahl rats, cardiac dysfunction
Introduction
Vitamin D deficiency has been shown to be associated with an increased incidence of left ventricular hypertrophy and congestive heart failure (CHF) [1]. Vitamin D deficiency and hyperparathyroidism are regularly found in patients with severe CHF [2], and there is an inverse relationship between plasma atrial natriuretic factor (ANF) levels and circulating levels of vitamin D [3]. The experimental evidences that suggest direct causal-effect relationship between vitamin D signaling and cardiac hypertrophy are also accumulating. Vitamin D receptor (VDR), 1α-HO, and 24-hydroxylase have been shown to be present in heart [4], the expression of VDR is upregulated during cardiac hypertrophy [4], and VDR has been shown to regulate both ANF and brain natriuretic peptide (BNP) expression by interacting with their promoters [4–6]. Vitamin D deficiency also has been shown to results in abnormalities in contraction and proliferation in cardiomyocytes in culture [7, 8], and endothelin-1-induced cardiac hypertrophy has been shown to be blocked by vitamin D treatment [9]. In addition, VDR knockout mice exhibit baseline cardiac hypertrophy, and activation of the renin angiotensin system [10–12]. These findings suggest that the key components required for a functional vitamin D-dependent signaling system are present in the heart, and that vitamin D signaling may have an anti-hypertrophic effect as well as a cardioprotective effect during the transition to heart failure.
We previously showed that administering activated vitamin D paricalcitol, an activated vitamin D3 analog, attenuated the development of cardiac hypertrophy and cardiac dysfunction in Dahl salt sensitive (DSS) rats which were fed a high salt (HS) diet [13]. In the present study, we examine whether anti-hypertrophic effects of vitamin D compounds can be applied to other forms of vitamin D analogs. Specifically, we examined if doxercalciferol (1α-hydroxyvitamin D2), a vitamin D2 pro-hormone that needs to be converted to an activated form, possesses similar anti-hypertrophic effects in the DSS rat model.
Methods
Animal model
Male DSS rats (Harlan Sprague–Dawley) were bred and fed a normal diet to 6 weeks of age. To generate cardiac hypertrophy, animals were fed a HS diet (6% NaCl) for the following 6 weeks as described previously [13–15]. To study the effects of doxercalciferol, the animals were divided into four groups: (i) normal diet (ND) + vehicle (V) (ND+V), (ii) ND + doxercalciferol (150 ng i.p. three times per wk) (ND+D), (iii) HS diet + V (HS+V), and (iv) HS diet + doxercalciferol (150 ng i.p. three times per wk) (HS+D). Doxercalciferol was prepared with 95% propylene glycol / 5% ethyl alcohol solution and injected i.p. 3 days before the initiation of HS diet and continued at 150 ng three times per week (Monday, Wednesday, and Friday) thereafter. Controls were age-matched male DSS rats fed normal chow receiving an equal volume of the vehicle three times per week for 6 weeks starting at 6 weeks of age. After 6 weeks of normal diet or HS diet, the rats were sacrificed for various analyses. The Institutional Animal Care and Use Committee (IACUC) of the Beth Israel Deaconess Medical Center approved this study.
Echocardiography
Echocardiography was performed under isoflurane anesthesia as described previously [13, 14]. An Agilent Sonos 5500 sector scanner equipped with a 7.5-MHz phased-array transducer was used to record 2D-guided M-mode tracings. The leading-edge method was used to determine anterior and posterior wall thickness and LV internal dimensions. LV mass was calculated using the corrected American Society of Echocardiography (ASE) simplified cubed equation – LV mass (grams) = 0.8 [1.05 (LVDd+AWd+PWd)3 – (LVDd)3].
Serum BNP measurement
Tail vein blood was collected at the time of sacrifice. Serum was collected by centrifuging 1.5 Eppendorf tubes containing blood at 13,000 rpm for 10 minutes and saving the supernatant. Serum BNP was measured by using BNP ELISA kit (Assaypro, St. Charles, MO) according to the manufacturer’s instructions.
Reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from rat ventricle tissue by using TRIzol (GIBCO–BRL, Gaithersburg, MD). RT-PCR was performed by PCR amplification of cDNA reverse-transcribed from mRNA by using rat ANF primers: 5’-TGG GCT CCT TCT CCA TCA CC-3’ and 5’-GCC AAA AGG CCA GGA AGA GG-3’.
Histology
Hearts were fixed in 10% formalin and paraffin-embedded. Sections were stained with hematoxylin and eosin (H&E) and Masson-Trichrome at the Histology Core facility at Beth Israel Deaconess Medical Center. The measurement of the cross-sectional area of cardiomyocytes and fibrosis was quantified at ×20 using a calibrated digital camera and software (DP 70 and DPController; Olympus America, Irving, TX) to survey entire heart sections. Relative size was quantified by measuring cardiomyocyte diameter, calculated as the distance between the two plasma membranes of a cell in longitudinal section. The measurement was done using ImageJ software. Data were obtained from >100 cells randomly selected from 3–5 microscopic fields of left ventricle slides. Areas of fibrosis were scored as as described previously [16].
