Abstract
In this short communication, we describe the scope and flexibility of using a novel device containing three coaxially arranged needles to form a variety of novel morphologies. Different combinations of materials are subjected to controlled flow through the device under the influence of an applied electric field. The resulting electrohydrodynamic flow allows us to prepare double-layered bubbles, porous encapsulated threads and nanocapsules containing three layers. The ability to process such multilayered structures is very significant for biomedical engineering applications, for example, generating capsules for drug delivery, which can provide multistage controlled release.
Keywords: electrohydrodynamic, biomedical, layered structures
1. Introduction
The basic electrohydrodynamic (EHD) process uses a flowing medium subjected to an applied electric field. The process can result in the formation of a jet, which may subsequently break up to form fine droplets or remain intact to produce fibres or threads (Zeleny 1914; Formhals 1934). Using this basic principle, processes such as EHD printing (Jayasinghe et al. 2002; Wang et al. 2005), EHD microbubbling (Farook et al. 2007a) and various types of EHD micro-encapsulation (Farook et al. 2008) have evolved. The last two processes are different manifestations of coaxial EHD atomization in which two fluids simultaneously undergo EHD flow, in coaxial needles subject to the same voltage to generate coaxial jetting or spinning. The final product of this process can result in encapsulated materials on the meso-, micro- and nano-scales (Loscertales et al. 2002).
The key benefit of EHD processing is the fact that very fine (usually a few micrometres in diameter) relics can be obtained using relatively large (several hundred micrometres in diameter) needle sizes (Gomez et al. 1998; Li et al. 2007). We have shown that coaxial jetting is a viable method for encapsulating materials, which have potential for use in drug delivery applications (Pareta & Edirisinghe 2006). Furthermore, the same principle can be used to prepare less than 10 μm diameter microbubbles from suspensions, with a near-monodisperse distribution (Farook et al. 2007a). Microbubble generation is an area of significant interest in a range of application areas, in particular food science and biomedical engineering. The properties of these have to be tailored to strict specifications in certain medical applications such as ultrasound imaging and therefore the ability to engineer their structure and surface characteristics by further modifying the processing is highly desirable (Stride et al. 2008).
Both of the above-mentioned processes, microbubbling and nano/micro-encapsulation, arise from the break up of the coaxial jet. However, when the viscosity of the medium is significantly increased to overcome jet instabilities, break up does not occur and electrospinning or threading can prevail (Gupta et al. 2005; Zhang et al. 2006a). This process results in the formation of fibres or threads and also has the potential to be used in biomedical engineering, e.g. in drug delivery systems (Zhang et al. 2006b) and cell deposition (Jayasinghe et al. 2007). Again, the ability to control the characteristics of the fibres formed through the processing is very important.
The present report describes substantial initial experimental results from a novel coaxial tri-needle device used to generate a variety of multilayer encapsulated structures using a range of materials. This represents a significant advance on coaxial work and offers considerable potential benefits, particularly in the healthcare sector with possible applications within pharmaceutics and tissue engineering, for the preparation of encapsulated fibres or threads, carrier vesicles and multilayered nano- or microcapsules. In the following sections, we describe the details of the novel device and demonstrate its use in preparing different types of structures.
2. Materials and methods
All materials were purchased from Sigma-Aldrich (Poole, UK) unless stated otherwise.
2.1 Polymer synthesis
Polyurethane (PU) was synthesized based on a two-step process, carried out under flowing nitrogen. Twenty grams of polyethylene glycol with an average molecular weight of 2×103 were dissolved in 30 ml of dimethylacetamide (DMAC) with mechanical stirring and the temperature was gradually increased to 60°C. Approximately 5 g of diphenylmethane diisocyanate were dissolved in 20 ml of DMAC and added dropwise to the former solution over a period of 60 min at 60°C. One gram of butanediol was dissolved in 10 ml of DMAC and added dropwise to the reaction over a period of 30 min. The temperature was then gradually increased to 130°C and the reaction mixture was stirred for a further 6 hours. The resultant PU solution was then allowed to stand under nitrogen until it reached ambient temperature (22°C). The polymer was then precipitated out of the solution by dropwise addition of it into methanol and purified by precipitating it twice; first in tetrahydrofuran (THF), and then again in methanol before drying under vacuum and silica gel.
