Abstract
Climate change affects plants in natural and agricultural ecosystems throughout the world but little work has been done on the effects of climate change on plant disease epidemics. To illustrate such effects, a weather-based disease forecasting model was combined with a climate change model predicting UK temperature and rainfall under high- and low-carbon emissions for the 2020s and 2050s. Multi-site data collected over a 15-year period were used to develop and validate a weather-based model forecasting severity of phoma stem canker epidemics on oilseed rape across the UK. This was combined with climate change scenarios to predict that epidemics will not only increase in severity but also spread northwards by the 2020s. These results provide a stimulus to develop models to predict the effects of climate change on other plant diseases, especially in delicately balanced agricultural or natural ecosystems. Such predictions can be used to guide policy and practice in adapting to effects of climate change on food security and wildlife.
Keywords: climate change scenarios, Leptosphaeria maculans, phoma stem canker, plant disease epidemiology, weather-based disease forecasts
1. Introduction
Climate change is affecting plants in natural and agricultural ecosystems throughout the world (Stern 2007). However, little work has been done to model the effects of predicted twenty-first-century climate change on plant disease epidemics (Garrett et al. 2006). Changing weather (e.g. temperature, rainfall) can induce severe plant disease epidemics (Coakley et al. 1999; Chakraborty 2005), which threaten food security if they affect staple crops (Luo et al. 1995; Chakraborty et al. 2000; Anderson et al. 2004) and can damage landscapes if they affect amenity species (Brasier & Scott 1994; Bergot et al. 2004). Severity of human, animal and plant disease epidemics is greatly affected by climatic factors, especially temperature and rainfall (Wint et al. 2002; Fitt et al. 2006a; Thomson et al. 2006; Bosch et al. 2007). Therefore, weather-based forecasts have been developed to guide control strategies for many important diseases worldwide (Wint et al. 2002; Garrett et al. 2006; Thomson et al. 2006). There is now an opportunity to link weather-based plant disease forecasts with recent climate change models, to predict the effects of climate change scenarios on the distribution and severity of plant disease epidemics.
Much discussion on the impact of climate change on plant disease epidemics has used qualitative, rule-based reasoning, which cannot easily accommodate the complex host–pathogen–environment interactions involved (Coakley et al. 1999; Anderson et al. 2004). Modelling approaches have included those matching existing climates in one region with climates predicted for another (Brasier & Scott 1994) or combinations of simulation models for crop growth and disease development (Luo et al. 1995). Before 1999, no work had used predicted climate variables generated by new more sophisticated general circulation models (GCMs); most studies had relied on predictions of fixed changes in temperature and rainfall (Coakley et al. 1999). Recently, GCMs have been used to predict the increase in range of Phytophthora cinnamomi (Bergot et al. 2004). Whereas empirical modelling has been used to produce disease epidemic models for combining with climate change predictions (Chakraborty et al. 1998; Coakley et al. 1999), few models have been based on datasets including both regional and seasonal variations that are sufficiently extensive to allow both model development and validation.
Phoma stem canker (blackleg, Leptosphaeria maculans) is an internationally important disease of wild and cultivated brassicas; during each growing season, it causes yield losses of millions of tonnes of brassica oilseed and vegetable crops in Europe, North America, Australia and Africa (Fitt et al. 2006b). It has spread across North America and eastern Europe in the last 20 years and now threatens 10 Mha of highly susceptible oilseed and vegetable brassicas in China, mostly grown by subsistence farmers. Temperature and rainfall affect not only the development of the pathogen (Huang et al. 2005) but also the resistance response of the host (Huang et al. 2006). Globally, the most severe epidemics occur in oilseed rape (Brassica napus) growing areas of Australia, with their Mediterranean climate, where susceptible crops can be destroyed by the disease (Howlett et al. 2001; Sprague et al. 2006). However, much of the world's oilseed rape crop is grown in cooler climates. In the UK, the most severe phoma stem canker epidemics occur in southern England; in Scotland, where the climate is colder, phoma leaf spotting does occur but damaging phoma stem cankers do not subsequently develop. To illustrate the effects of predicted climate change on the range and severity of plant disease epidemics, weather-based models predicting the development of phoma stem canker epidemics were combined with climate change models to generate scenarios for the future severity of epidemics in the UK.