Membrane fractionation and Immunoblot
The membrane fractions from heart tissues were obtained as described previously [16]. Briefly, the heart tissues were homogenized in ice-cold buffer A (20mM Tris·HCl, pH 7.5/2 mM EDTA/0.5 mM EGTA/1 mM phenylmethlysulfonyl fluoride/1 mM DTT/0.3 M sucrose/25 µg/ml leupeptin) with a Polytron electric homogenizer for 20 sec and then with a Dounce homogenizer (60 strokes). The homogenates were centrifuged at 2500 rpm for 10 min at 4°C, and the supernatant was ultracentrifuged at 40,000 rpm for 30 min at 4°C. The resulting supernatant was retained as the cytosolic fraction and the pellets were resuspended with buffer B (buffer A without sucrose) and solubilized with 1% Triton X-100. After rotating for 45 min at 4°C, soluble membrane fractions were obtained by ultracentrifugation at 40,000 rpm for 30 min. The membrane pellet was resuspended in buffer B. Immunoblotting was performed as described previously [16, 17]. Protein concentration of whole heart tissue lysate was determined by the Bradford method. Antibodies against Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling, Danvers, MA) and Na+/Ca2+ Exchanger (NCX) (Thermo Fisher Scientific, Rockford, IL) were used as cytosolic and membrane fraction loading controls, respectively.
Statistical Analysis
Data were expressed as means ±SEM. Comparisons between and within groups were conducted with unpaired Student t tests and repeated-measures ANOVA, respectively. P values of <0.05 were considered statistically significant.
Results
Doxercalciferol attenuates cardiac hypertrophy and cardiac dysfunction
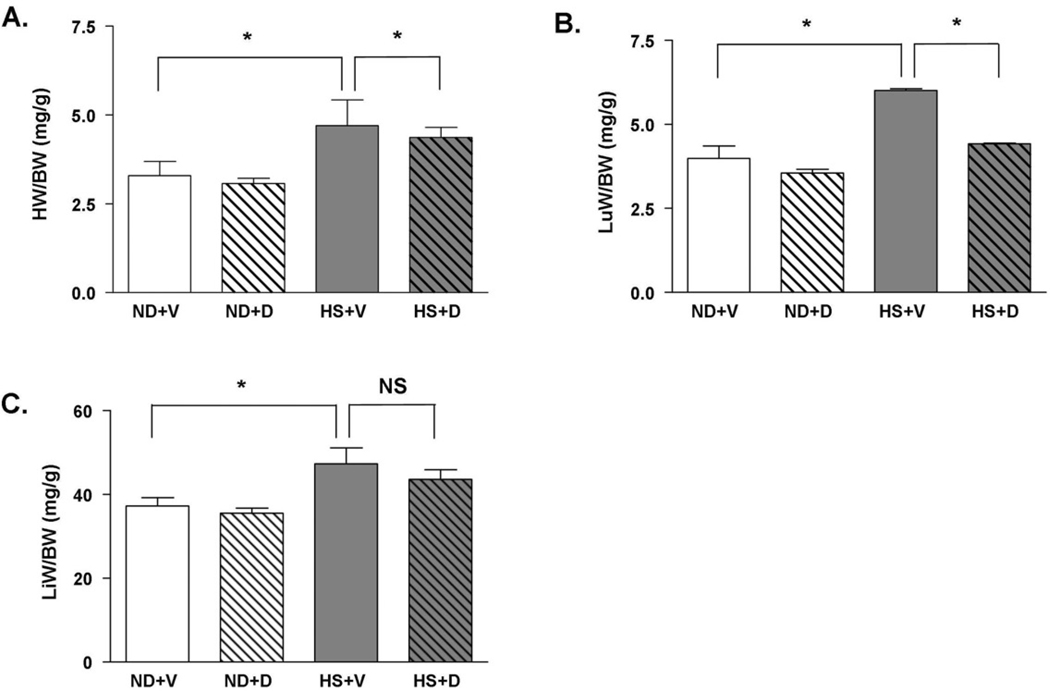
There was no significant difference in organ weights between rats fed normal diet with or without doxercalciferol, demonstrating that doxercalciferol did not have a significant effect on normal cardiac structure. The HS diet resulted in a significant increase in heart weight (HW) to body weight (BW), lung weight (LuW) to BW and liver weight (LiW) to BW ratios suggesting cardiac hypertrophy, left-sided heart failure, and right-sided heart failure, respectively (Figure 1A – 1C). In HS fed rats, doxercalciferol treatment resulted in 11% reduction in HW/BW and 29% reduction in LuW/BW ratios compared to the vehicle treated group. There was a modest but not significant decrease in LiW/BW ratio in the doxercalciferol treated group.
Figure 1.