2.2 Solution preparation
A number of different solutions were prepared for use in the experiments as shown in table 1. Ten grams of starch (National Starch and Chemical Ltd, Manchester, UK) were added to 120 ml of dimethyl sulphoxide (DMSO) and were sealed and stirred for 24 hours at 130°C. Five grams of bovine serum albumin (BSA) were dissolved in 50 ml of deionized water and allowed to stir for 24 hours. Two grams of the synthesized dry PU were dissolved in 25 ml of DMAC and were also stirred for 24 hours. Ten grams of polyethylene oxide (PEO) with an average molecular weight of 2×106 were dissolved in 60 ml of deionized water and 30 ml of THF by sealing the mixture and applying rapid stirring for 48 hours. Glycerol and olive oil (Salov SpA, Lucca, Italy) were used in their as-received states without dilution.
Table 1.
Physical properties of solutions and other liquids used in this work. (Values are at an ambient temperature of 22°C. The electrical conductivity of the albumin is extrapolated from the values quoted by Pareta et al. (2005).)
| concentration (mg ml−1) | surface tension (mN m−1) | electrical conductivity (S m−1/10−4) | viscosity (mPa s) | |
|---|---|---|---|---|
| polyurethane solution | 80 | 36 | 0.8 | 38 |
| starch solution | 83 | 40 | 1.1 | 12 |
| albumin solution | 100 | 56 | 1050 | 28 |
| PEO solution | 111 | 66 | 0.9 | 3054 |
| glycerol | — | 65 | 20 | 988 |
| olive oil | — | 42 | ≪1 | 80 |
2.3 Electrohydrodynamic processing
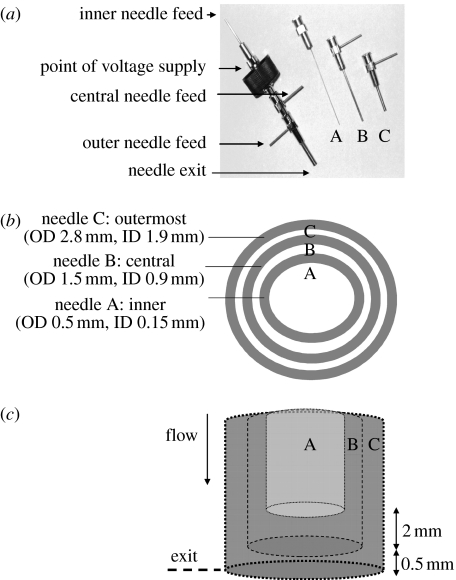
EHD processing was carried out using a stainless steel coaxial device that contained three separate needles (hereinafter referred to as the device) as shown in figure 1a, in both the assembled and dismantled forms. The dimensions of the needles are shown in figure 1b and the needle configuration is shown in figure 1c, where the innermost needle exit is approximately 2 mm above the central needle exit and 2.5 mm above the outer needle exit. The device was coupled to a high-voltage power supply (Glassman Europe Ltd, Tadley, UK) and the required solutions were made to flow into each orifice using specially designed Perfusor pumps (Harvard Apparatus, Edenbridge, UK). In the experiments reported in this work, all carried out at ambient temperature, the use of the device is exemplified by supplying a variety of solutions to the individual needles.
Figure 1.
Details of the three-needle coaxial device: (a) needle assembly, (b) needle dimensions and (c) relative placement of needles in the device. ID and OD represent internal and outer diameters, respectively.
The first set of experiments used air, glycerol and olive oil. The second maintained the use of air and olive oil, but more viscous PEO solution was used instead of glycerol. Samples from these experiments were collected on a standard glass microscope slide for subsequent characterization. Finally, PU, starch and BSA solutions were used to prepare nanocapsules, which were collected in a stirred mixture of ice-water/methanol. The solution was filtered (Whatman filter paper, retention size 3 μm) and the capsules were then allowed to dry in air over a period of 72 hours.
2.4 Characterization
Liquids and solutions subjected to EHD processing were characterized by measuring their surface tension, electrical conductivity and viscosity. Each characterization procedure was carried out three times at ambient temperature and pressure and a mean value was taken. Surface tension was evaluated using a Kruss K9 tensiometer (KRÜS GmbH, Hamburg, Germany). Electrical conductivity was obtained using a HANNA conductivity meter (Camlab Ltd, Cambridge, UK) and the viscosity was measured using a ViscoEasy concentric cylinder rotational viscometer (Camlab Ltd, Cambridge, UK) working at 100 rpm.
