Abstract
Young children often perseverate, engaging in previously correct, but no longer appropriate behaviors. One account posits that such perseveration results from the use of stimulus-specific representations of a situation, which are distinct from abstract, generalizable representations that support flexible behavior. Previous findings supported this account, demonstrating that only children who flexibly switch between rules could generalize their behavior to novel stimuli. However, this link between flexibility and generalization might reflect general cognitive abilities, or depend upon similarities across the measures or their temporal order. The current work examined these issues by testing the specificity and generality of this link. In two experiments with 3-year-old children, flexibility was measured in terms of switching between rules in a card-sorting task, while abstraction was measured in terms of selecting which stimulus did not belong in an odd-one-out task. The link between flexibility and abstraction was general across (1) abstraction dimensions similar to or different from those in the card-sorting task and (2) abstraction tasks that preceded or followed the switching task. Good performance on abstraction and flexibility measures did not extend to all cognitive tasks, including an IQ measure, and dissociated from children’s ability to gaze at the correct stimulus in the odd-one-out task, suggesting that the link between flexibility and abstraction is specific to such measures, rather than reflecting general abilities that affect all tasks. We interpret these results in terms of the role that developing prefrontal cortical regions play in processes such as working memory, which can support both flexibility and abstraction.
Keywords: executive functions, cognitive flexibility, abstract reasoning, cognitive development
Introduction
Children demonstrate remarkable limitations in executive functions, including cognitive flexibility. For example, when asked to sort cards first by one rule (e.g., shape) and later sort the same cards by another rule (e.g., color) in the dimensional change card-sort task (DCCS), 3-year-olds reliably perseverate by continuing to sort cards by the previously correct, but no longer appropriate rule, even after explicit, repeated instructions to switch to the new rule (Zelazo et al., 1996; Kirkham et al., 2003; Yerys and Munakata, 2006; Kloo et al., 2008). Even 8- to-10-year-old children often perseverate on the old rule in the related Wisconsin Card Sort Task (Crone et al., 2006), and perseveration is not completely eliminated even in typical adults (e.g., Miyake et al., 2000).
What causes such limitations in executive function? Prominent accounts of perseveration posit that children who successfully switch to new behaviors and children who perseverate on old behaviors differ in processes that operate on representations of the basic sorting rules, and not in how the basic rules themselves are represented. For example, the attentional inertia account posits that perseverators may know the new rule they should be following, but fail to inhibit attending to the previously correct dimension (Kirkham et al., 2003). The redescription account posits that perseverators can conceptualize a conflicting stimulus in a single way (e.g., as a red one), but lack the understanding that objects can be described in several ways and thus fail to redescribe stimulus objects in terms of alternative dimensions or perspectives (e.g., as a truck; Perner and Lang, 2002; Kloo and Perner, 2003; Kloo et al., 2008). A version of the cognitive complexity and control account (CCC-r; Zelazo et al., 2003) posits that perseveration results in part from an inability to activate new rules (e.g., to sort by color), as a result of having ignored the associated features (e.g., red and blue) while following the previous rule (e.g., shape; Muller et al., 2006).
Alternative accounts posit that switchers and perseverators are relying on distinct types of basic rule representations (Munakata, 1998; Zelazo et al., 2003; Marcovitch et al., 2007; Towse et al., 2007; Chevalier and Blaye, 2008). For example, according to an active–latent account (Morton and Munakata, 2002), switchers represent task-relevant information (e.g., this is the “shape” game) actively, via neuronal firing in prefrontal cortical (PFC) regions. These active representations serve to maintain currently task-relevant information in working memory, thus supporting flexible switching to new tasks (Cohen and Servan-Schreiber, 1992), as well as faster responses to questions about the task (Blackwell et al., 2009). Consistent with this working memory account, when children and adults switch to a new rule on the DCCS, they activate the frontoparietal network, including the dorsolateral PFC and the inferior frontal junction (Morton et al., 2009), regions that are activated in working memory tasks (Cole and Schneider, 2007).
In addition to supporting flexible switching, these prefrontal active working memory representations can encode information in an abstract or categorical form, collapsing across specific idiosyncratic details in the service of forming explicit superordinate categories, which could be applied outside of the learned context and in novel situations (Bunge et al., 2003; Badre et al., 2010). For example, relatively abstract categories of “color” or “shape” or “orientation” should help to generalize information across distinct features, such as “red,” “blue,” “truck,” “flower,” or “vertical”). Similarly, the PFC also supports integrating abstract information to form analogies, or abstract relations between items or categories (Green et al., 2006; Wendelken et al., 2008; Badre et al., 2010; Speed, 2010). In contrast, perseverators represent information latently according to the active–latent account, through changes in neuronal connections in posterior cortical areas, which are thought to encode information in a more stimulus-specific, detailed, and graded form (Miller and Desimone, 1994; Jog et al., 1999).