Effect of doxercalciferol therapy in attenuating the development of cardiac hypertrophy. In HS animals, doxercalciferol treatment resulted in a significant prevention of HS-induced increase in (A) HW/BW and (B) LuW/BW ratios. (C) LiW/BW ratio did not significantly change. HW=heart weight, LuW=lung weight, LiW=liver weight, BW=body weight, ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol. *p<0.05, NS=not significant. N=6–9.
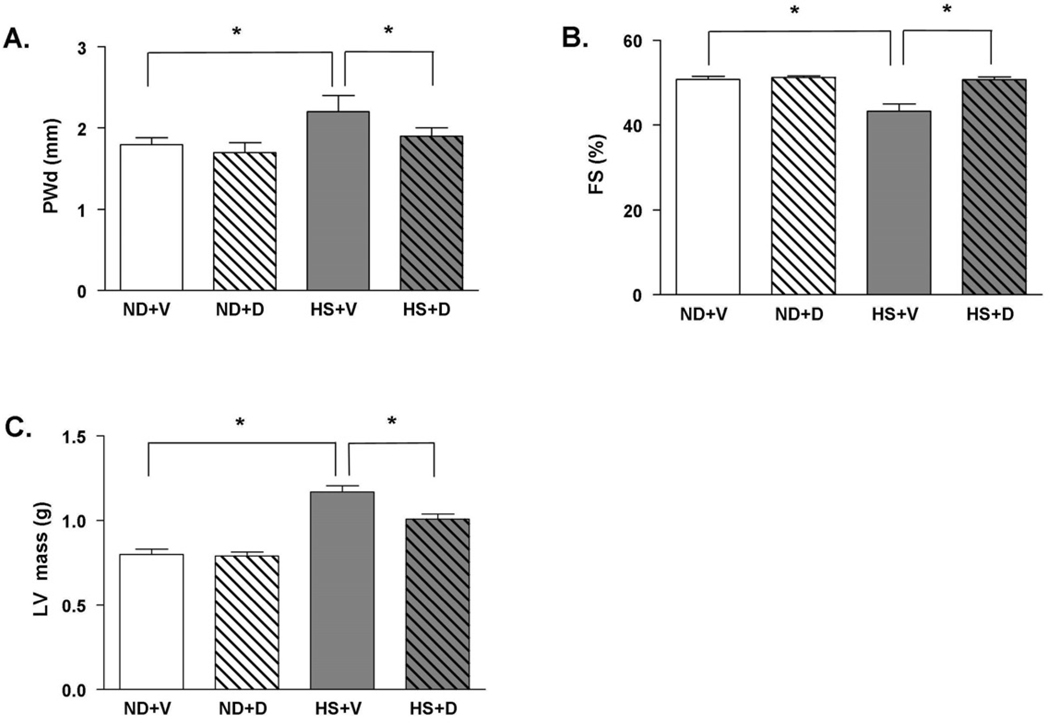
To further examine the effect of doxercalciferol on development of cardiac hypertrophy and cardiac dysfunction, we next examined cardiac function in DSS rats with M-mode echocardiograms. In the HS + V group, the development of cardiac hypertrophy was evident by a significant increase in diastolic left ventricle posterior wall thickness (LVPWd) (Figure 2A). There was also a significant decrease in fractional shortening in the HS + V group compared to the ND groups suggesting the development of cardiac dysfunction in HS + V group. Doxercalciferol treatment markedly decreases posterior wall thickness by 13% and improves fractional shortening by 17% (Figure 2A – 2B). In addition, calculated LV mass using echocardiogram parameters was significantly increased in the HS + V group compared to the ND groups; doxercalciferol therapy significantly attenuated the increase in LV mass (Figure 2C). These findings suggest that doxercalciferol treatment significantly attenuates cardiac hypertrophy and improves cardiac dysfunction in the HS diet-induced model of cardiac hypertrophy and cardiac dysfunction.
Figure 2.
Echocardiographic parameters in DSS rats with or without doxercalciferol. (A) Doxercalciferol treated animals showed a significant reduction in PWd, (B) as well as a marked improvement in fractional shortening (FS) compared to the vehicle group. (C) Left ventricular (LV) mass is also significantly reduced with doxercalciferol treatment. PWd=posterior wall thickness in diastole, ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol. *p<0.05. N=6–9.
