Abstract
Anemia prevalence is highest in preschool children, reproductive age women and women who are pregnant. While etiology is multifactorial, a deficiency in iron is the most commonly recognized nutritional cause. Observational studies imply that supplementation with iron or iron-folic acid should be started early in pregnancy, if not before, in order to prevent low birth weight and preterm delivery. Despite this clinical trials, even those from early pregnany, are equivocal. Recent follow up studies of children born to iron-folic acid supplemented women suggest that mortality is decreased and that the infant’s iron endowment reflects the mother’s iron status during pregnancy.
Keywords: iron deficiency, anemia, pregnancy
INTRODUCTION
Anemia, as measured by low hemoglobin or hematocrit, affects nearly one quarter of the world’s population. Worldwide prevalence is highest in preschool children (47.4%), reproductive age women (30.2%) and women who are pregnant (41.8%). During pregnancy, prevalence exceeds 50% in gravidae from Africa, 40% from Asia, 30% from Latin America and Oceania. Anemia is generally less frequent among pregnant women from Europe (18.7%) and North America (6%) except for low income and minority women from the United States where prevalence is high (7.6%,12.1%,33.8% trimesters 1,2 and 3).1–3 The etiology of anemia is multifactorial-including hemoglobiopathies, acute infections, chronic inflammation, and diets poor in nutrients such as folate, B12 and vitamin A, along with iron, the most commonly recognized nutritional cause.
PREVALENCE AND ETIOLOGY OF IRON DEFICIENCY
Iron deficiency is defined by three stages of increasing severity: depletion of iron stores (Stage 1), iron deficiency without anemia (ID) (Stage 2), and iron deficiency anemia (IDA) (Stage 3). Changes in erythropoiesis are a late manifestation of iron deficiency evidenced by abnormally low concentration of hemoglobin or hematocrit. Like anemia, risk of iron deficiency is increased during early childhood, in reproductive age women as well as during pregnancy. Iron deficiency is associated with an inadequate intake of absorbable iron in the face of a circumstance that increases the need for extra iron - rapid growth during the first two years of life, loss of iron in menstrual blood in women and the increase in red cell mass and growth of the conception during pregnancy.
Many women of childbearing age have a dietary intake of absorbable iron which is too low to offset losses from menstruation and the increased requirement associated with gestation. Data from NHANES III (1988–94) show a median dietary intake of 14.7 mg/day of iron for pregnant women, suggesting that approximately 90% were below the estimated average requirement or EAR for pregnancy (22 mg/day).4 Recent participants (2001–2007) from the Camden Study had a similar iron intake from food −15 mg/day at the median - with 83% below the pregnancy EAR (Table 1).
Table 1.
Usual intake of Iron from Food (mg/d): Pregnant Women from NHANES III (1988–1994) and Camden (2001–2007)
| Percentiles | NHANES III | Camden Study |
|---|---|---|
| 95% | 24 mg/d | 28 mg/d |
| 90 | 21 | 24 |
| 75% | 17.6 | 19 |
| 50% (Median) | 14.7 | 15 |
| 25% | 12.2 | 12 |
| 10% | 10.2 | 9 |
| 5% | 9 | 8 |
| Mean | 15 | 16 |
| SEM | 0.75 | 0.23 |
| N | 346 | 997 |
| % Below | ||
| Estimated Average | ||
| Requirement (EAR) | 90% | 83% |
Dietary intake of iron and other micronutrients is associated with iron deficiency during pregnancy. While energy adjusted intakes of protein and carbohydrate were similar, Camden gravidae with 3rd trimester iron deficiency, based upon two abnormal tests: ferritin (<12 ng/ml) and transferrin saturation (<15%), had diets (average of three 24 hour recalls) that were significantly lower in iron, B12, B6, riboflavin and folate (p=0.07) and higher in fat than gravidae who were not iron deficient (Table 2). In addition to a poor quality diet, poor iron absorption and blood loss from menstruation, with labor and delivery or from rapid repeat pregnancy are other important causes of iron deficiency in women.4–6
Table 2.