The active–latent account makes a counterintuitive prediction regarding the development of flexibility: despite the fact that perseverators seem completely stuck on sorting by a particular rule, they should struggle with the task of sorting a new item by that rule. That is, perseverators’ more stimulus-specific representations should preclude generalization of their behavior to novel cards, whereas switchers’ more abstract representations should allow them to apply their sorting rule to novel cards. This prediction has been confirmed (Kharitonova et al., 2009). Across two experiments, only 3-year-olds who switched flexibly in the DCCS applied their behavior to sorting novel cards. For example, after switching from sorting blue trucks and red flowers by color to sorting them by shape, children next generalized their behavior to novel cards (e.g., sorting a teal TV with a similarly boxy red truck target, and sorting an orange ball with a similarly round blue flower target). In contrast, perseverators performed at chance in generalizing their perseverative behavior to these novel cards.
This study provided the first demonstration of a striking difference between switchers’ and perseverators’ representations of rules guiding their behavior. These findings are consistent with theoretical perspectives that emphasize the importance of abstract representations in supporting cognitive flexibility and other domains of higher-order cognition (Vygotsky, 1962; Jacques and Zelazo, 2001, 2005; Wallis et al., 2001; Gentner, 2003; Zelazo et al., 2003; Rougier et al., 2005; Bunge and Zelazo, 2006; Pasnak et al., 2009; Fisher, 2011)1. In contrast, these findings are counterintuitive from the perspective of alternative accounts that assume switchers and perseverators represent basic rules in the same way, and differ only in processes that operate on these rules. If switchers and perseverators are sorting by the color or shape rule in the same way, why can only switchers sort novel colors or shapes correctly? That is, why should perseverators fail to generalize the rule they are currently using, if they simply lack: (a) the inhibitory mechanisms to stop sorting cards by this rule (e.g., Kirkham et al., 2003), or (b) ability to redescribe stimuli according to a new rule (e.g., Perner and Lang, 2002; Kloo and Perner, 2003; Kloo et al., 2008), or (c) the ability to activate a new rule as a result of having ignored it earlier (Muller et al., 2006)? Thus, the finding that switchers and perseverators represent sorting rules at different levels of abstraction poses a challenge to prominent accounts of cognitive flexibility.
However, other interpretations are possible. For example, alternative accounts might explain the observed link between flexibility and abstraction by appealing to factors that are not specific to flexibility or abstraction. This relationship might reflect a general cognitive advantage for switchers who might excel across tasks, regardless of whether they depend on the development of the PFC. To test this possibility, we investigate the specificity of the flexibility–abstraction link, by also including a perceptual priming task thought to rely on maturity of occipital regions rather than PFC regions (Gabrieli et al., 1995; Schacter and Buckner, 1998; Schott et al., 2002; Henson, 2003). If switchers’ advantage is not specific to tasks that require prefrontal active and abstract representations (contrary to Kharitonova et al.’s interpretation), switchers should also perform better on the perceptual priming measure. In addition, we include the spatial reasoning subtest of an established IQ measure, KABC-II (Kaufman and Kaufman, 2004) to test if the flexibility–abstraction link is mediated by aspects of intelligence.
Alternative accounts might also posit that the observed link between flexibility and abstraction is a by-product of the two tasks relying on the same dimensions, instead of reflecting a domain–general relationship. That is, the previously reported link suggests that the ability to maintain an active representation that supports switching to a particular dimension (e.g., color) is linked to having an abstract representation and subsequent generalization to that same dimension (e.g., color). It is unclear whether this link holds only when the dimension relevant for abstraction is identical to the dimension relevant for switching. Much of knowledge acquired early in development is gained in a piecemeal fashion (e.g., Tomasello, 2000), such that the ability to flexibly switch to one dimension or category (e.g., color) might not be linked to abstracting another dimension (e.g., orientation). In contrast, these links might develop in a more integrated fashion, based on their reliance on common mechanisms, such that active representations of one dimension are linked to abstract representations of other dimensions. One example of how this process might occur was demonstrated in a neural network model, in which a simulated PFC system that could actively maintain information was also able to extract all the relevant dimensions in the environment (e.g., shape, size, color) after a sufficient breadth of exposure (Rougier et al., 2005). Thus, to determine the generality of the earlier-reported link, we include a task that requires abstraction of a dimension (orientation) that is different from the dimensions used in the DCCS flexibility task (color or shape).