2.5 Microscopy
Optical micrographs were obtained using a Nikon Eclipse ME600 optical microscope. Scanning electron microscopy (SEM) was carried out using a JEOL JSM-6301F, operated at an accelerating voltage of 5 kV. Samples were coated with a thin layer of gold before electron microscopy. Transmission electron microscopy (TEM) was performed on a JEOL 100-CX microscope, with samples mounted on carbon-coated TEM grids (Agar Scientific, Stansted, UK).
3. Results and discussion
3.1 Material characteristics and device
Table 1 presents the measured surface tension, electrical conductivity and viscosity of all the materials used in this work, which are important properties in EHD processing (Gupta et al. 2005; Barrero & Loscertales 2007). The solutions displayed a range of electrical conductivities. It is imperative to couple materials with extremely low electrical conductivities (such as olive oil) with those which have comparably higher values and are capable of atomization, under the influence of an electric field. For example, co-flow of olive oil and glycerol is achievable due to the higher electrical conductivity of glycerol, whereas olive oil has an exceptionally low electrical conductivity and it is not possible to atomize this material alone. The surface tension values varied from 35 to 70 mN m−1. A broad range of viscosities was encountered, with the PEO solution being the most viscous (3058 mPa s) and the starch solution being the least viscous (12 mPa s); it is this parameter which largely controls EHD spinning or threading (Zhang et al. 2006a).
The characteristics of the needles in EHD processing can be varied and this will have a direct impact on the operating conditions and products (Borra et al. 1999; Chen et al. 1999). For example, changing the needle diameter while processing very similar suspensions has been found to change the applied voltage required to yield a stable jet (Li et al. 2007; Ahmad et al. 2008). The device that we have used is more complex when compared with a conventional electrospraying nozzle. In the first instance, the inner diameter (ID) of the largest needle (1900 μm) is significantly larger than the needle diameters (685 μm) used in our previous work (Farook et al. 2007a,b, 2008). Secondly, there are three needles, which results in an added dimension to the processing when compared with one or two needles, in terms of material co-flow and jetting viability (Farook et al. 2007b).
3.2 Electrohydrodynamic behaviour
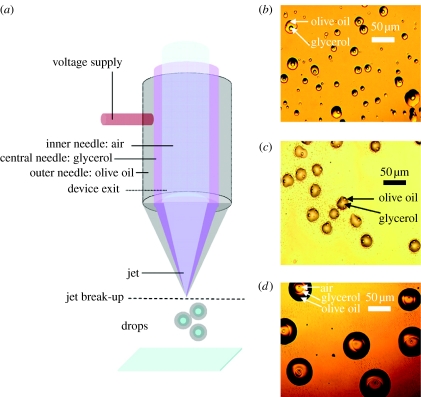
Figure 2a illustrates the process of droplet encapsulation using air, glycerol and olive oil. Encapsulated droplet relics of olive oil (central needle) and glycerol (inner needle) are shown in figure 2b. This process used coaxial flow using only two needles, with the flow of air switched off. The flow of glycerol was maintained at 25 μl min−1 and the flow of olive oil was fixed at 50 μl min−1. The voltage required to enable encapsulation was in the range 6.6–7.2 kV. The spatial arrangement of needles was found to be a key to this process. When the needles are made to touch, so that one side of a needle is in contact with an adjacent needle, perfect encapsulation does not occur, but rather ‘inconsistent’ encapsulation results as shown in figure 2c. When air is perfused through the inner needle and the simultaneous flows in the needles are set at air=250, glycerol=30 and olive oil=50 μl min−1, an applied voltage of 6.5–8 kV generated a jet. Under these conditions, concentric encapsulation of air results as shown in figure 2d. The encapsulation is visible, with two layers around the air core.
Figure 2.
Droplet encapsulation resulting in layered relics. (a) Flow arrangement of materials during processing, (b) coaxial two-needle product with olive oil and glycerol, (c) non-concentric coaxial two-needle encapsulation and (d) coaxial tri-needle encapsulation with air, glycerol and olive oil.