Doxercalciferol attenuates biochemical markers of cardiac hypertrophy and cardiac dysfunction
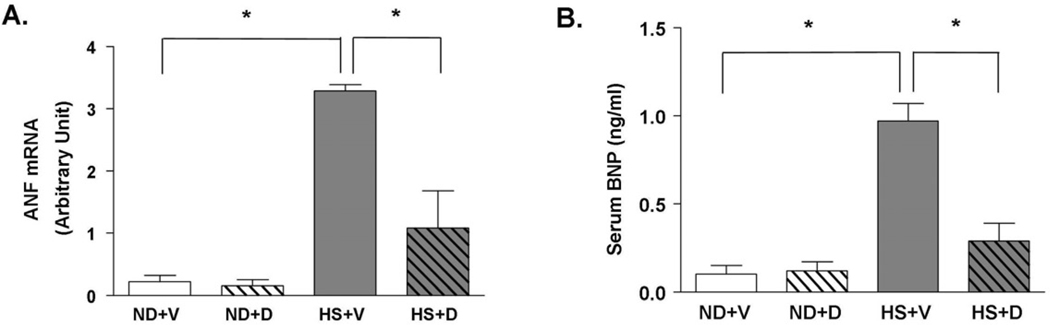
To confirm the pathological findings, we examined the level of serum BNP level and LV tissue mRNA expression of ANF, which are reliable markers of cardiac hypertrophy. There was a significant increase in serum BNP and tissue ANF mRNA levels in the HS + V compared to the ND groups (Figure 3). Treatment with doxercalciferol in the context of a HS diet significantly decreased serum BNP and tissue ANF mRNA levels compared to the vehicle treated group although not to the ND baseline level. These biochemical analyses confirm our pathological findings that doxercalciferol treatment significantly attenuates cardiac hypertrophy and cardiac dysfunction.
Figure 3.
Effect of doxercalciferol on biochemical markers of cardiac hypertrophy and cardiac dysfucntion. (A) Serum BNP level was significantly attenuated in doxercalciferol treated group than in vehicle treated group. (B) LV tissue ANF mRNA level was also reduced with doxercalciferol treatment. Serum BNP and tissue ANF levels were determined using ELISA and qRT-PCR, respectively. ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol. *p<0.05, N=6–9.
Vitamin D promotes calcium absorption in intestine and kidney to increase serum calcium level, a most common side effect of vitamin D therapy. To determine if there is a significant increase in serum calcium level after doxercalciferol treatment, we measured serum calcium levels after different doses of doxercalciferol treatments. At the dose used in the study (150 ng/rat three times a week), there was no significant change in serum calcium levels between vehicle and doxercalciferol treated animals (Table 1). There was a modest but non-significant increase in serum calcium in rats treated with a higher dose of doxercalciferol at 250 ng/rat administered three times a week.
Table 1.
Doxercalciferol treatment, even at high doses, did not significantly alter serum [Ca2+]. HS + doxercalciferol 150 =high salt diet + doxercalciferol (150ng/rat 3×/wk), HS + doxercalciferol 250 =high salt diet + doxercalciferol (250ng/rat 3×/wk). NS= not significant.
| GROUP | [Ca2+] (mg/dL) | P value |
|---|---|---|
| ND + vehicle | 10.2 ± 0.7 | NS |
| ND + doxercalciferol 150 | 11.2 ± 1.4 | NS |