Energy Adjusted Nutrient Intakes from Food are Associated with 3rd Trimester Iron Deficiency in Camden (2001–2007)
| Iron Deficient 1,2 | Not Iron Deficient 1,2 | P | |
|---|---|---|---|
| Protein g/d | 88 ± 1.5 | 88 ± 1.8 | 0.77 |
| Carbohydrate g/d | 286 ± 4.8 | 294 ± 4 | 0.15 |
| Fat g/d | 87 ± 1.6 | 83 ± 1.3 | 0.04 |
| Iron mg/d | 15.5 ± 0.5 | 16.9 ± 0.4 | 0.02 |
| Folate mcg/d | 307 ± 12.7 | 337 ± 10.5 | 0.07 |
| B12 mcg/d | 4.0 ± 0.4 | 5.1 ± 0.3 | 0.03 |
| Vitamin A IU/d | 5072 ± 3.51 | 5530 ± 287 | 0.30 |
| B6 mg/d | 1.9 ± 0.05 | 2.0 ± 0.03 | 0.02 |
| Riboflavin mg/d | 2.0 ± 0.04 | 2.2 ± 0.03 | 0.01 |
| Zinc mg/d | 12.1 ± 0.32 | 12.5 ± 0.26 | 0.41 |
| Calcium mg/d | 917 ± 25 | 967 ± 21 | 0.13 |
Mean of three, 24 hour recalls (entry, 20, 28 weeks gestation). Adjusted for age, parity, ethnicity, smoking, BMI and energy intake.
Iron deficiency based upon 2 abnormal tests: serum ferritin (<12 mg/nl) and transferrin saturation (<15%) at week 28.
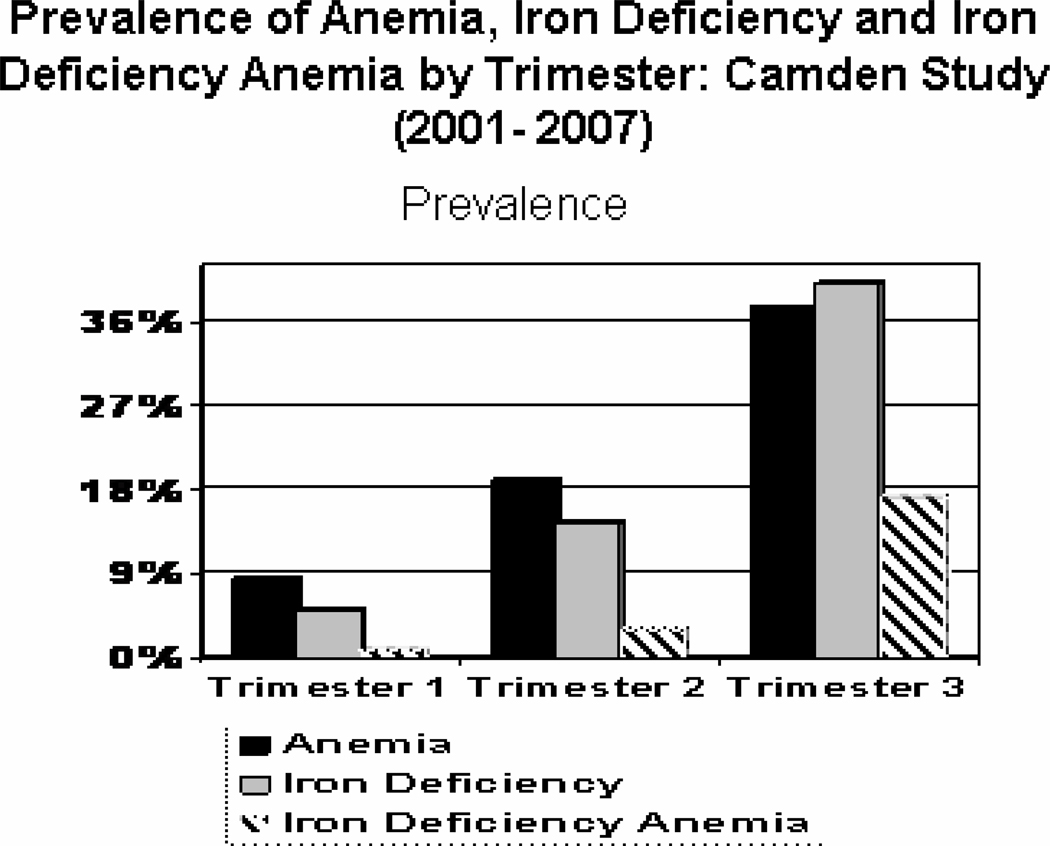
During pregnancy the maternal body requirement for iron increases to approximately 1000 mg on average.4, 6 Of this 350 mg is associated with fetal and placental growth, 500 mg with expansion in the red cell mass and 250 mg with blood loss at delivery. The increased requirement needs to be supported by higher maternal iron intake – increasing from 6 mg/d in the 1st trimester, to 19 mg/day in the 2nd trimester to 22 mg/day in the 3rd trimester of pregnancy.4, 6 In order to meet these increased requirement gravidae must draw upon iron stores, consequently increasing risk of iron deficiency and iron deficiency anemia. Among non pregnant women aged 16–49 from NHANES, the prevalence of iron deficiency anemia, estimated from low hemoglobin and two of three additional laboratory tests for iron deficiency, was 2–5%, while iron deficiency occurred in 11–16%.7, 8 A body iron model which utilized soluble transferrin receptors and serum ferritin gave a lower prevalence for iron deficiency amounting to 9.2 % in reproductive age women aged 20–49.8 Among Camden gravidae (2001–2007), there was a substantial rise in maternal anemia between trimesters 1 and 3. Likewise, iron deficiency [based upon low serum ferritin (<12 ng/ml) and transferrin saturation (<15%)], rose by trimester from 5% to 14.4% to 40% and iron deficiency anemia (CDC criteria for anemia by trimester with iron deficiency)6 increased from 0.9%, to 3% to 17% during trimesters 1,2 and 3 respectively (Figure 1). Predictive values would of course be higher in the developing countries where IDA is more prevalent. While supplementation with iron or iron-folic acid is beneficial for improving maternal hemoglobin levels during pregnancy and reducing risk of maternal anemia, daily supplementation may increase risk of a maternal hemoglobin which exceeds 130 g/L.9
Figure 1.
Iron deficiency is based upon 2 abnormal texts: serum ferritin (<12 ng/ml) and transferrin saturation (<15%). Anemia utilizes CDC definition.6 Iron deficiency anemia is anemia with iron deficiency.
PREGNANCY OUTCOME AND MATERNAL ANEMIA
The best time to detect risk associated with maternal anemia may be early in pregnancy before the plasma volume is fully expanded. While the maternal red cell mass and maternal plasma volume increases with gestation, they do not do so simultaneously. Hemoglobin and hematocrit decline throughout the 1st and 2nd trimesters, reach their lowest point late in the second to early in the 3rd trimester and then rise again nearer to term with peak hemodilution occurring at 24 to 26 weeks.6 As a result the physiologic anemia from the normal expansion of the maternal plasma volume is difficult to distinguish from true anemia, particularly late in pregnancy.