Previously, using a two-needle coaxial EHD device, we prepared microbubbles with a glycerol–air system and classified the various modes of EHD flow (Farook et al. 2007b). These modes were bubble dripping, coning and microbubbling, and occur as a function of the applied voltage. The microbubbling mode is desirable for continuous microbubble formation. In addition to this, by gradually adjusting the flow rate of the individual materials and observing any changes, mode mapping can also be achieved (Farook et al. 2007b). In this work, bubbling was almost continuous; however, a few interruptions were noted and this resulted in some scattered relics outside the main deposited features. Mode mapping using the current device is a more complex process, when compared with a two-needle device, and this is currently under investigation. The introduction of a selected third medium, exemplified by olive oil, in the outermost needle offers a method of directly preparing multiple layered bubbles, building directly upon our existing coaxial technology for possible drug delivery via microbubbles. Considering the fact that the outermost needle's ID is 1900 μm (figure 1b), the ability to generate 50 μm features (figure 2d) is remarkable; however, we intend to further improve the resolution to a few micrometres as this is crucial for investigations such as the preparation of coated microbubbles for ultrasound contrast agents and drug delivery (Stride et al. 2008).
Bubbles, which are commonly used as contrast agents for ultrasound (Wu & Nyborg 2008), are being developed in the treatment and assessment of cancerous tumours (Suzuki et al. 2008). This is achieved by loading a desired agent on the surface of a bubble or cavity, which collapses with the application of ultrasound at the desired site, enabling location-specific release (Husseini et al. 2005). Using EHD processing, the preparation of mono- (Farook et al. 2007a) and multilayered bubbles is now achievable, the latter an outcome of the present work. With the prospect of multilayered bubbles, multiple loading is possible and so is the ability to vary the surface properties of such bubbles, making them hydrophilic or hydrophobic. Some bubbles may not be able to access certain regions due to this property, which may be a problem when considering the nature of the drug itself. In such cases, an additional coating would be desirable, while maintaining the drug on the surface within the adjacent layers and the cavity still intact. The other benefit is that the properties of bubbles can be enhanced for ultrasound applications by modifying the surface of bubbles (Stride et al. 2008), and with the use of a multilayering device, as elucidated in the present work, this will offer greater coating options and this contributes towards several characteristics, e.g. stability of bubbles and adsorption of materials (Murray 2007).
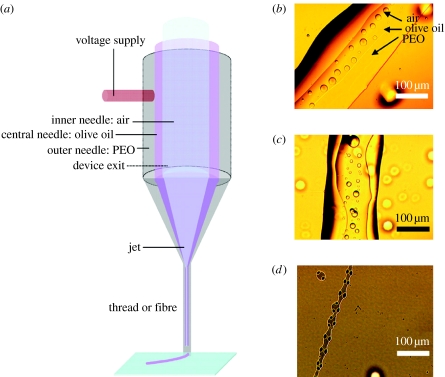
When a more viscous solution is used as the outermost medium, such as the high-molecular-weight PEO solution, jet break up may not be as likely to occur as it would be for a less viscous medium, enabling the formation of fibres or threads. Figure 3a illustrates the process and figure 3b shows air bubbles trapped in a multilayered thread, output by EHD jetting. Simultaneous flow at air=150, olive oil=70 and PEO solution=100 μl min−1 required an applied voltage of 7.1–7.4 kV to fabricate such structures. Hence, it is possible to go from droplet encapsulation to thread encapsulation by adjusting the viscosities of the flowing solutions. Instabilities in the jetting process, which may arise, for example, due to intermittent bubbling, can give rise to irregular thread morphologies as shown in figure 3c. As was the case with droplet encapsulation, when the needles are made to touch there is non-concentric thread formation. This is shown in figure 3d, where liquid-emitting needles were in contact during co-flow of materials, olive oil and glycerol, with the flow of air switched off. The process demonstrates that encapsulated threads with a width of 100–200 μm are fabricated from a device having a maximum ID of 1900 μm. It is noteworthy that encapsulated fibres with diameters of approximately 300 nm (Zhang et al. 2006b) have been prepared and our work in progress aims to improve the resolution of our present results.
Figure 3.
Thread encapsulation. (a) Flow arrangement, (b) air encapsulation in twin-layered thread with olive oil and PEO solution, (c) instabilities during thread formation and (d) two-needle (third needle switched off) coaxial non-concentric thread encapsulation using olive oil and PEO solution.