| HS + vehicle | 11.4 ± 1.0 | NS |
| HS + doxercalciferol 150 | 10.8 ± 2.5 | NS |
| HS + doxercalciferol l250 | 12.1 ± 4.0 | NS |
Increased heart weight is due to increased size of cardiomyocytes
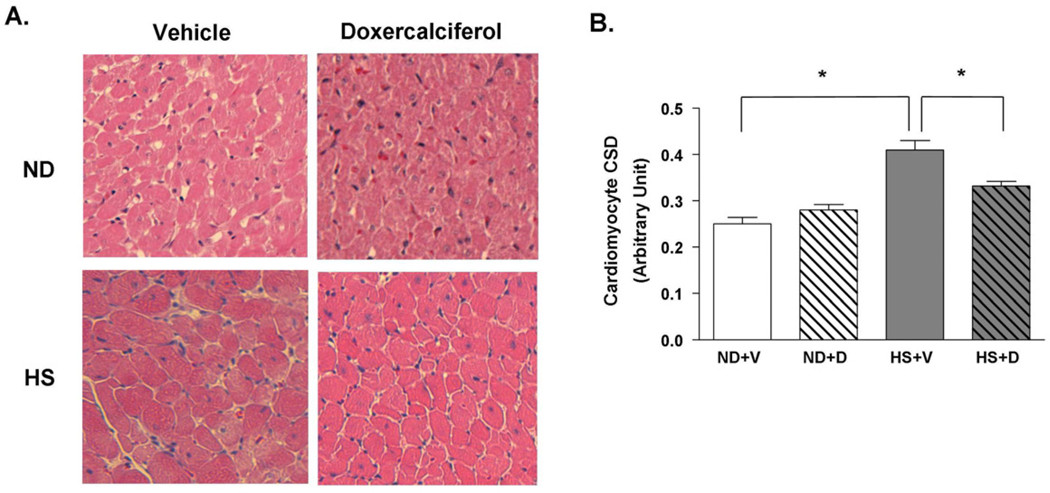
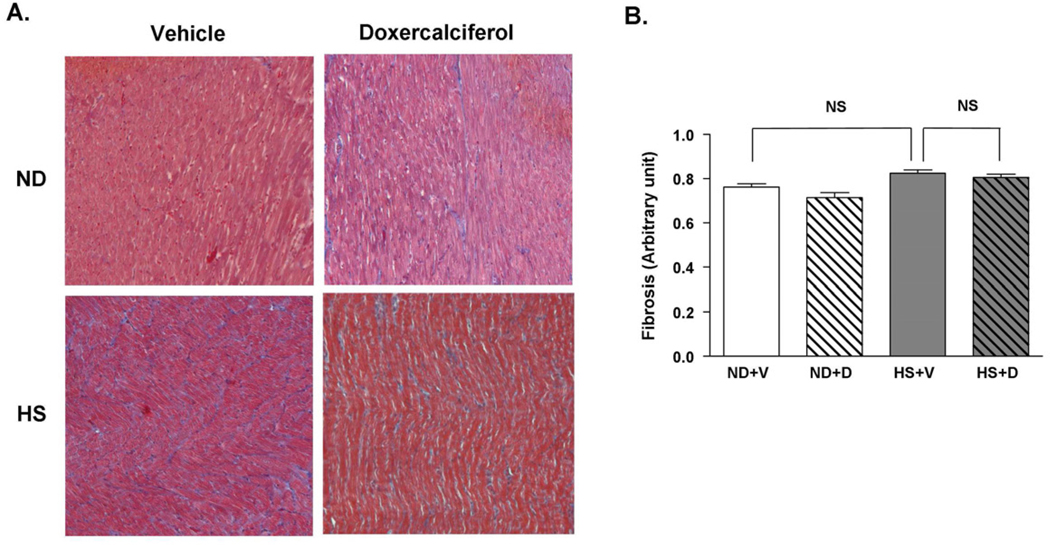
We further evaluated the presence of cardiac hypertrophy using histological analysis. The cross sectional diameter of individual cardiomyocytes showed a 65% increase in the HS + V group compared to the ND groups (Figure 4A and 4B). The increase in cardiomyocyte size due to the HS diet was mitigated after 6 weeks of doxercalciferol treatment, resulting in a 22% reduction in cell size compared to the HS + V group. In addition, we analyzed the presence of cardiac fibrosis since increase in heart weight could be due to increased interstitial fibrosis. There was no significant difference in myocardial fibrosis between ND and HS diet groups, which is consistent with our previous data when DSS rats were fed HS diet for 6 weeks [13]. Administration of doxercalciferol did not alter the amount of fibrosis in the heart (Figure 5A and 5B). These findings demonstrate that doxercalciferol treatment resulted in specific decrease in cardiomyocyte size, and the increase in heart weight by the HS diet was due to an increase in cardiomyocyte hypertrophy and not due to significant increased interstitial fibrosis in this model.
Figure 4.
Histological analysis of cardiomyocyte hypertrophy with H&E staining in DSS rats with or without doxercalciferol. (A) HS diet leads to cardiomyocyte hypertrophy, whereas doxercalciferol attenuates this response. (B) More than 100 randomly selected cells in 3–5 fields at 20× objective per animal were imaged, and the cross-sectional dimension (CSD) of cardiomyocytes was measured by using ImageJ program. ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol. *p<0.05, N=6–9.
Figure 5.
Histological analysis of cardiac fibrosis in DSS rats with or without doxercalciferol. (A) Representative Masson’s trichrome staining of the heart sections. (B) There was no significant difference in cardiac fibrosis in all experimental groups. 25–30 fields at ×10 objective per animal were measured. Fields with >5% fibrosis area were counted and divided by the total number of fields observed. ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol *p<0.05, N=6–9.