In addition, we test the generality of the flexibility–abstraction link by testing whether it depends upon switching preceding generalization. The previous finding showed that switchers generalized their behavior to a subsequent task, while perseverators did not. In the current studies, we assess abstraction before testing switching, using an odd-one-out task in which children were asked to select one picture that did not belong with three others2. This task requires abstraction because it cannot be solved based purely on the perceptual characteristics of the attributes themselves; the same stimulus can be the odd-one-out in one grouping but not in another grouping. Thus, relations between attributes need to be considered to determine the “odd” stimulus (Chalmers and Halford, 2003); this comparison process might be essential for promoting abstract thought (Christie and Gentner, 2010; Son et al., 2010). If children who flexibly switch from one rule to another also show better abstraction in this preceding odd-one-out task, this would suggest that the flexibility–abstraction relationship is more general rather than being dependent on first activating representations for switching.
Based on the active–latent framework, we predict both specificity and generality of the link between abstraction and flexibility. We predict that this link is general across different measures of abstraction and flexibility and orderings of these measures, while being specific to these measures over other measures that do not depend on active, abstract PFC representations. Addressing these questions about the specificity and generality of the link between children’s flexible switching and abstract representations is a critical step toward reconciling conflicting theories about the development of cognitive flexibility and better understanding of the mechanisms supporting the development of flexible thought.
Experiment 1
We tested the generality of the flexibility–abstraction link by giving children: (1) an odd-one-out shape or color task just before they needed to flexibly switch to sorting cards by the same dimension, and (2) an odd-one-out task in a new dimension (orientation) after they needed to flexibly switch to sorting cards by shape or color. We tested the specificity of the flexibility–abstraction link by assessing performance on a perceptual priming measure.
Material and methods
Participants
Forty-seven 3-year-olds (M = 3.3 years, range: 2.9–3.8 years; 33 boys) participated (22 in the shape to color condition 25 in color to shape). One child failed to finish all tasks included in the session, and is therefore only included in analyses of those tasks that were completed. Two additional children were excluded from all analyses due to mixed behavior in the postswitch phase. Participants were recruited from a departmental participant pool or from local preschools. This study was approved by the University of Colorado Institutional Review Board and informed consent was obtained from a parent of each participant before testing. Children received a small prize and the parents of participants tested in the laboratory were paid $5 for travel expenses.
Design and procedure
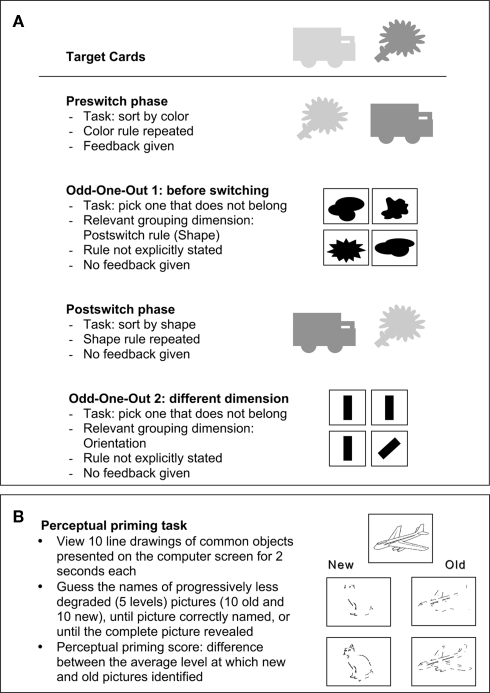
The experiment consisted of five phases (Figure 1) and lasted 20–30 min.
Figure 1.
(A) Dimensional Change Card Sort and Odd-one-out tasks: The DCCS sorting cards are shown under the corresponding target cards, for a sample preswitch and postswitch. (B) Perceptual priming task: Pictures were presented in a random, but constant order. “Old” and “new” lists were counterbalanced, and constructed such that each list contained the same number of items from various categories, such as animals and household items.
-
(1)
Preswitch: The experimenter first named the game and explained the rule (“Today we are going to play a game called the shape game. In the shape game, trucks go here and flowers go here.”). The experimenter then demonstrated the game by sorting two sorting cards facedown into the appropriate trays. The sorting cards (blue trucks and red flowers) matched each target (red truck or blue flower) on only one dimension. The child was then invited to participate (“Now it’s your turn to play!”) and for each of the subsequent six preswitch trials, the rule was reiterated (“Remember, in the shape game, trucks go here and flowers go here.”) and feedback was given (“Good job!” if correct or “No, trucks go here in the shape game.”).
-
(2)
Odd-one-out, preswitch: To assess the generality of the flexibility–abstraction link, we adapted a measure that would allow us to test abstraction separate from the card-sorting task. In this measure (based on Chalmers and Halford, 2003; Pasnak et al., 2009; also reviewed in Henry and Bettenay, 2010), children were asked to select the one picture that did not belong with the others. Each of the five odd-one-out trials included four color patches or four shapes, arranged in a two-by-two table, and printed on a single sheet of paper. In each set, one of the four pictures was the “odd-one-out” on the basis of either color (e.g., three warm colors and one cool color) or shape (e.g., three angular and one rounded shape). This task is thought to measure abstraction because it requires participants to select an answer based on overarching category, such as “warm color,” instead of based on specific, idiosyncratic features of each item.