Simple threads have been used in the preparation of scaffolds for tissue engineering constructs (Gregory et al. 2007), and they have several other uses such as sutures (Woffles 2004) and the computer-controlled patterning of different scaffold geometries (Gupta et al. 2007). Some of these structures have been encapsulated and have demonstrated the controlled release of proteins from electrospun fibres (Zhang et al. 2006b; Maretschek et al. 2008), which offer the possibility of controlled drug release during polymer degradation. Hence, with the possibilities presented in this report, multilayer encapsulation opens up the potential to offer greater drug delivery options primarily due to the presence of extra layers. The idea of loading a polymeric fibre or thread with model proteins to demonstrate the suitability of such devices for drug delivery applications is also applicable to cells, and has been shown using EHD or electrospinning processing to be a viable method (Stankus et al. 2006). This can result in fibres or threads that have a good degree of cell integration. Controlled multilayered encapsulation has the potential to offer an efficient direct seeding process during the preparation of scaffolds and will also permit the optional loading of required materials for cell proliferation within the actual matrix, e.g. growth factors. It has been shown that the presence of two different fibres within a matrix can induce different cellular responses (Chua et al. 2007), which favours and projects multilayer threads as possible smart devices, spatially incorporating several materials resulting in different cellular responses during the device lifespan. The advantage that this method possesses over a conventional mix-spun material is that the location of adjacent materials (drug, growth factors or cells) can be controlled in threads and, with the usage of air, the threads can also be made porous, which is an essential property for scaffolds.
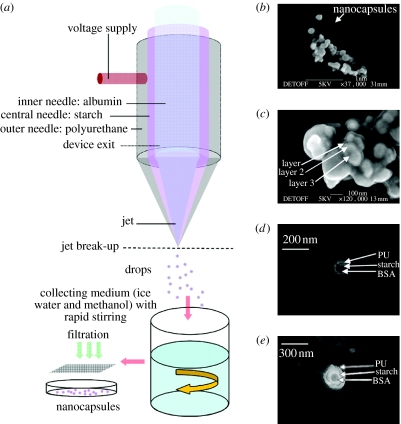
A further possibility with this device was to build upon existing coaxial drug delivery work (Pareta & Edirisinghe 2006), where starch and BSA solutions were used to prepare microcapsules. Here, the synthesized PU solution was used in the outermost needle and this precipitates from its solvent (DMAC) to form solid polymer when in contact with methanol or water. The process is illustrated in figure 4a. When the solutions are co-flowing (BSA solution=15 μl min−1, starch solution=25 μl min−1 and PU solution=40 μl min−1) cone-jet spraying is observed. Nanocapsules obtained from the filtrate were in the range of 100–300 nm, as shown in the scanning electron micrograph in figure 4b. A closer inspection of these nanocapsules (figure 4c) clearly shows the presence of two and in some cases three different densities, indicating the presence of multiple layers. TEM of these capsules clearly confirms the presence of multiple layers, with non-concentric (figure 4d) and concentric encapsulation (figure 4e), which correlates well with the different densities observed in the micrographs.
Figure 4.
Nanocapsule formation. (a) Flow arrangement of mediums used, (b) scanning electron micrograph of nanocapsules, (c) high magnification scanning electron micrograph of nanocapsules showing different regions (densities), (d) transmission electron micrograph of nanocapsule with non-concentric multiple layers and (e) transmission electron micrograph of nanocapsule with concentric multiple layers.
The prospects of such encapsulated materials could benefit drug delivery formulations. Large capsules, such as those used for loading commercial drugs, are recognizable by the general public. These comprise an outer organic shell that breaks down to release the encapsulated material. The preparation of such structures at a much higher resolution, such as the micro- and nano-scales, is less appreciated but can be more advantageous, i.e. due to the increased surface area and several modes of delivery. Factoring this in with the ability to control the number of layers and the material from which the layers can be made, be it a drug or drug enhancing material, the possibilities offered can be numerous. The encapsulation of materials, using electrospraying, has been used to prepare micro- and nanocapsules with applications in drug delivery (Loscertales et al. 2002; Hwang et al. 2008). The natural progression from here would lead to multilayered structures that could then have potential for multiple loading of drugs, prodrugs or other active agents required at a later time.
This method is a commercially viable direct method to prepare multiple layered polymeric capsules at the nano-scale. Further experimentation is required to deliver a well-defined size distribution of uniform multiple layered capsules. We believe that this is critically dependent on the solution flow rates and this can be determined by mapping the process.