This was originally examined in Camden by separating anemia by time (early vs later gestation) and etiology (iron deficiency anemia and anemia from other causes).10–11 Early in pregnancy there were clear differences in mean corpuscular volume (MCV) and diet in women with iron deficiency anemia that were absent during the 3rd trimester. Early iron deficiency anemia was associated with greater then 2-fold increases in the risks of low birth weight and preterm delivery, while anemia from other causes was associated with a small but non-significant increase in risk. In the 3rd trimester, risk of preterm delivery was reduced for women with iron deficiency anemia; there was no increased risk for women with other anemias.
Findings with early anemia were confirmed with observational data from Chinese textile workers from before pregnancy. Approximately 83% of the workers who became pregnant had anemia; but iron deficiency anemia - anemia accompanied by a low levels of ferritin - was less common occurring in 8%. Pregravid anemia, regardless of etiology, was associated with significantly lower birth weight (100–125 g). Iron deficiency anemia, (hemoglobin <120 g/L and ferritin <12 ng/ml) was associated with the largest deficit of all −240 g infant birth weight.12 Data from the Pregnancy Nutritional Surveillance showed that while preterm delivery was increased for anemic women and women with low hemoglobin during the 1st or 2nd trimester, women with moderate to severe anemia had a risk which was approximately double; for the other women, risk of preterm delivery was increased between 10–40%.13 During the 3rd trimester the association reversed – anemia and low hemoglobin were each associated with a decreased risk of preterm birth. The association of early pregnancy IDA or early maternal anemia with preterm delivery and other poor outcomes was consistent with data from the Collaborative Perinatal Project in the United States, and studies from England, Wales, and the developing world including China, Nepal and Egypt.13–17
High maternal hemoglobim has consistently been associated with failure of the plasma volume to expand and adverse outcomes related to maternal pathology - maternal hypertension, preeclampsia or diabetes.9 Recent studies have raised the possibility that giving too much iron to nonanemic women, i.e. daily administration of 50–60 mg/d, can increase hemoconcentration and associated poor outcomes.9,18–19 Increased levels of the iron storage protein ferritin also are associated with preterm delivery but likely via infection and inflammation.20 Thus, both extremes of the maternal hemoglobin distribution are associated with adverse pregnancy outcomes with low hemoglobin reflecting a mix of true and physiologic anemia and high hemoglobin reflecting failure of the plasma volume to expand. Poorly nourished animals have reduced maternal plasma volume expansion during pregnancy, and low cardiac output, lower uteroplacental blood flow and reduced nutrient transmission to the fetus.21 It is possible that hypovolemia, along with anemia, has nutritional antecedents.