2. Phoma stem canker model development and validation
A weather-based model to describe the development of phoma stem canker epidemics was constructed, using disease and weather data from 40 winter oilseed rape field experiments done under different weather conditions in growing seasons between 1992–1993 and 2001–2002 at a wide range of UK sites, each with one to three cultivars (electronic supplementary material, table 1). The development of a phoma stem canker epidemic was considered in three stages. In the first stage, the date when phoma leaf spot epidemics start in autumn was predicted from the preceding summer weather data. Since phoma stem canker is a monocyclic disease (one cycle per growing season), the date in autumn when leaf spotting starts (Dl; table 1; figure 1d) is a crucial factor affecting the severity of phoma stem canker epidemics on stems the following summer (West et al. 2001). The date when phoma leaf spotting starts in autumn (Dl) was estimated from weekly records of disease incidence (percentage of plants affected) between the end of September and mid-December on 25 randomly selected plants per crop (40 datasets for different cultivars in different seasons, untreated with fungicides), using linear interpolation between the two assessment dates with disease incidences that were above and below the 10% incidence threshold. Multiple linear regression was used to examine the relationship between the start of phoma leaf spot in autumn (Dl) and the temperature and rainfall during the intercrop period between the harvest of the previous crop and the establishment of the new crop, because maturation in pseudothecia (fruiting bodies; figure 1a) on crop debris of L. maculans ascospores (figure 1b) and infection of oilseed rape leaves through stomata (figure 1c) are dependent on both temperature and wetness. The dates for the start and end of this period, selected by model optimization over a range of dates to give the optimal regression fit, were 15 July and 26 September, respectively, and the dates in the growing season were then expressed as days after 15 July. These dates are assumed to represent the current average dates of harvest in summer (15 July, when the maturation of L. maculans ascospores starts) and the establishment of the new crop in autumn (26 September, when new leaves are available to be infected). Location and year (growing season) were included as random effects to account for the hierarchical nature of the data. The analysis of variance was used to investigate differences between cultivars in the start of phoma leaf spotting.
Table 1.
Parameters/terms used in developing phoma stem canker models.
| parameter/variable | term/abbreviation | unit | explanation |
|---|---|---|---|
| 15 Jul (day 196) | start of optimized prediction period | day of the year | selected by model optimization (approx. date of harvest) |
| 26 Sep (day 289) | end of optimized prediction period | day of the year | selected by model optimization (approx. date for establishment of new crop, sown late Aug/early Sep) |
| Rsum | total rainfall | mm | total rainfall for the period 15 Jul–26 Sep |
| Tmax | mean maximum temperature | °C | mean maximum daily temperature for the period 15 Jul–26 Sep |
| Dl | date, start of leaf spottinga | day of the yearb | date (autumn) when 10% of plants in crop affected by phoma leaf spotting |
| Dc | date, start of stem cankera | day of the yearb | date (spring) when 10% of plants in crop affected by phoma stem canker |
| Dh | date of pre-harvest sample | day of the yearb | date in the period between Dc and harvest |
| Sc | severity of stem cankera | 0–4 scale | severity of phoma stem canker on date Dh |
| thermal time | °C-days | accumulated °C-days above 0°C between date a and date b; to estimate Dc and Sc, multiplied by a constant depending on cultivar resistance | |
| climate | mean weather over a 30-year period (comparisons made with the period 1960–1990) | ||
| HadCM3 | HadCM3 is a coupled atmosphere–ocean global climate model with a spatial resolution of 300 km developed at the Hadley Centre | ||
| HadRM3 | HadRM3 is a regional climate model with a spatial resolution of 50 km developed at the Hadley Centre | ||
| LO | low-emission scenario (less than 1100 Gt carbon per annum) | ||
| HI | high-emission scenario (1450–1800 Gt carbon per annum) | ||
| LARS-WG | stochastic weather generator, used to generate climate change scenarios |
For these parameters, the values predicted by the model (p) are compared with the observed values (o).
Dates used are assessed as ‘days after 15 July’.
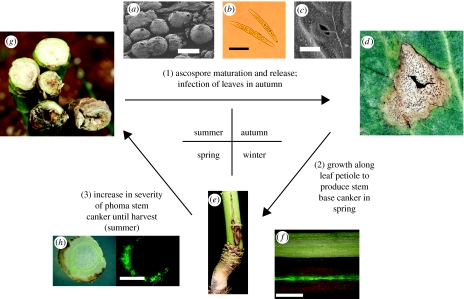
Figure 1.