The dimension of this first odd-one-out task (i.e., color or shape) matched the dimension to be used in the subsequent postswitch phase, because one of our original goals was to investigate the effect of exposure to different levels of abstraction (broad vs. narrow) on subsequent switching3. However, the manipulation of broad vs. narrow categories did not influence switch rates (Wald’s < 0.1), possibly because the broad categories were not sufficiently broad to activate an abstract category of color. Therefore, we focus on the more meaningful individual differences in children’s performance on the odd-one-out task (collapsed across broad and narrow categories) and their ability to switch on DCCS. Exposure to the same dimension used in postswitch also did not contribute to any flexibility–abstraction link by priming children to the postswitch dimension, a point elaborated in the Section “Discussion.”
The odd-one-out phase started with the experimenter removing the target cards to minimize potential conflict from extraneous stimuli4. The experimenter then announced, “Okay, now I’m going to show you four things, and one of the things is not like the other things. One thing is different. And I want you to tell me which one is different.” The experimenter then demonstrated by saying, “First it’s my turn. I’m looking very carefully at these four things and I think THIS one is different,” while pointing to the correct odd-one-out picture. The child was then invited to complete five odd-one-out trials and select the “different” picture. The rule by which the odd-one-out should be chosen was not explicitly stated and feedback was not given. Data in the odd-one-out tasks were analyzed in terms of proportion of trials answered correctly.
-
(3)
Postswitch: The postswitch phase started with the experimenter reattaching the target cards to the sorting tray. The experimenter then strongly emphasized the change of the game: “Remember these? Now we are going to switch and play a new game, called the color game. We are not going to play the shape game anymore. No way! We are going to play the color game and the color game is different. In the color game, red ones go here and blue ones go here.” During the postswitch phase, the rule was repeated for each of the six trials (“Remember, in the color game, red ones go here and blue ones go here.”). However, in postswitch no feedback was given – the experimenter neutrally said “OK” after the child placed each card into a tray.
-
(4)
Odd-one-out, different dimension: This phase was identical to the first odd-one-out, but the relevant dimension was line orientation. The experimenter once again removed the target cards and invited the child to complete four trials by selecting the picture that was “different.” The rule was not explained and feedback was not provided.
-
(5)
Perceptual priming (Figure 1B): Children saw a series of 10 complete (non-fragmented) line drawings of common objects (selected from Cycowicz et al., 2000) on a computer screen, presented for 2 s each5. Children were then shown 10 new and 10 old pictures, fragmented as in Cycowicz et al. (2000) and were asked to guess the names of each picture.
Results
As in previous studies (e.g., Kirkham et al., 2003), children were classified as switchers or perseverators due to non-normal data in the postswitch phase. Twenty-two children switched (sorted at least five out of six postswitch trials correctly), and 25 children perseverated (sorted no more than one out of six postswitch trials correctly). The relationship between performance on the first odd-one-out task and switching did not depend on age [Wald(1) = 1.4, p = 0.2] or the type of DCCS game [switching from color to shape, or from shape to color; Wald(1) = 0.92, p = 0.3]. Hence, the following analyses collapse across these factors.
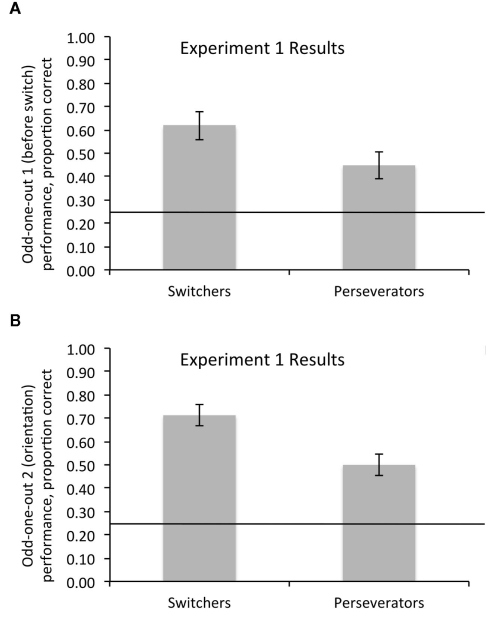
Across both of the odd-one-out tasks, both switchers and perseverators performed above chance (i.e., above 25% correct), both ps < 0.006, demonstrating a rudimentary ability to compare and contrast items based on category membership. However, switchers picked the odd-one-out more accurately than perseverators on both the color/shape odd-one-out task that preceded switching [Switchers’ M = 0.62, SD = 0.28; Perseverators’ M = 0.45, SD = 0.29; t(45) = 2.0, p = 0.048; Figure 2A] and on the unrelated orientation odd-one-out task that followed switching [Switchers’ M = 0.71, SD = 0.2; Perseverators’ M = 0.50, SD = 0.2; t(44) = 3.3, p = 0.002; Figure 2B].