Doxercalciferol blocked protein kinase C-α (PKCα) activation induced by a high salt diet
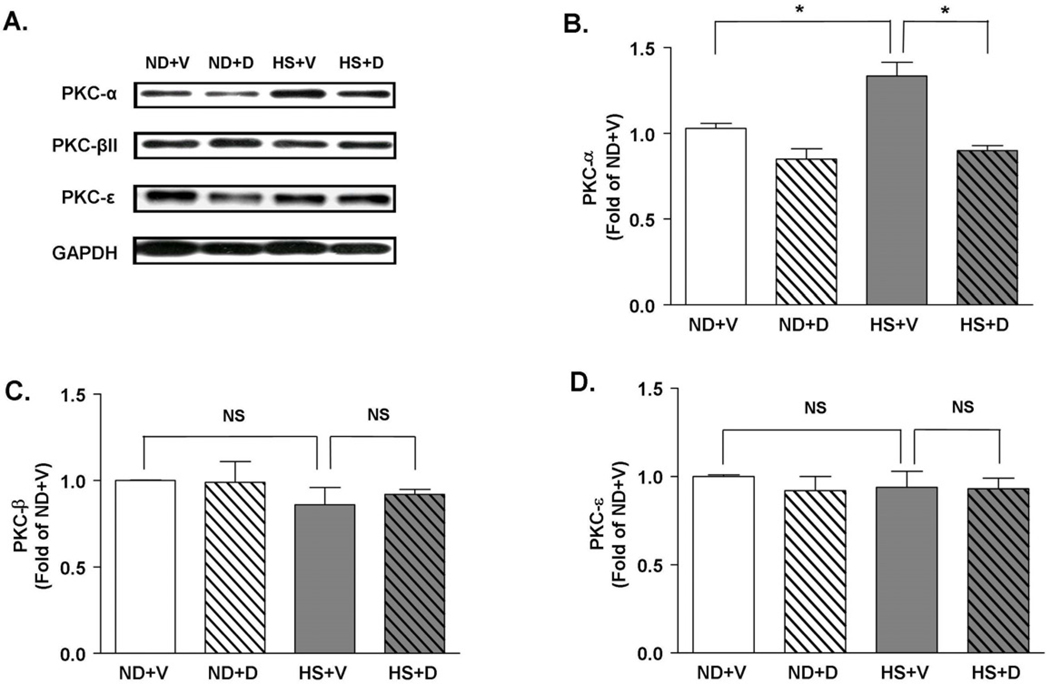
PKC signaling has been shown to mediate hypertrophic signaling in heart. To analyze whether PKC signaling is involved in vitamin D’s anti-hypertrophic effect, we analyzed the level of various PKC isoforms in heart. We found that PKCα protein was significantly increased in the heart of rats fed 6 weeks of high salt diet (Figure 6A and 6B). In comparison, PKCβII and PKCε were not significantly altered after 6 weeks of HS diet compared to the ND groups (Figure 6A, 6C and 6D). Doxercalciferol treatment significantly blocked the increase in PKCα level compared to the vehicle treated group suggesting the potential anti-hypertrophic mechanism of doxercalciferol. Expression level of PKCβII and PKCε did not change with doxercalciferol.
Figure 6.
Expression of PKC isoforms. (A) Representative immunoblots of PKCα, PKCβII and PKCε. GAPDH was used as an internal control. (B) HS diet caused a 1.5 fold increase in PKCα level, which was attenuated by doxercalciferol treatment. HS diet and doxercalciferol therapy did not significantly alter the expression levels of either (C) PKCβII or (D) PKCε. The intensity of each band was quantified by ImageJ software. The arbitrary values were calculated by dividing the intensity level of each PKC band by the intensity level of a corresponding GAPDH band. Measurements were represented in fold of ND+V level. ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol. *p<0.05, N=6–9.
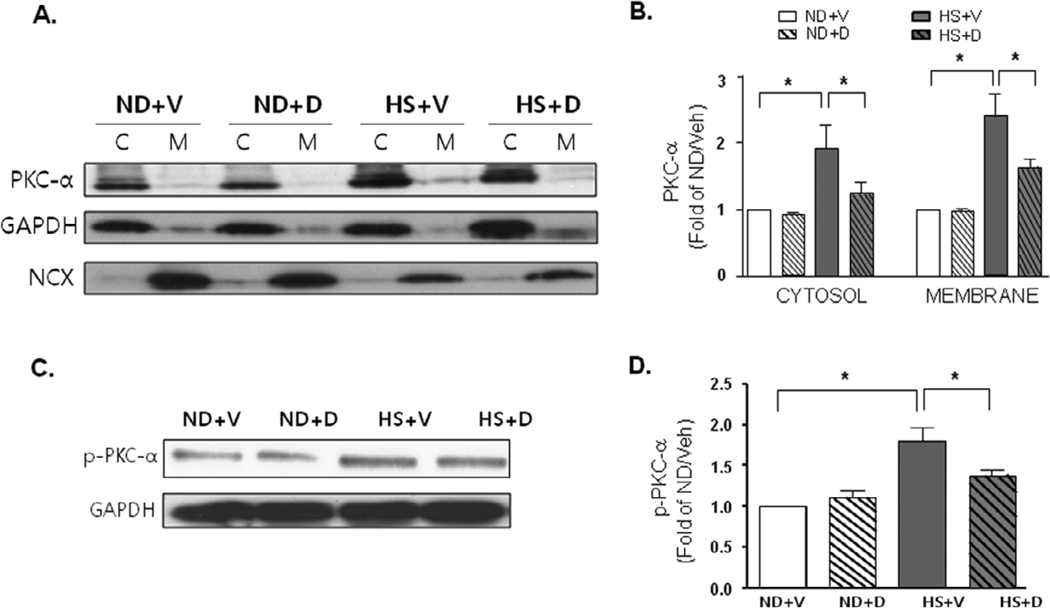
To demonstrate if there is truly an activation of PKCα, we examined the membrane translocation of PKCα, as well as the increase in the expression of phosphorylated PKCα (p-PKCα). We found that PKCα showed significant membrane translocation with 6 weeks of HS diet compared to the ND groups (Figure 7A and 7B). There was also significant increase in p-PKCα with 6 weeks of HS diet compared to the ND groups (Figure 7C and 7D). Doxercalciferol treatment significantly blocked the membrane translocation of PKCα and increase in p- PKCα expression compared to the vehicle treated group suggesting the potential anti-hypertrophic mechanism of doxercalciferol.