RANDOMIZED TRIALS WITH IRON OR IRON- FOLIC ACID IN PREGNANT WOMEN
Observational data on anemia imply that supplementation should be started early in pregnancy, if not before, in order to prevent low birth weight and preterm delivery. There have been two such trials in the US and several others from China, Nepal, Mexico and Australia. In both US studies low income and minority non-anemic women were enrolled before 20 weeks gestation and randomly assigned to supplemental iron or placebo or to multivitamins with or without iron.22–23 In one trial, the proportion with absent iron stores and IDA at week 28 was improved by the supplemental iron in another there was little change. In both studies, supplemented gravidae had significantly longer gestations and higher infant birth weights with a reduced risk of low birth weight or preterm low birth weight infants.
In Nepal, a comparison of four micronutrient regimens showed that the iron-folic acid supplement with vitamin A significantly decreased low birth weight (by 19%) and small for gestation births (by nearly 10%) compared to vitamin A alone.24 In a second Nepalese trial, a comparison of multiple micronutrients to the iron-folic acid control showed increased infant birth weight (+77 g) and decreased risk of low birth weight (45%) for the group on micronutrients.25 Neither study showed an effect of the supplements on risk of preterm delivery or gestation duration.
In China, gravidae from Hong Kong with hemoglobin levels between 80 and 140 g/L at baseline (<16 weeks) were randomly assigned to either 60 mg supplemental iron or placebo to determine if iron increased risk of gestational diabetes.26 While gestational diabetes risk was not altered with supplemental iron, birth weight was increased significantly (+ 96 g), and risk of small for gestation births (SGA) significantly reduced (3.6% vs 7.5%) with gestation duration (38.8 vs 38.7 weeks) and preterm delivery (6.4% vs 6.7%) remaining unchanged. A second trial took place in the rural northwest China, with villages randomly assigned to folic acid (control), iron-folic acid or multiple micronutrient supplements at week 14 gestation on average.27 Supplementation with iron-folic acid increased gestation duration (+0.23 weeks) and reduced risk of very preterm delivery <34 weeks (0.98% vs 1.8%) compared to the folic acid alone. Infant birth weight was increased significantly with multiple micronutrients (+42g) but not with iron-folic acid (+20 g). Both Chinese trials thus showed higher hemoglobin levels and improvement in birthweight or gestation duration among supplemented women. However, a randomized trial of non-anemic Australian gravidae, supplemented before 20 weeks gestation, showed no improvement in birth weight or gestation among iron supplemented pregnant women.28
Early pregnancy anemia and iron deficiency anemia are associated with an increased risk of adverse outcomes of pregnancy but clinical trials, even those from early pregnany are equivocal. A recent Cochrane review, found that women receiving iron folic acid supplements had infants significantly heavier (+58 g) than women on placebo or other regimens but no difference in preterm delivery, SGA or low birth weight.9 The authors opined that pooling results may not be the best way to evaluate studies that are not only statistically heterogeneous but also vary in the dose and/or duration of iron or iron folic acid supplementation as well as in maternal background. A poor maternal diet that results in anemia is unlikely to occur in isolation even in developed countries; effects may not be correctable by a brief period of supplementation. In the developing world hookworm and malaria infestation compound effects of chronic undernutrition, early childbearing and short intervals between pregnancies on maternal anemia. Supplementation with iron or iron-folic acid may need to be started before pregnancy and continued throughout the reproductive years, in order to prevent low birth weight and preterm delivery.