Figure 2.
Switchers performed better than perseverators at abstracting the relevant dimension on odd-one-out tasks, (A) before the switching task, and (B) requiring the abstraction of a dimension (orientation) not previously used in the switching task. Both groups performed better than chance (ps < 0.006), indicated by the horizontal lines. Error bars represent SEM.
The perceptual priming measure was successful in eliciting priming, since old items (M = 5.3, SD = 0.44) were recognized at a less fragmented level than new items (M = 5.8, SD = 0.44), F(1,41) = 40.7, p < 0.001. Critically, perceptual priming did not interact with switch status, F(1,41) = 1.1, p = 0.3 and also was unrelated to both odd-one-out tasks, both ps > 0.3. Thus, even though both switchers and perseverators performed better than chance on the perceptual priming task (i.e., better than zero difference between levels at which old and new items were recognized), both ps < 0.001, the two groups did not differ in their ability to remember precise perceptual details of pictures.
Discussion
Experiment 1 demonstrated that the developmental link between flexibility and abstraction is: (1) specific – performance on these measures is unrelated to performance on a perceptual priming task that does not depend on the maturity of PFC regions (Gabrieli et al., 1995; Schacter and Buckner, 1998; Schott et al., 2002; Henson, 2003), (2) general across orthogonal dimensions, such that switching from one dimension to another (e.g., from color to shape) is related to the ability to reason abstractly within a third, unrelated dimension (e.g., orientation), and (3) general across temporal orderings, such that the link between flexibility and generalization holds even when the abstraction task precedes the switching task. Although these findings fit within frameworks emphasizing the link between abstract and flexible thought, the extent of this link is nonetheless surprising. The orientation odd-one-out task should be quite easy: children only need to pick out one line (e.g., a horizontal one) as different from three other lines that are identical to each other (e.g., all vertical). Thus, it is remarkable that children have difficulty with this task, and moreover, that their performance is linked to their ability to switch on DCCS.
Why do children have such difficulty with the orientation odd-one-out task? After all, even young infants show perceptual pop-out effects for line orientation (Atkinson and Braddick, 1992; Rieth and Sireteanu, 1994), which might be expected to support accurate performance on this task in the much older children tested here. However, pre-attentive perceptual processes like those that support pop-out effects are not always sufficient to support performance in other tasks (Schneider and Shiffrin, 1977; Sagi and Julesz, 1985; Hansen and Hansen, 1988; Lachter et al., 2004; Ghorashi et al., 2010). For example, people can pre-attentively detect target stimuli that vary on only one dimension, such as vertical and horizontal lines among diagonal ones, and yet fail to identify the orientation of these lines without deploying selective attention (Sagi and Julesz, 1985). Specifically, as the number of target stimuli is increased, reaction times for the detection task remain constant (consistent with pre-attentive, parallel processing), while reactions times for the identification task increase (consistent with serial, attentive processing).
Thus, distinct stimuli can pop-out pre-attentively, but further processing of this information in the service of goals (e.g., for identification in Sagi and Julesz, 1985, or manual selection in our odd-one-out task) requires selective attention and likely recruits distinct systems including PFC regions (Lieberman et al., 2004; McClure et al., 2004; O’Reilly, 2010). For children with less developed PFC regions, these systems may not support such further processing despite sensitivity in pre-attentive processes. If so, all children in our odd-one-out task may exhibit sensitivity to the target item in where they gaze, even though switchers are better than perseverators at manually selecting the odd-one-out. We test this possibility in Experiment 2 by using an eye-tracker.
Alternatively, it is possible that children’s poor performance (and perseverators performing worse than switchers) reflected a difficulty in switching to attending to the new dimensions, such as the postswitch dimension in the first odd-one-out, or the orientation dimension in the second odd-one-out that came after dealing with color and shape dimensions. We test this possibility in Experiment 2, by administering the orientation odd-one-out task first, before the color/shape card sort. If the link between color/shape switching and orientation odd-one-out were driven by perseverators’ difficulty with switching to the orientation dimension, then this link should no longer hold when the order of the tasks is reversed.
The new ordering allows us to test another possibility regarding Experiment 1. Results from the color/shape odd-one-out task suggested that the link between flexibility and generalization holds even when the abstraction task precedes the switching task. It is possible, however, that this odd-one-out task primed the postswitch dimension, thus helping children switch to it and leading to the link between odd-one-out and switching. We do not think this possibility is likely, because the color/shape odd-one-out task in the present study did not appear to significantly inflate switch rates6, minimizing the possibility that our effects were driven by priming children to the postswitch dimension. Experiment 2 tests this issue more directly, by using an unrelated abstraction task prior to the switching task. If the results of Experiment 1 were driven by priming the relevant dimension with the color/shape odd-one-out game, or by asking children to switch to a new dimension, then the link between flexibility and abstraction should disappear when the abstraction task requiring the use of one dimension precedes the flexibility task that requires use of different dimensions.