Figure 7.
Activation of PKCα. (A) Representative immunoblots of cytosolic and membrane fraction expression of PKCα. GAPDH and NCX were used as cytosolic and membrane fraction loading controls, respectively. (B) Quantitative analyses of cytosolic and membrane fraction expression of PKCα. Measurements were represented in fold of ND+V level. ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol. *p<0.05, N=4–6. (C) Representative immunoblots of phosphorylated PKCα expression. (B) Quantitative analyses of phosphorylated PKCα expression. Measurements were represented in fold of ND+V level. ND=normal diet, HS=high salt diet, V=vehicle, D=doxercalciferol. *p<0.05, N=4–6.
Discussion
Our study showed that administration of doxercalciferol three times a week reduced cardiac hypertrophy due to high salt diet in DSS rats. There was also evidence of improvement in cardiac function in this model with 6 weeks of doxercalciferol treatment as found by improved fractional shortening and attenuation of biochemical evidence of cardiac hypertrophy and cardiac dysfunction, such as increase in serum BNP and tissue ANF mRNA levels. The current findings and our previous finding, which showed a similar degree of anti-hypertrophic effects with paricalcitol, suggest that the anti-hypertrophic effects of vitamin D are not limited to one specific class of vitamin D formulation. In addition, we found that the activation of PKCα signaling was significantly attenuated by doxercalciferol treatment, suggesting the role of PKC signaling is a vitamin D-mediated anti-hypertrophic effect.
Clinically, the relationship between vitamin D deficiency and cardiovascular diseases are strongest in renal failure patients. Since the critical conversion of the storage form to the active form of vitamin D by 1α-hydroxylase (1α-HO) occurs in the kidney, patients with kidney failure are typically vitamin D deficient [18, 19]. In fact, in patients with end stage renal disease, the prevalence of left ventricular hypertrophy and diastolic dysfunction is over 80%, and the rate of cardiovascular-related mortality is 10–20 times higher than in the general population [20, 21]. In a seminal clinical observational study, hemodialysis patients treated with vitamin D showed an improved survival [22] associated with a decrease in cardiovascular mortality [23]. These data suggest that vitamin D deficiency may be a contributing factor in the pathogenesis of CHF.
The two main sources of vitamin D are dietary foods and endogenous production. Vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) usually come from plant and animal sources, respectively [24]. Vitamin D3 is synthesized endogenously, in the skin, by the photochemical conversion of 7-dehydrocholesterol. It then undergoes hydroxylation in the liver to form 25-dihydroxyvitamin D3 (25(OH)VitD3), the main circulating storage form of vitamin D [24]. The conversion of 25(OH)VitD3 to the hormonally active form, 1,25-dihydroxyvitamin D3 (1,25(OH)2VitD3) by 1α-hydroxylase (1α-HO) occurs mainly in the kidneys. Currently, there are two vitamin D analogs that are commercially available in U.S.: paricalcitol (Zemplar®) and doxercalciferol (Hectorol®). It appears that both forms of vitamin D have a beneficial effect on preventing cardiac hypertrophy and cardiac dysfunction, at least, in animal models of cardiac hypertrophy.
Since vitamin D helps the body absorb calcium ions from the intestine and increase the renal calcium reabsorption rate, its analogues are often used to treat secondary hyperparathyroidism. However, a high level of circulating vitamin D level can be toxic by causing hypercalcemia and, therefore, it is crucial to check whether the drug therapy elicits a rise in calcium level. By measuring the calcium levels in all groups, we observed that there is no significant alteration in serum calcium level after doxercalciferol treatment, even at high doses. These data suggest that doxercalciferol therapy is as effective as paricalcitol therapy in preventing the development of left ventricular hypertrophy and further bolsters the evidence of vitamin D’s protective role in cardiovascular diseases.
Hypertrophic signaling pathway via PKC, particularly via the conventional PKC isoforms (α, β, γ) that are characterized by their requirement for calcium for activation, has been suggested to play an important role in heart [25]. Supporting this notion, PKCα is elevated during pathologic cardiac hypertrophy [26, 27], and the activation of PKCα is both necessary and sufficient to induce cardiomyocyte hypertrophy [28]. Transgenic mice with cardiac specific overexpression of PKCα results in cardiac hypertrophy and cardiac dysfunction by alterations in calcium homeostasis in transgenic mice [29, 30]. These findings suggest that PKCα is an important signaling molecule that regulates cardiac function by sensing intracellular calcium. In our study, PKCα protein expression, but not PKCβ2 or PKCε, were significantly elevated in high salt fed rats. However, other reports are somewhat conflicting. Inagaki et al. reported that PKCβ and PKCε, but not PKCα, are increased during cardiac hypertrophy in DSS rats [31]. In contrast, Koide et al. reported that all three isoforms, PKCα, PKCβ, and PKCε, were increased after HS diet [26]. We believe that further studies using specific PKC isoform inhibitors or genetically altered PKC isoform mice need to be done to further clarify whether specific PKC isoforms are involved in cardiac hypertrophy and more importantly, whether inhibition of specific PKC isoform will be beneficial in the development of cardiac hypertrophy and heart failure.