IRON OR IRON - FOLIC ACID SUPPLEMENTATION AND INFANT MORTALITY
Since preterm delivery, fetal growth restriction and low birth weight are known causes of infant mortality, supplementation with iron, or iron-folic acid, if beneficial, might also be expected to increase infant survivorship. Offspring born to the iron-folic acid supplemented mothers from rural China and those supplemented with multiple micronutrients both had lower neonatal mortality compared to controls receiving folic acid alone.27 The difference was significant only for the iron -folic acid group where there was a 54% decrease in neonatal mortality associated with fewer deaths from complications of preterm birth (6.8% vs 15%) and birth asphyxia (4.1% vs 11%).27 In Niger, while women supplemented with iron from later gestation (week 28) onward did not show differences in birth weight or gestation, there were fewer fetal/neonatal deaths (1% vs 7%) among births to iron supplemented mothers.29 In Nepal, despite higher birth weights with multiple micronutrients, fewer early neonatal deaths (23.4/1000 vs 9.1/1000) and perinatal dealths (49.0/1000 vs 40.5/1000) occurred among births to controls-the iron supplemented women.25 A post-hoc comparison of the two Nepalese trials suggested that neonatal and perinatal mortality were significantly lower among offspring of women supplemented with iron-folic acid than micronutrients.30 Long term follow up of Nepalese children showed a significant 31% decrease in mortality between birth and age 7 years for children born to mothers supplemented with iron-folic acid while pregnant.31
IRON STORES IN PRETERM AND SMALL FOR GESTATION INFANTS
The preterm delivery, and low (or lower) birth weight associated with anemia and iron deficiency anemia likely contribute to poor neonatal iron stores. From week 32 until delivery fetal iron stores increase as the fetal liver grows. Most (>66%) of the infant’s total body iron is acquired during the final trimester of pregnancy. An early preterm birth deprives the infant of the opportunity to acquire iron. Total body iron, hemoglobin, and ferritin are all lower in preterm infants.32–34 Between 25% and 85% of preterm infants develop iron deficiency during the first year and it develops earlier than in term infants.32–33 An infant weighing 1500 g has half the body iron content of an infant with a birth weight of 3000 g.34 A term infant of low birth weight also should have a smaller liver size and poorer iron stores than one that is appropriately grown. Small for gestation (SGA) infants born to women with hypertension or preeclampsia also have decreased iron stores due to poor placental function with decreased iron transport.35 A study of 84 low birth weight Chilean infants who were preterm [appropriate for gestation (AGA) or small for gestation (SGA)] or term SGA showed that term SGA infants had higher cord hemoglobin levels than preterm AGA infants who, in turn, had higher levels of ferritin that infants both preterm and SGA.36 After birth, limited iron stores of preterm infants are unable to support postnatal erythropoesis and catch-up growth. Preterm infants, both AGA and SGA, had lower hemoglobin concentrations by four months of age compared to term SGA infants.36
MATERNAL IRON STORES AND INFANT IRON ENDOWMENT
Another influence on infant iron stores, apart lower birth weight and shorter gestation duration, is maternal iron deficiency. Studies on rhesus macaques suggest that the iron status of the infant after birth reflects that of the mother before pregnancy.37 Serum ferritin levels but not hemoblobin or hematocrit, were lower in infants born to iron deficient macaques and correlated with the mother’s pregravid transferrin saturation. In humans, mothers with low levels of ferritin had cord blood ferritins 30–60% below those with better iron stores.38–39 The data reviewed by Allen suggest that ferritin in cord blood usually correlates with maternal ferritin or hemoglobin measured at delivery;40 however, there appears to be little relation between maternal hemoglobin and cord blood hemoglobin except in the case of severe anemia.39–43 Mild maternal anemia is associated with increased serum erythropoetin in mother41–42 and in the cord.41,44 Chronic fetal hypoxia from reduced uteroplacental blood flow increases hemoglobin production in the fetus via increased erythropoetin45 and occurs in SGA infants, a condition where hypoxia has been documented.46 Greater erythropoetin production may be the underlying reason for similar levels of hemoglobin in the cords of infants born to anemic and non-anemic women, i.e. to enhance oxygen delivery and promote growth of fetal organs and tissues in an hypoxic environment.