Finally, Experiment 2 also tests the possibility that the link between flexibility and abstraction is driven by cognitive processes that are not measured by perceptual priming. We examine this possibility by including a standardized intelligence measure.
Experiment 2
We test the generality of the flexibility–abstraction link, by having the orientation odd-one-out task precede the color/shape card-sorting task. We test the specificity of the flexibility–abstraction link by assessing whether the link is driven by individual differences in general intelligence. We also examine the basis for children’s poor performance during the orientation odd-one-out task by measuring where they look during the task. If children can use pre-attentive perceptual processes to detect the odd-one-out, and perseverators’ difficulty with the task stems from limitations in processing the relevant dimensional information in goal-relevant, prefrontal regions, then perseverators and switchers should show comparable sensitivity to the target item in where they look, despite differences in their pointing performance.
Material and methods
Participants
Forty-nine 3-year-olds (M = 3.4 years, range: 3.25–3.7 years; 22 boys) participated (23 in the shape to color condition 26 in color to shape). Additional children were excluded from all analyses due to failing the preswitch phase (N = 1) and for mixed performance on the postswitch phase (N = 3). Participants were recruited from a departmental participant pool. This study was approved by the University of Colorado Institutional Review Board and informed consent was obtained from a parent of each participant before testing. Children received a small prize and the parents of participants tested in the laboratory were paid $5 for travel expenses.
Design and procedure
The experiment consisted of five phases and lasted approximately 30 min.
-
(1)
Odd-one-out, orientation dimension: The odd-one-out task was programmed and executed using E-Prime (version 1.1), and all responses were collected through a MagicTouch touchscreen interface, in order to allow eye-gaze data to be collected using the Tobii X50 eye-tracker, at the frequency of 50 Hz. Participants viewed the stimuli from a 60-cm distance and used a wooden “magic pointing stick” to respond on the touchscreen.
Participants were first familiarized with the touch screen and trained on responding using the stick by tapping one of the four empty frames on the screen at a time, presented in the same locations as the experimental stimuli. Experimenter pointed to a location and asked participants to “poke” there, making the frames disappear.
Participants were then told, “Now that you know how to poke, we are going to play a game. In this game, you will see pictures, and one of the pictures is going to be not like the other, one of them is going to be different. I want you to poke the one that looks different.”
The experimenter then demonstrated one trial by saying, “First it’s going to be my turn to play. I am looking very carefully at all of these pictures [experimenter points to all four pictures, in the order different from the tapping training], and you know what? I think this one is different.” Experimenter pointed to the picture that was different, holding the pointer there for several seconds to making sure the child saw where the different picture was, and then “poked” it with the stick, making it disappear. The experimenter then said, “Now it’s your turn to play. Can you poke the one that looks different?”
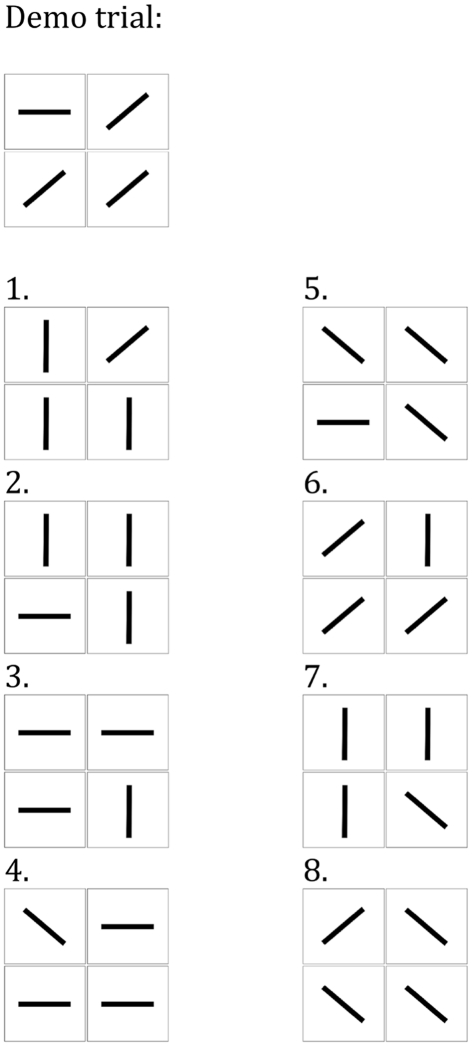
The stimuli then appeared on the screen and remained there until the child made a response. The children were invited to complete eight odd-one-out trials (the number of trials was increased relative to Experiment 1 to increase task reliability) and select the “different” picture. As in Experiment 1, the rule by which the odd-one-out should be chosen was not explicitly stated and feedback was not given. As in the orientation odd-one-out task in Experiment 1, in each trial three lines were identical and one had a different orientation. The order of target location was counterbalanced across eight trials (Figure 3). On each trial, we quantified the number of eye gazes in each of the four regions of the screen where the stimuli appeared, until the trial was terminated by the subject’s response (Figures 5A,B).