Stages in modelling phoma stem canker epidemics on winter oilseed rape in the UK. (1) Predicting the date in autumn when 10% of plants have (d) phoma leaf spotting (Dl), from data for temperature and rainfall which affect maturation of (a) L. maculans pseudothecia (scale bar, 300 μm) on affected oilseed rape stems after harvest to produce (b) ascospores (scale bar, 10 μm) that infect leaves through (c) stomata (scale bar, 10 μm). (2) Predicting the first date in spring when 10% of plants have (e) phoma stem canker lesions (Dc), from autumn/winter temperature data and cultivar resistance which affect the rate of pathogen growth along the leaf petiole ((f) shown with GFP-labelled L. maculans growing along the centre of symptomless petiole; scale bar, 5 mm). (3) Predicting increase in severity of phoma stem canker lesions until (g) harvest (Sc), from spring/summer temperature data and cultivar resistance rating which affect growth through oilseed rape stem tissues ((h) shown with GFP-labelled L. maculans growing in stem cortex; scale bar, 5 mm). Images (f) and (h) each show the same tissue viewed under white light or with a GFP filter.
In the second stage, the date when phoma canker starts to develop on stems in spring (Dc; figure 1e) was predicted from the start of phoma leaf spotting (Dlp) and accumulated thermal time (°C-days) from Dlp, which affects the rate of progress of the pathogen along leaf petioles to the stem in autumn/winter (figure 1f; Sun et al. 2000). The date when phoma stem canker starts in spring (Dc) was estimated from weekly records of stem canker incidence (percentage of plants affected) in March, April and May (18 datasets). This work suggested that the thermal time accumulated between the two dates (Dl and Dc) was dependent on the resistance of the winter oilseed rape cultivar to L. maculans, so the dates of first appearance of phoma stem canker were estimated separately for cultivars with high-(6–9) and low (1–5)-resistance ratings (www.hgca.com).
In the third stage, the increase in severity of phoma stem canker (Sc, 0–4 severity scale; figure 1g) was predicted from the start of stem cankers in spring (Dcp), using accumulated thermal time (°C-days) from Dcp, which affects colonization of stem tissues by the pathogen (figure 1h). The increase in severity of phoma stem canker in the period before harvest (mean of all plants sampled) was assessed on a 0–4 scale (0, healthy; 4, plant dead; Zhou et al. 1999) on plants regularly sampled from untreated crops in the period March to July (32 datasets). The severity of phoma stem canker on the pre-harvest date of sampling (Sc) was predicted from Dc as a function of thermal time and cultivar resistance to L. maculans (Sun et al. 2000) by applying a model and treating each experiment as a random effect. Analyses were done with the statistical software GenStat (Anon. 2006).
Multiple linear regression, using the 40 datasets (electronic supplementary material, table 1), indicated that mean maximum daily temperature [Tmax] and total rainfall [Rsum] between 15 July and 26 September produced the best prediction of the start of the phoma leaf spotting epidemic (Dlp) for each site and growing season. The mean maximum daily temperature discriminated well between different sites and the sum of rainfall discriminated well between different growing seasons (electronic supplementary material, figure 1). The variables crop sowing date, cultivar resistance to L. maculans (www.hgca.com) and proximity to a previous season's oilseed rape crop did not account for additional variation in these data. The relationship was described by the equation
| (2.1) |
The 95% CI of the parameters (estimate±interval; 216.5±52.84, −0.24±0.08 and −4.55±2.36) show that both Tmax and Rsum were significant (p<0.001). When the observed start of phoma leaf spotting (Dlo) was regressed against the date predicted by equation (2.1) (Dlp), 63% of the variance was accounted for (electronic supplementary material, figure 2).
The prediction of the start of phoma stem canker in spring (Dcp), using the start of phoma leaf spotting in the previous autumn (Dlp) and a derived function of °C-days (T′) from Dlp, was improved by taking account of cultivar resistance to L. maculans (Sun et al. 2000). The mean thermal time (±s.e.) was 1097(±66.0) °C-days for susceptible cultivars (resistance rating 1–5; www.hgca.com, A=0) and 1386(±74.5) °C-days for resistant cultivars (resistance rating 6–9; A=1), and Dcp is the first day where the accumulated thermal time (°C-days) exceeds the threshold (min(x))
| (2.2) |
where Ti is the temperature in °C on day i and is the accumulated °C-days from start of leaf spotting (Dlp) to day x (=Dlp, Dlp+1, …).