Infants born at term who are appropriate for gestation have iron stores adequate for approximately 6 months; while those born preterm or who are SGA have smaller stores. As rate of postnatal growth increases infant hematological status declines. In macaques, the infant’s MCV was significantly lower across the first 6 months if the mother was iron deficient before pregnancy.37 In humans one common assumption has been that the iron status of the offspring is independent of maternal iron status during pregnancy.40 In the study by Colomber, 156 infants were followed during their first year; risk of developing anemia was increased 6.57 fold for those infants whose mothers had anemia, defined by low hemoglobin with low ferritin(<12 ng/ml) at delivery. A stratified analysis suggested that risk was independent of infant birth weight, feeding practice and other variables.47 Term infants born to anemic and non-anemic Jordanian mothers were followed from delivery to age 12 months. While there were no differences in cord blood levels for any hematologic variables, by 9 months of age infants of anemic women had lower hemoglobin and red cell indices; the cumulative incidence of IDA, as defined by low hemoglobin and (low) ferritin or (high) zinc protoporphyrin, was 81% in infants of anemic mothers and 65% in controls.48 Infants of anemic Javanese mothers weighing more than 2,500 g at birth had approximately a 2-fold increased risk of low hemoglobin (<100 g/L) at 3–5 months (AOR=1.81, 95% CI 1.34–2.43) compared to non-low birth weight infants of non-anemic mothers; those weighing <2500 grams were at higher risk of anemia (AOR=3.7, 95% CI 1.69–8.02). However risk was not greatly increased (AOR 1.15, 95% CI 0.61–2.16) for low birth weight infants of mothers without anemia.49 As might be expected, the smaller iron stores associated with low birth weight interact with anemia in the mother to increase the offspring’s anemia risk nearly 4- fold. Thus, follow up studies of infants born to anemic women suggest that the infant’s iron endowment mirrors that of the mother. Additional research is required to confirm or deny whether increased erythropoetin production is associated with maternal anemia and has given rise to the notion that the iron endowment of the offspring is unrelated to that of the mother during pregnancy.
CONCLUSION
Iron supplementation during pregnancy increases maternal iron status and stores; it is plausible that iron supplementation improves pregnancy outcome when mother is anemic or from a population where prevalence of anemia is high. A poor maternal diet resulting in anemia is unlikely to occur in isolation and effects may not be correctable by a brief period of supplementation. Iron or iron-folic acid supplements may need to be started before pregnancy and continued throughout the reproductive years in order to reduce the risk of adverse pregnancy outcomes and to improve the iron stores of the infant. Since preterm delivery, fetal growth restriction and low birth weight are known causes of infant mortality, supplementation with iron, or iron-folic acid, might also increase infant survivorship as recent research is beginning to suggest.
Follow up studies of infants born to anemic women also indicate that the infant’s iron endowment mirrors that of the mother despite the fact many investigators find similar levels of hemoglobin in infants born to anemic and non-anemic women. Increased erythropoetin production - an adaptation which enhances oxygen delivery and promotes growth of fetal organs and tissues in the hypoxic environment-may be responsible but more research is needed.
At the other extreme, high maternal hemoglobin and high levels of the iron storage protein ferritin have consistently been associated with an increased risk adverse outcomes. It is possible that giving too much iron to nonanemic women, i.e. daily administration of 50–60 mg/day, can increase hemoconcentration and risk of poor outcomes like preeclampsia or gestational diabetes.