4. Concluding remarks
In this paper, we report on our initial experimental findings from a novel tri-needle coaxial EHD device, successfully building upon the existing coaxial technologies already used to fabricate twin-layered structures at both the nano- and micro-scales. The feasibility of using such a device for fabricating multilayered structures is clearly shown in the preparation of multilayered bubbles, encapsulated threads and multilayered nanocapsules. Our current work focuses on improving the resolution, mode mapping the process and other possible novel processing possibilities offered by this device.
Acknowledgments
We are grateful to the archaeology and chemistry departments at University College London for use of their SEM and TEM equipments, respectively. We also acknowledge the EPSRC grant EP/E045839 and Royal Academy of Engineering for supporting this work. Collaboration between UCL and the University of Padua was funded by the Royal Society and we wish to acknowledge the exchange visits made possible by this grant.
References
- Ahmad Z, Thian E.S, Huang J, Edirisinghe M.J, Jayasinghe S.N, Best S.M, Bonfield W, Brooks R.A, Rushton N. Deposition of nano-hydroxyapatite utilising direct and transitional electrohydrodynamic processes. J. Mater. Sci. Mater. Med. 2008;19:3093–30104. doi: 10.1007/s10856-008-3436-z. [DOI] [PubMed] [Google Scholar]
- Barrero A, Loscertales I.G. Micro- and nanoparticles via capillary flows. Annu. Rev. Fluid Mech. 2007;39:89–106. doi: 10.1146/annurev.fluid.39.050905.110245. [DOI] [Google Scholar]
- Borra J.P, Tombette Y, Ehouarn P. Influence of electric field profile and polarity on the mode of EHDA related to electric discharge regimes. J. Aerosol Sci. 1999;30:913–925. doi: 10.1016/S0021-8502(98)00779-4. [DOI] [Google Scholar]
- Chen C.H, Emond M.H.J, Kelder E.M, Meester B, Schoonman J. Electrostatic sol-spray deposition of nanostructured ceramic thin films. J. Aerosol Sci. 1999;30:959–967. doi: 10.1016/S0021-8502(98)00075-5. [DOI] [Google Scholar]
- Chua K.N, Tang Y.N, Quek C.H, Ramakrishna S, Leong K.W, Mao H.Q. A dual-functional fibrous scaffold enhances P450 activity of cultured primary rat hepatocytes. Acta Biomater. 2007;3:643–650. doi: 10.1016/j.actbio.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Farook U, Zhang H, Edirisinghe M, Stride E, Saffari N. Preparation of microbubble suspensions by co-axial electrohydrodynamic atomization. Med. Eng. Phys. 2007a;29:749–754. doi: 10.1016/j.medengphy.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Farook U, Stride E, Edirisinghe M.J, Moaleji R. Microbubbling by co-axial electrohydrodynamic atomization. Med. Biol. Eng. Comp. 2007b;45:781–789. doi: 10.1007/s11517-007-0210-1. [DOI] [PubMed] [Google Scholar]
- Farook U, Edirisinghe M.J, Stride E, Colombo P. Novel co-axial electrohydrodynamic in-situ preparation of liquid-filled polymer-shell microspheres for biomedical applications. J. Microencap. 2008;25:241–247. doi: 10.1080/02652040801896666. [DOI] [PubMed] [Google Scholar]
- Formhals, A. 1934 Process and apparatus for preparing artificial threads. US Patent 1,975,504.