The increasing severity of phoma stem canker in the period until harvest (Sc) was predicted as a function of °C-days since the start of stem canker, with different slopes for susceptible (0.00135(±0.00011)) and resistant (0.001(±0.00017)) cultivars. The equation to predict the canker severity on a specific date (e.g. last sample before harvest) was
| (2.3) |
where Scp is the mean stem canker severity of plants (0–4 scale; Zhou et al. 1999) and Dh is the pre-harvest sample date.
The weather-based model for describing the development of phoma stem canker epidemics was validated with data from 21 winter oilseed rape field experiments done under different weather conditions in growing seasons between 2004–2005 and 2006–2007 at a wide range of UK sites, each with 1–17 cultivars (electronic supplementary material, table 2). Linear regressions were done for the observed values against the predicted values for the start of phoma leaf spotting (autumn), start of phoma stem canker (spring) and severity of phoma stem canker (pre-harvest). The values of the percentage of variance accounted for were used as measures of the goodness of fit of the model. When the observed start of phoma leaf spotting (Dlo) was regressed against the date predicted by the model (Dlp), 33% of the variance was accounted for (electronic supplementary material, figure 3a). Regression of observed (Dco) against predicted (Dcp) start of stem canker accounted for 21% of the variance (electronic supplementary material, figure 3b). Regression of observed (Sco) against predicted (Scp) pre-harvest severity of phoma stem canker accounted for 36% of the variance, using data from the field experiments (electronic supplementary material, figure 3c).
3. Modelling the effects of climate change on phoma stem canker epidemics
To predict the effects of climate change on the range and severity of phoma stem canker epidemics, we used UKCIP02 climate change predictions for the years 2020 and 2050 (Hulme et al. 2002), by comparison with a baseline period (1960–1990), under high (HI)- and low (LO)-emission scenarios as defined by global IPCC emission scenarios (Nakicenovic 2000). The UKCIP02 climate change predictions for the year 2080 were available but not used since predicted average values are outside the current boundary values within which the phoma stem canker model was validated. The UKCIP02 scenarios are based on the HadCM3 global and HadRM3 regional climate models (Collins et al. 2001). The HadRM3 model has a horizontal resolution of 0.44°×0.44° (50 km). For each grid cell, UKCIP02 provides predicted changes in monthly climate variables. A weather generator (LARS-WG; Semenov & Barrow 1997) produced 70 yearly site-specific daily weather datasets for both 2020 and 2050 for each emission scenario, based on UKCIP02 projections and daily output from the HadRM3 climate model. For selected sites (15 locations evenly distributed over the UK), we calculated weather generator parameter sets, representing the baseline climate, using spatial interpolation of parameters, previously derived from the observed daily weather for 1960–1990 (Semenov & Brooks 1999). To adjust 1960–1990 parameters for climate change predictions, changes in mean and variability of climate variables for a site were required. The mean monthly changes in total rainfall, maximum and minimum temperatures were provided by UKCIP02. Changes in duration of monthly mean dry and wet series were calculated using HadRM3 daily rainfall output. New sets of parameters adjusted for changed climate were used to generate 70 yearly site-specific daily weather sets for each emission scenario for both 2020 and 2050 at each location.
These data were used as input in the phoma stem canker model to predict the effects of climate change on the start of phoma leaf spotting (Dlp), start of stem canker (Dcp) and severity of stem canker at harvest (Scp; initially assumed to be on 15 July). The results of simulations at 15 sites were spatially interpolated over the UK. As data were skewed, the median date rather than the mean date for the start of phoma leaf spotting was calculated for each scenario (the date was calculated as the number of days from 15 July). From this predicted date (Dlp), the predicted start of phoma stem canker (Dcp) and phoma stem canker severity at harvest (Scp) were calculated. The start of phoma leaf spotting in autumn predicted for 2020 and 2050 using the climate change model scenarios under high- or low-emission scenarios was only 5–10 or 10–15 days, respectively, earlier than in the 1960–1990 period (electronic supplementary material, figure 4). Since the start of leaf spotting is dependent on both temperature and rainfall (equation (2.1); West et al. 2001; Huang et al. 2005), the effects of increasing summer temperature were counteracted by the effects of decreasing summer rainfall.