Acknowledgments
HD38329 and HD58128 from the National Institutes of Health
REFERENCES
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Publ Hlth Nutr. 2008;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Worldwide prevalence of anaemia report 1993–2005: WHO global database on anaemia. Geneva: World Health Organization; 2008. [Accessed September 15, 2010]. Available at: http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf. [Google Scholar]
- 3.Polhamus B, Dalenius K, Mackintosh H, Smith B, Grummer-Strawn L. Paediatric Nutrition Surveillance 2008. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 4.Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zin. Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Recommendations to prevent and control iron deficiency in the United States. MMWR Recommendations and Report. 1998;47(RR-3):1–36. [PubMed] [Google Scholar]
- 6.Institute of Medicine. Nutrition during Pregnancy. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 7.U.S. Preventive Services Task Force. Screening for Iron Deficiency Anemia in Childhood and Pregnancy: Update of the 1996 U.S. Preventive Task Force Review. Rockville, MD: Agency for Healthcare Research and Quality; 2006. Apr, [Accessed September 3, 2010]. Report No.: 06-0590-EF-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20722137. [PubMed] [Google Scholar]
- 8.Cogswell ME, Looker AC, Pfeiffer CM, et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr. 2009;89:1334–1342. doi: 10.3945/ajcn.2008.27151. [DOI] [PubMed] [Google Scholar]
- 9.Pena-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database of Systematic Reviews. 2009;4:CD004736. doi: 10.1002/14651858.CD004736.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Scholl TO, Heldiger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr. 1992;55:985–988. doi: 10.1093/ajcn/55.5.985. [DOI] [PubMed] [Google Scholar]
- 11.Scholl TO, Heldiger ML. Anemia and iron deficiency anemia, compilation of data on pregnancy outcome. Am J Clin Nutr. 1994;59:492S–501S. doi: 10.1093/ajcn/59.2.492S. [DOI] [PubMed] [Google Scholar]
- 12.Ronnenberg AG, Wood RJ, Wang X, et al. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. J Nutr. 2004;134:2586–2591. doi: 10.1093/jn/134.10.2586. [DOI] [PubMed] [Google Scholar]
- 13.Scanlon KS, Yip R, Schieve LA, Cogswill ME. High and low hemoglobin level during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol. 2000;96:741–748. doi: 10.1016/s0029-7844(00)00982-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol. 1998;148:998–1006. doi: 10.1093/oxfordjournals.aje.a009577. [DOI] [PubMed] [Google Scholar]
- 15.Bondevik GT, Lie RT, Kvale G. Maternal hematological status and risk of low birth weight and preterm delivery in Nepal. Acta Obstet Gynecol Scnad. 2001;80:402–408. [PubMed] [Google Scholar]
- 16.Arafa M, Abou-Zied H, Attia AF, Youssof M. Maternal haemoglobin and premature child delivery. East Mediterr Health J. 1998;4:480–486. [Google Scholar]
- 17.Brabin B, Ginny M, Supau J, Galme K, Paino J. Consequences of maternal anaemia on outcome of pregnancy in a malaria endemic area in Papua New Guinea. Ann Trop Med Parasitol. 1990;84:11–24. doi: 10.1080/00034983.1990.11812429. [DOI] [PubMed] [Google Scholar]
- 18.Casanueva E, Viteri FE, Mares-Galindo M, et al. Weekly iron as a safe alternative to daily supplementation for nonanemic pregnant women. Arch Med Res. 2006;37:674–682. doi: 10.1016/j.arcmed.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomized placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin ≥ 13.2 g/dl. BJOG. 2007;114:684–688. doi: 10.1111/j.1471-0528.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 20.Scholl TO. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol. 1998;92:161–165. doi: 10.1016/s0029-7844(98)00157-4. [DOI] [PubMed] [Google Scholar]
- 21.Rosso P, Salas SP. Mechanisms of fetal growth retardation in the underweight mother. Adv Exp Med Biol. 1994;352:1–9. doi: 10.1007/978-1-4899-2575-6_1. [DOI] [PubMed] [Google Scholar]
- 22.Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003;78:773–781. doi: 10.1093/ajcn/78.4.773. [DOI] [PubMed] [Google Scholar]