This study, in conjunction with our earlier study with paricalcitol [13], is important because it showed that administration of different forms of vitamin D compounds can attenuate the development of cardiac hypertrophy and cardiac dysfunction in animal models. Further studies are needed to confirm whether this finding in animal model can translate directly into the clinical setting.
Acknowledgments
Disclosures
The study was supported in part by Genzyme Corporation, which employs Cynthia Arbeeny. Drs. Peter M. Kang and Ravi Thadhani received research funding and honoraria from Genezyme Corporation. Doxercalciferol (Brand name Hectorol®) is marketed by Genzyme Corporation.
Research Support: The study was supported in part by the grants from National Institutes of Health RO1 HL091998 and Genzyme Corporation (PMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Lee W, Kang PM. Vitamin D deficiency and cardiovascular disease: Is there a role for vitamin D therapy in heart failure? Curr Opin Investig Drugs. 2010;11:309–314. [PubMed] [Google Scholar]
- 2.Shane E, Mancini D, Aaronson K, Silverberg SJ, Seibel MJ, Addesso V, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197–207. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 3.Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens. 1990;3:903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, et al. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–1112. doi: 10.1161/HYPERTENSIONAHA.108.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)(2)-vitamin D(3) actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103:533–537. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Wu J, Hsieh JC, Whitfield GK, Jurutka PW, Haussler MR, et al. Suppression of ANP gene transcription by liganded vitamin D receptor: involvement of specific receptor domains. Hypertension. 1998;31:1338–1342. doi: 10.1161/01.hyp.31.6.1338. [DOI] [PubMed] [Google Scholar]
- 7.O'Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272:H1751–H1758. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 8.O'Connell TD, Weishaar RE, Simpson RU. Regulation of myosin isozyme expression by vitamin D3 deficiency and 1,25-dihydroxyvitamin D3 in the rat heart. Endocrinology. 1994;134:899–905. doi: 10.1210/endo.134.2.8299585. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 11.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–524. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H72–H80. doi: 10.1152/ajpheart.00556.2003. [DOI] [PubMed] [Google Scholar]
- 15.Siu PM, Bae S, Bodyak N, Rigor DL, Kang PM. Response of caspase-independent apoptotic factors to high salt diet-induced heart failure. J Mol Cell Cardiol. 2007;42:678–686. doi: 10.1016/j.yjmcc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigor DL, Bodyak N, Bae S, Choi JH, Zhang L, Ter-Ovanesyan D, et al. Phosphoinositide 3-kinase Akt signaling pathway interacts with protein kinase Cbeta2 in the regulation of physiologic developmental hypertrophy and heart function. Am J Physiol Heart Circ Physiol. 2009;296:H566–H572. doi: 10.1152/ajpheart.00562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhury S, Bae S, Kumar SR, Ke Q, Yalamarti B, Choi JH, et al. Role of AIF in cardiac apoptosis in hypertrophic cardiomyocytes from Dahl salt-sensitive rats. Cardiovasc Res. 2010;85:28–37. doi: 10.1093/cvr/cvp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley RN. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial. 2003;16:111–117. doi: 10.1046/j.1525-139x.2003.160271.x. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 20.US Renal Data Systems. Causes of death in ESRD. Am J Kidney Dis. 1999;34:S87–S94. doi: 10.1053/AJKD034s00087. [DOI] [PubMed] [Google Scholar]
- 21.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 22.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 23.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 24.Dusso AS, Brown AJ, Slatopolsky E, Vitamin D. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 25.Sabri A, Steinberg SF. Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol Cell Biochem. 2003;251:97–101. [PubMed] [Google Scholar]
- 26.Koide Y, Tamura K, Suzuki A, Kitamura K, Yokoyama K, Hashimoto T, et al. Differential induction of protein kinase C isoforms at the cardiac hypertrophy stage and congestive heart failure stage in Dahl salt-sensitive rats. Hypertens Res. 2003;26:421–426. doi: 10.1291/hypres.26.421. [DOI] [PubMed] [Google Scholar]
- 27.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 28.Vijayan K, Szotek EL, Martin JL, Samarel AM. Protein kinase C-alpha-induced hypertrophy of neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H2777–H2789. doi: 10.1152/ajpheart.00171.2004. [DOI] [PubMed] [Google Scholar]
- 29.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca(2+)-dependent transgenic model of cardiac hypertrophy: A role for protein kinase Calpha. Circulation. 2001;103:140–147. doi: 10.1161/01.cir.103.1.140. [DOI] [PubMed] [Google Scholar]
- 30.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 31.Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, et al. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol. 2002;34:1377–1385. doi: 10.1006/jmcc.2002.2089. [DOI] [PubMed] [Google Scholar]