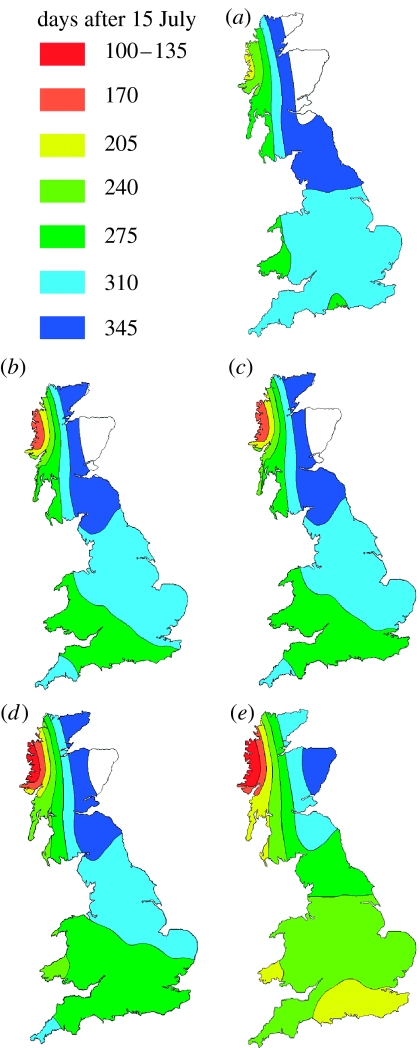
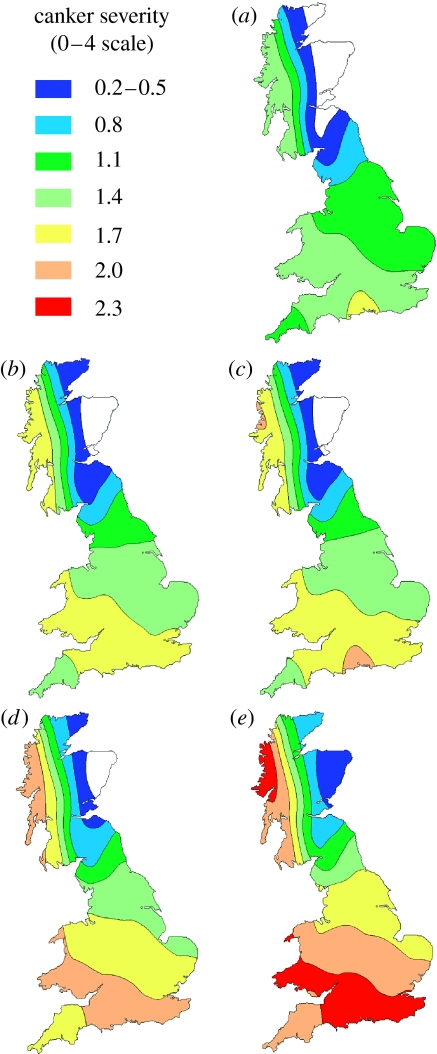
There was a large effect of predicted climate change on the start of phoma stem canker in spring, with predicted dates often 80 days earlier than during 1960–1990 (figure 2). The range of the damaging stem canker phase of epidemics was predicted to extend northwards from England into oilseed rape growing areas in eastern Scotland (white area in figure 2, currently unaffected by phoma stem canker). Furthermore, the predicted severities of phoma stem canker at harvest for 2020 and 2050 were much greater than during 1960–1990; the UK maximum mean severity increased from 1.7 (1960–1990) to 2.0 (2020) and 2.3 (2050) on the 0–4 scale for a harvest date of 15 July (figure 3). These increases in severity of epidemics still occurred if harvest dates were earlier, as predicted for sites in southern England, when harvest dates under these scenarios were estimated for Rothamsted from predicted wheat harvest dates (table 2). The effects of climate change on the range and severity of the disease can already be observed by comparing the values for periods 1960–1990 and 1975–2005 at Rothamsted, UK; the simulated start of canker is 10 days earlier during 1975–2005 than during 1960–1990 and canker severity is 18% greater. These large effects on the start and severity of stem canker were related to predicted increase in temperature, especially in winter, which greatly influences this stage of epidemic development (equation (2.2); Sun et al. 2000), although the effects are less on resistant than on susceptible cultivars (table 2).
Figure 2.
Predicted start of phoma stem canker (L. maculans) in spring. Predicted number of days after 15 July until the date in spring when the first phoma stem canker is observed on winter oilseed rape crops (mean of resistant and susceptible cultivars) affected with L. maculans for (a) baseline 1960–1990, (b) 2020LO, (c) 2020HI, (d) 2050LO and (e) 2050HI climates. Areas unaffected by the disease are white.
Figure 3.