- 23.Siega-Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, Cogswell ME. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. Am J Obstet Gynecol. 2006;194:512–519. doi: 10.1016/j.ajog.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Christian P, Khatry SK, Katz, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomized community trial. BMJ. 2003;326:571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osrin D, Vaidya A, Shrestha Y, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomized controlled trial. Lancet. 2005;365:955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 26.Chan KKL, Chan BCP, Lam KF, Lao TT. Iron supplementation in pregnancy and development of gestational diabetes – a randomized placebo-controlled trial. BJOG. 2009;116:789–798. doi: 10.1111/j.1471-0528.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L, Cheng Y, Dang S, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomized controlled trial. BMJ. 2008;337:a2001. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low-dose iron supplements during pregnancy: a randomized controlled trial. Am J Clin Nutr. 2003;78:145–153. doi: 10.1093/ajcn/78.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am J Clin Nutr. 1997;66:1178–1182. doi: 10.1093/ajcn/66.5.1178. [DOI] [PubMed] [Google Scholar]
- 30.Christian P, Osrin D, Manandhar DS, Khatry SK, de L Costello AM, West KP., Jr Antenatal micronutrient supplements in Nepal. Lancet. 2005;366:711–712. doi: 10.1016/S0140-6736(05)67166-8. [DOI] [PubMed] [Google Scholar]
- 31.Christian P, Stewart CP, LeClerq SC, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow-up in a randomized, controlled community trial. Am J Epidemiol. 2009;170:1127–1136. doi: 10.1093/aje/kwp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol. 2009;36:27–42. doi: 10.1016/j.clp.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao, Georgieff MK. Perinatal aspects of iron metabolism. Acta Paediatr Suppl. 2002;438:124–129. doi: 10.1111/j.1651-2227.2002.tb02917.x. [DOI] [PubMed] [Google Scholar]
- 34.Faldella G, Corvaglia L, Lanari M, Salvioli GP. Iron balance and iron nutrition in infancy. Acta Paediatr Suppl. 2003;441:82–85. doi: 10.1111/j.1651-2227.2003.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 35.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatol. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivares M, Llaguno S, Marin V, Hertrampf E, Mena P, Milad M. Iron status in low-birth-weight infants, small and appropriate for gestational age: a follow-up study. Acta Paediatr. 1992;81:824–828. doi: 10.1111/j.1651-2227.1992.tb12111.x. [DOI] [PubMed] [Google Scholar]
- 37.Lubach GR, Coe CL. Preconception maternal iron status is a risk factor for iron deficiency in infant Rhesus monkeys (Macaca mulatta) J Nutr. 2006;136:2345–2349. doi: 10.1093/jn/136.9.2345. [DOI] [PubMed] [Google Scholar]
- 38.Ilyes I, Jezerniczky J, Kovacs J, Dvoracsek E, Csorba S. Relationship of maternal and newborn (cord) serum ferritin concentrations measured by immunoradiometry. Acta Paediatr Hung. 1985;26:317–321. [PubMed] [Google Scholar]
- 39.Jaime-Perez JC, Herrera-Garza JL, Gomez-Almaguer D. Sub-optimal fetal iron acquisition under a maternal environment. Arch Med Res. 2005;36:598–602. doi: 10.1016/j.arcmed.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71:1280S–1284S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 41.Erdem A, Erdem M, Arslan M, Yazici G, Eskandari R, Himmetoglu O. The effect of maternal anemia and iron deficiency on fetal erythropoiesis: comparison between serum erythropoietin, hemoglobin and ferritin levels in mothers and newborns. J Matern Fetal Neonatal Med. 2002;11:329–332. doi: 10.1080/jmf.11.5.329.332. [DOI] [PubMed] [Google Scholar]
- 42.Mahajan S, Aalinkeel R, Shah P, Singh S, Kochupillai N. Nutritional anaemia dysregulates endocrine control of fetal growth. Br J Nutr. 2008;100:408–417. doi: 10.1017/S000711450889438X. [DOI] [PubMed] [Google Scholar]
- 43.Singla PN, Tyagi M, Shankar R, Dash D, Kumar A. Fetal iron status in maternal anemia. Acta Paediatr. 1996;85:1327–1330. doi: 10.1111/j.1651-2227.1996.tb13919.x. [DOI] [PubMed] [Google Scholar]
- 44.Finne PH. Erythropoietin levels in cord blood as an indicator of intrauterine hypoxia. Acta Paediatr Scand. 1966;55:478–489. doi: 10.1111/j.1651-2227.1966.tb15239.x. [DOI] [PubMed] [Google Scholar]
- 45.Blackburn ST. Maternal, fetal, & neonatal physiology: a clinical perspective. 3rd ed. New York, NY: Elsevier Health Sciences; 2007. [Google Scholar]
- 46.Amarilyo G, Mimouni FB, Oren A, et al. Prohepcidin concentrations and erythroid progenitors in cord blood of appropriate versus small for gestational age neonates. J Perinatol. 2010;30:396–398. doi: 10.1038/jp.2009.179. [DOI] [PubMed] [Google Scholar]
- 47.Colomer J, Colomer C, Gutierrez D, et al. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinatal Epidemiol. 1990;4:196–204. doi: 10.1111/j.1365-3016.1990.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 48.Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: a case-control study in Jordan. Intl J Epidemiol. 1999;28:461–468. doi: 10.1093/ije/28.3.461. [DOI] [PubMed] [Google Scholar]
- 49.de Pee S, Bloem MW, Sari M, Kiess L, Yip R, Kosen S. The high prevalence of low hemoglobin concentration among Indonesian infants aged 3–5 months is related to maternal anemia. J Nutr. 2002;132:2215–2221. doi: 10.1093/jn/132.8.2215. [DOI] [PubMed] [Google Scholar]