-
(2)
Preswitch, (3) Postswitch, and (4) Perceptual priming phases were administered in the same way as in Experiment 1, minus the detaching and reattaching of the target cards between preswitch and postswitch phases, because the odd-one-out task no longer intervened between them.
-
(5)
KABC-II IQ measure. To test whether the link between flexibility and abstraction is related to individual differences in general intelligence, the triangles subset of the KABC-II test (Kaufman and Kaufman, 2004) was administered to participants. This subtest of the IQ test requires children to assemble plastic and foam shapes to match increasingly more complex abstract designs. The administration of this subtest stopped when children answered three consecutive questions incorrectly. We used the raw scores to control for absolute level of intelligence proficiency. Results were identical with standardized scores, because raw scores and standardized scores were highly correlated (R = 0.96, N = 44, p < 0.001), given the narrow age range of participants.
Figure 3.
The setup of the odd-one-out task in Experiment 2, include a demonstration trial (performed by experimenter) and eight experimental trials.
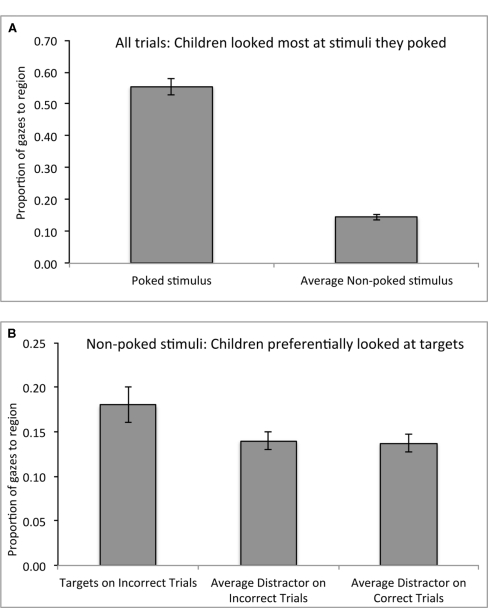
Figure 5.
Eye-gaze data on the orientation odd-one-out task. (A) Unsurprisingly, children looked most at the items they pointed to in each trial, regardless of accuracy. (B) Critically, even when children poked the wrong stimulus, they looked more at the target than at both the average non-poked distractor on those incorrect trials and the average distractor on the correct trials, indicating some sensitivity to the correct answer. Switchers and perseverators did not differ in this pattern, suggesting that visually detecting these targets is independent of cognitive flexibility. The proportions do not add to 1.0 because gazes to Non-reached stimuli and Distractors were computed as averages (i.e., as the total number of gazes to all of the candidate stimuli divided by the number of stimuli), for comparison to the single Reached or single Target stimulus. Error bars represent SEM.
Results
Children again were classified as switchers (N = 29) and perseverators (N = 20), based on the same criteria as in Experiment 1.
Manual selection results
As in Experiment 1, both switchers and perseverators performed above chance on the odd-one-out task (i.e., above 25% correct), both ps < 0.001, again indicating at least a rudimentary ability to pick an odd-one-out item. The relationship between performance on the odd-one-out task and switching did not depend on age [Wald(1) = 0.9, p = 0.3] or the type of DCCS game [switching from color to shape, or from shape to color; Wald(1) = 0.11, p = 0.7]. Hence, the following analyses collapse across these factors.
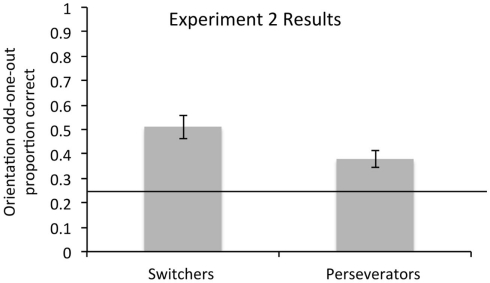
Consistent with our predictions and our results from Experiment 1, switchers picked the odd-one-out more accurately than perseverators on the orientation odd-one-out task (Switchers’ M = 0.51 correct, SD = 0.26; Perseverators’ M = 0.38, SD = 0.15), t(47) = 2.0, p = 0.047 (Figure 4). The perceptual priming task again successfully elicited priming, such that old items (M = 5.5, SD = 0.60) were recognized at a less fragmented state than new items (M = 6.1, SD = 0.41), F(1,44) = 63.0, p < 0.001. However, once again, perceptual priming did not interact with switch status, F(1,44) = 2.1, p = 0.2 and it was also unrelated to odd-one-out performance, F(1,44) = 0.04, p = 0.9. The similarity in the results across Experiments 1 and 2 was confirmed by adding Experiment (1 or 2) as a factor into the regression. Only switch status significantly predicted odd-one-out performance, F(1,91) = 4.0, p = 0.048; the interaction between experiment and switch status was not significant, F(1,91) = 0.76, p = 0.4. There was also a marginal main effect of experiment [F(1,91) = 3.3, p = 0.07], such that odd-one-out performance was marginally better in Experiment 1 than Experiment 2. There are many possible reasons for why the overall levels of performance would differ, including the computerized setup of Experiment 27. However, because the relationship between switching and odd-one-out performance did not depend on the experiment, we can conclude that this relationship holds across task orders and other sources of variability in experiments (e.g., different groups of children, computerized vs. paper-based setup, etc.).