Predicted severity of phoma stem canker (L. maculans) at harvest (Sc) on winter oilseed rape crops (mean of resistant and susceptible cultivars) for (a) baseline 1960–1990, (b) 2020LO, (c) 2020HI, (d) 2050LO and (e) 2050HI climates. Stem canker severity on a 0–4 scale (0, no disease; 4, plant dead; Zhou et al. 1999). Areas unaffected by the disease are white.
Table 2.
Effect of cultivar resistance on predicted number of days after 15 July until the date in spring when the first phoma stem canker is observed and the severity of stem canker at harvest (on 15 July or on predicted harvest date) at Rothamsted, Hertfordshire, UK for winter oilseed rape crops affected with L. maculans for 2020 and 2050 climates under low (LO)- and high (HI)-emission scenarios.
| cultivar resistance (ratinga) | emission scenario | ||||
|---|---|---|---|---|---|
| baseline | 2020 LO | 2020 HI | 2050 LO | 2050 HI | |
| date of first phoma stem canker (days after 15 Jul) | |||||
| resistant (6–9) | 309 | 295 | 292 | 281 | 264 |
| susceptible (1–5) | 279 | 264 | 261 | 246 | 227 |
| mean | 296 | 275 | 278 | 257 | 242 |
| phoma stem canker severity at harvest on 15 Jul (day 196; 0–4 scale) | |||||
| resistant (6–9) | 1.14 | 1.43 | 1.49 | 1.71 | 2.04 |
| susceptible (1–5) | 1.55 | 1.83 | 1.88 | 2.11 | 2.44 |
| mean | 1.36 | 1.63 | 1.69 | 1.98 | 2.31 |
| phoma stem canker severity at predicted harvest date (0–4 scale) | |||||
| predicted harvest dateb | 190 | 186 | 185 | 178 | |
| resistant (6–9) | 1.27 | 1.22 | 1.44 | 1.56 | |
| susceptible (1–5) | 1.68 | 1.63 | 1.83 | 1.97 | |
| mean | 1.47 | 1.43 | 1.64 | 1.77 | |
Resistance rating on a 1–9 scale (www.hgca.com).
Day of the year, obtained from the predicted harvest date for winter wheat under these climate change scenarios and difference in harvest dates between winter wheat and winter oilseed rape in oC-days.
4. Discussion
These results demonstrate how predicted global warming can increase the range and severity of plant diseases of worldwide importance within the next 20 years. The effects of climate change may be on the pathogen, the host or the host–pathogen interaction (Coakley et al. 1999; Huang et al. 2005, 2006; Garrett et al. 2006). The long-term effects of human-made environmental change on plant diseases may be masked by short-term seasonal fluctuations (Bearchell et al. 2005; Fitt et al. 2006c). To ignore such effects may result in devastating epidemics on staple food crops, with far-reaching socio-economic consequences, or on important plants in natural ecosystems, threatening wildlife (Luo et al. 1995; Chakraborty et al. 2000; Anderson et al. 2004).
The evidence that climate change will increase the range and severity of phoma stem canker is supported by the observations that phoma stem canker epidemics are currently most severe in oilseed rape growing regions with Mediterranean climates (e.g. in Australia or France; Howlett et al. 2001; Fitt et al. 2006a). There is a need for new cultivars destined for the future UK market to be tested under such climates. Whereas some predicted effects of climate change may be anticipated by qualitative reasoning (Coakley et al. 1999), others, such as the small effect on the date of phoma leaf spotting in autumn, may not. It is important to recognize that climate change effects on complex host–pathogen–environment interaction may also decrease severity of epidemics (Chakraborty et al. 1998).
These predictions about the effects of climate change on range and severity of phoma stem canker were possible only because of the availability of both knowledge about the epidemiology of this monocyclic disease and datasets on epidemic development and weather for a range of sites and growing seasons. These datasets encompass a wide range of dates of establishment in autumn (late August to mid-October) and harvest dates in summer (early July to mid-August). To improve accuracy of predictions, there is a need to improve the model by further validation against data obtained in a wide range of climates, such as those predicted for the UK, and by incorporating into the model a weather-based crop growth model to describe the effects of climate change on crop growth that influence disease development (Steed et al. 2007). A high priority over the next decade should be the collation of accurate disease and weather data and development of models to forecast the effects of climate change on other plant diseases to provide the necessary foresight for strategic adaptation to climate change. These models can guide policy and practice to counter such emerging threats to delicately balance natural and agricultural ecosystems.