- Gomez A, Bingham D, de Juan L, Tang K. Production of protein nanoparticles by electrospray drying. J. Aerosol Sci. 1998;29:6561–6574. doi: 10.1016/S0021-8502(97)10031-3. [DOI] [Google Scholar]
- Gregory C, Rutledge A, Sergey V, Fridrikh A. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007;59:1384–1391. doi: 10.1016/j.addr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Gupta P, Elkins C, Long T.E, Wilkes G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer. 2005;46:4799–4810. doi: 10.1016/j.polymer.2005.04.021. [DOI] [Google Scholar]
- Gupta A, Seifalian A.M, Ahmad Z, Edirisinghe M.J, Winslet M.C. Novel electrohydrodynamic printing of nanocomposite biopolymer scaffold. J. Bioact. Compat. Polym. 2007;22:265–280. doi: 10.1177/0883911507078268. [DOI] [Google Scholar]
- Husseini G.A, de la Rosa M.A.D, Richardson E.S, Christensen D.A, Pitt W.G. The role of cavitation in acoustically activated drug delivery. J. Control. Rel. 2005;107:253–261. doi: 10.1016/j.jconrel.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y.K, Jeong U, Cho E.C. Production of uniform-sized polymer core–shell microcapsules by coaxial electrospraying. Langmuir. 2008;24:2446–2451. doi: 10.1021/la703546fS0743-7463(70)03546-1. [DOI] [PubMed] [Google Scholar]
- Jayasinghe S.N, Edirisinghe M.J, de Wilde T. A novel ceramic printing technique based on electrostatic atomization of a suspension. J. Mater. Res. Innovat. 2002;6:92–99. doi: 10.1007/s10019-002-0192-4. [DOI] [Google Scholar]
- Jayasinghe S.N, Irvine S, McEwan J.R. Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomedicine. 2007;2:555–567. doi: 10.2217/17435889.2.4.555. [DOI] [PubMed] [Google Scholar]
- Li X, Huang J, Ahmad Z, Edirisinghe M. Electrohydrodynamic coating of metal with nano-sized hydroxyapatite. Biomed. Mater. Eng. 2007;17:335. [PubMed] [Google Scholar]
- Loscertales I.G, Barrero A, Guerrero I, Cortijo R, Marquez M, Gañán-Calvo A.M. Micro/nano encapsulation via electrified coaxial liquid jets. Science. 2002;295:1695–1698. doi: 10.1126/science.1067595. [DOI] [PubMed] [Google Scholar]
- Maretschek S, Greiner A, Kissel T. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. J. Control. Rel. 2008;127:180–187. doi: 10.1016/j.jconrel.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Murray B.S. Stabilization of bubbles and foams. Curr. Opin. Coll. Interface Sci. 2007;12:232–241. doi: 10.1016/j.cocis.2007.07.009. [DOI] [Google Scholar]
- Pareta R, Edirisinghe M.J. A novel method for the preparation of biodegradable microspheres for protein drug delivery. J. R. Soc. Interface. 2006;3:573–582. doi: 10.1098/rsif.2006.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareta R, Brindley A, Edirisinghe M.J, Jayasinghe S.N, Luklinska Z.B. Electrohydrodynamic atomization of protein (bovine serum albumin) J. Mater. Sci. Mater. Med. 2005;16:919–925. doi: 10.1007/s10856-005-4426-z. [DOI] [PubMed] [Google Scholar]
- Stankus J.J, Guan J, Fujimoto K, Wagner W.R. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27:735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride E, Pancholi K, Edirisinghe M.J, Samarasinghe S. Increasing the nonlinear character of microbubble oscillations at low acoustic pressures. J. R. Soc. Interface. 2008;5:807–811. doi: 10.1098/rsif.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, et al. Tumor specific ultrasound enhanced gene transfer in vivo with novel liposomal bubbles. J. Control. Rel. 2008;125:137–144. doi: 10.1016/j.jconrel.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Wang D.Z, Jayasinghe S.N, Edirisinghe M.J. Instrument for electrohydrodynamic print-patterning three-dimensional complex structures. Rev. Sci. Instrum. 2005;76:075105. doi: 10.1063/1.1942531. [DOI] [Google Scholar]
- Woffles T.L. Barbed sutures in facial rejuvenation. Aesthet. Surg. J. 2004;24:582–587. doi: 10.1016/j.asj.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Wu J, Nyborg W.L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008;60:1103–1116. doi: 10.1016/j.addr.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Zeleny J. The electrical discharge from liquid points, and a hydrostatic method of measuring the electric intensity at their surfaces. Phys. Rev. 1914;3:69. doi: 10.1103/PhysRev.3.69. [DOI] [Google Scholar]
- Zhang H.B, Jayasinghe S.N, Edirisinghe M.J. Electrically forced microthreading of highly viscous dielectric liquids. J. Electrostat. 2006a;64:355–360. doi: 10.1016/j.physletb.2003.10.071. [DOI] [Google Scholar]
- Zhang Y.Z, Wang X, Feng J, Li J, Lim C.T, Ramakrishna S. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(ϵ-caprolactone) nanofibers for sustained release. Biomacromolecules. 2006b;7:1049–1057. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]