Acknowledgments
We thank the UK Department for Environment, Food and Rural Affairs (OREGIN and PASSWORD projects), Biotechnology and Biological Sciences Research Council, Home-Grown Cereals Authority, ProCam Ltd, DuPont Ltd, Syngenta Ltd and the Perry Foundation for funding this work. We thank J. S. West for his comments on the paper and for figure 1b, Y. J. Huang for figure 1c,d,f,h, and B. Hall, J. Hood, E. Pirie, J. M. Steed and J. S. West for provision of phoma leaf spot/stem canker data and many Rothamsted and ADAS staff involved in collecting the data from 1992–1993 to 2005–2006.
Supplementary Material
References
- Anderson P.K, Cunningham A.A, Patel N.G, Morales F.J, Epstein P.R, Daszak P. Emerging infectious diseases of plants: pathogen, pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004;19:535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Anon. 2006 Genstat release 9 reference manual. In Release 9 reference manual. Oxford, UK: VSN International.
- Bearchell S.J, Fraaije B.A, Shaw M.W, Fitt B.D.L. Wheat archive links long-term fungal pathogen population dynamics to air pollution. Proc. Natl Acad. Sci. USA. 2005;102:5438–5442. doi: 10.1073/pnas.0501596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergot M, Cloppet E, Pérarnaud V, Déqué M, Marçais B, Desprez-Loustau M.-L. Simulation of potential range expansion of oak disease caused by Phytophthora cinnamomi under climate change. Global Change Biol. 2004;10:1539–1552. doi: 10.1111/j.1365-2486.2004.00824.x. [DOI] [Google Scholar]
- Bosch J, Carrascal L.M, Duran L, Walker S, Fisher M.C. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc. R. Soc. B. 2007;274:253–260. doi: 10.1098/rspb.2006.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier C.M, Scott J.K. European oak declines and global warming: a theoretical assessment with special reference to the activity of Phytophthora cinnamomi. EPPO Bull. 1994;24:221–232. [Google Scholar]
- Chakraborty S. Potential impact of climate change on plant–pathogen interactions. Aust. Plant Pathol. 2005;34:443–448. doi: 10.1071/AP05084. [DOI] [Google Scholar]
- Chakraborty S, et al. Potential impact of climate change on plant diseases of economic significance to Australia. Aust. Plant Pathol. 1998;27:15–35. doi: 10.1071/AP98001. [DOI] [Google Scholar]
- Chakraborty S, Tiedemann A.V, Teng P.S. Climate change: potential impact on plant diseases. Environ. Pollut. 2000;108:317–326. doi: 10.1016/S0269-7491(99)00210-9. [DOI] [PubMed] [Google Scholar]
- Coakley S.M, Scherm H, Chakraborty S. Climate change and plant disease management. Annu. Rev. Phytopathol. 1999;37:399–426. doi: 10.1146/annurev.phyto.37.1.399. [DOI] [PubMed] [Google Scholar]
- Collins M, Tett S.F.B, Cooper C. The internal climate variability of HadCM3, a version of the Hadley centre coupled model without flux adjustments. Clim. Dynam. 2001;17:61–81. doi: 10.1007/s003820000094. [DOI] [Google Scholar]
- Fitt B.D.L, Brun H, Barbetti M.J, Rimmer S.R. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus) Eur. J. Plant Pathol. 2006a;114:3–15. doi: 10.1007/s10658-005-2233-5. [DOI] [Google Scholar]
- Fitt B.D.L, Evans N, Howlett B.J, Cooke M. Springer; Dordrecht, The Netherlands: 2006b. Sustainable strategies for managing Brassica napus (oilseed rape) resistance to Leptosphaeria maculans (phoma stem canker) [Google Scholar]
- Fitt B.D.L, Huang Y.-J, van den Bosch F, West J.S. Coexistence of related pathogen species on arable crops in space and time. Annu. Rev. Phytopathol. 2006c;44:163–182. doi: 10.1146/annurev.phyto.44.070505.143417. [DOI] [PubMed] [Google Scholar]
- Garrett K.A, Dendy S.P, Frank E.E, Rouse M.N, Travers S.E. Climate change effects on plant disease: genomes to ecosystems. Annu. Rev. Phytopathol. 2006;44:489–509. doi: 10.1146/annurev.phyto.44.070505.143420. [DOI] [PubMed] [Google Scholar]
- Howlett B.J, Idnurm A, Pedras M.S.C. Leptosphaeria maculans, the causal agent of blackleg disease of Brassicas. Fungal Genet. Biol. 2001;33:1–14. doi: 10.1006/fgbi.2001.1274. [DOI] [PubMed] [Google Scholar]
- Huang Y.-J, Fitt B.D.L, Jedryczka M, Dakowska S, West J.S, Gladders P, Steed J.M, Li Z.-Q. Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa) Eur. J. Plant Pathol. 2005;111:263–277. doi: 10.1007/s10658-004-4421-0. [DOI] [Google Scholar]
- Huang Y.-J, Evans N, Li Z.-Q, Eckert M, Chevre A.-M, Renard M, Fitt B.D.L. Temperature and leaf wetness duration affect phenotypic expression of Rlm6-mediated resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2006;170:129–141. doi: 10.1111/j.1469-8137.2006.01651.x. [DOI] [PubMed] [Google Scholar]
- Hulme, M. et al. 2002 Climate change scenarios for the United Kingdom: the UKCIP02 scientific report, pp. 120. Norwich, UK: University of East Anglia.
- Luo Y, Tebeest D.O, Teng P.S, Fabellar N.G. Simulation studies on risk analysis of rice leaf blast epidemics associated with global climate change in several Asian countries. J. Biogeogr. 1995;22:673–678. doi: 10.2307/2845969. [DOI] [Google Scholar]
- Nakicenovic N. Greenhouse gas emissions scenarios. Technol. Forecast. Soc. 2000;65:149–166. doi: 10.1016/S0040-1625(00)00094-9. [DOI] [Google Scholar]
- Semenov M.A, Barrow E.M. Use of a stochastic weather generator in the development of climate change scenarios. Clim. Change. 1997;35:397–414. doi: 10.1023/A:1005342632279. [DOI] [Google Scholar]
- Semenov M.A, Brooks R.J. Spatial interpolation of the LARS–WG stochastic weather generator in Great Britain. Clim. Res. 1999;11:137–148. [Google Scholar]
- Sprague S.J, Balesdent M.-H, Brun H, Hayden H.L, Marcroft S.J, Pinochet X, Rouxel T, Howlett B.J. Major gene resistance in Brassica napus (oilseed rape) is overcome by changes in virulence of populations of Leptosphaeria maculans in France and Australia. Eur. J. Plant Pathol. 2006;114:33–40. doi: 10.1007/s10658-005-3683-5. [DOI] [Google Scholar]
- Steed J.M, Baierl A, Fitt B.D.L. Relating plant and pathogen development to optimise fungicide control of phoma stem canker (Leptosphaeria maculans) on winter oilseed rape (Brassica napus) Eur. J. Plant Pathol. 2007;118:359–373. doi: 10.1007/s10658-007-9137-5. [DOI] [Google Scholar]
- Stern N. Cambridge University Press; Cambridge, UK: 2007. The economics of climate change: the Stern review. [Google Scholar]
- Sun P, Fitt B.D.L, Gladders P, Welham S.J. Relationships between phoma leaf spot and development of stem canker (Leptosphaeria maculans) on winter oilseed rape (Brassica napus) in southern England. Ann. Appl. Biol. 2000;137:113–125. doi: 10.1111/j.1744-7348.2000.tb00043.x. [DOI] [Google Scholar]
- Thomson M.C, Doblas-Reyes F.J, Mason S.J, Hagedorn R, Connor S.J, Phindela T, Morse A.P, Palmer T.N. Malaria early warnings based on seasonal climate forecasts from multi-model ensembles. Nature. 2006;439:576–579. doi: 10.1038/nature04503. [DOI] [PubMed] [Google Scholar]
- West J.S, Kharbanda P.D, Barbetti M.J, Fitt B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 2001;50:10–27. doi: 10.1046/j.1365-3059.2001.00546.x. [DOI] [Google Scholar]
- Wint G.R.W, Robinson T.P, Bourn D.M, Durr P.A, Hay S.I, Randolph S.E, Rogers D.J. Mapping bovine tuberculosis in Great Britain using environmental data. Trends Microbiol. 2002;10:441–444. doi: 10.1016/S0966-842X(02)02444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fitt B.D.L, Welham S.J, Gladders P, Sansford C.E, West J.S. Effects of severity and timing of stem canker (Leptosphaeria maculans) symptoms on yield of winter oilseed rape (Brassica napus) in the UK. Eur. J. Plant Pathol. 1999;105:715–728. doi: 10.1023/A:1008761219493. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.