Figure 4.
Odd-one-out performance in Experiment 2: Switchers again performed better than perseverators at abstracting the relevant dimension, even when the orientation odd-one-out task preceded the color/shape flexibility task. Error bars represent SEM.
Switchers performed marginally better than perseverators on the KABC spatial IQ subtest F(1,44) = 2.9, p = 0.094; however, KABC performance was unrelated to odd-one-out performance, F(1,44) = 0.94, p = 0.34, and did not mediate the relationship observed between switching and odd-one-out, as assessed through a formal mediation analysis (Preacher and Hayes, 2008). Specifically, the 95% confidence interval for the indirect (mediating) effect of IQ is −0.02 to 0.10 (with a point estimate of 0.016; SE = 0.028); because zero is included in the interval, the mediating effect of IQ on the flexibility–abstraction link is not significant. This mediation analysis generates this estimate via 5000 bootstrap samples, thus making no assumptions about the normality of the sampling distribution of the indirect effect of IQ on the relationship between switching and odd-one-out.
Eye-tracking results
Also consistent with our predictions, children showed high sensitivity in looking at the target items in the odd-one-out task, even on trials where they failed to “poke” these targets. Unsurprisingly, children looked most at the items they poked, such that poked stimuli received 56% of the total gazes to the four stimuli, while non-poked stimuli received only 14% of total gazes on average, F(1,47) = 188.3, p < 0.001 (Figure 5A). Critically, even when children poked the wrong stimulus, they looked more at the target (M = 18.2% of gazes, SD = 11.0) than at both the average non-poked distractor on those incorrect trials (M = 14.5% of gazes, SD = 6.3%), F(1,47) = 149.4, p < 0.001, and the average distractor on the correct trials (M = 13.7% of gazes, SD = 7.2), F(2, 94) = 101.4, p < 0.001 (Figure 5B). This pattern did not interact with switch status (both ps > 0.4), such that switchers and perseverators showed similar gaze sensitivity to the target item over non-reached distractors.
Finally, there was a marginal interaction between switch status and stimulus type [F(1,47) = 3.6, p = 0.06], such that perseverators spent more time looking at the target (M = 60.3%) relative to distractors (M = 13.2%) than did switchers (M = 51.7% of gazes to targets, 16.1% of gazes to distractors). This pattern suggests that switchers engaged in a more active comparison process across the four stimuli in order to select an answer. No other main effects or interactions were significant, all Fs < 1.6.
Discussion
Consistent with the active–latent framework that posits a strong link between flexible and abstract thought, we found that children who switched from one card-sorting rule to another performed better on the preceding orientation odd-one-out task than children who perseverated on the first rule in the card-sorting task. These results suggest that the flexibility–abstraction link is general across dimensions (orientation, color, or shape) and ordering of tasks (abstraction preceding or following switching). Moreover, this link may be specific to tasks that require the use of flexible and abstract representations: there again was no link between flexibility and perceptual priming, as in Experiment 1, and although switchers showed marginally higher IQs than perseverators, IQ was not related to performance on odd-one-out and did not mediate the relationship between switching and odd-one-out.
Critically, the eye-tracking results suggested that children’s difficulty with the odd-one-out task was not due to their failure to detect the target stimulus, given that children looked at the target items more than at the non-reached distractors, even for incorrectly answered trials. Looking at the target might reflect pre-attentive perceptual processes, which are well developed for children at this age. In contrast, goal-related processing, such as manual selection of the different item in the odd-one-out task, might require top-down selective attention toward the orientation dimension, which might be immature, particularly in perseverating children.
General Discussion
Across two experiments we demonstrated that the link between flexibility and abstraction is general across dimensions and task orders, but may also be specific to tasks that rely on the maturity of flexible and abstract mental representations. Experiment 1 showed the link holds when the abstraction task preceded the flexibility task, and when the abstraction task required the use of a different dimension than the flexibility task. Experiment 2 showed that this link holds even when the different dimension task (orientation odd-one-out) preceded the flexibility task.
In addition, our studies demonstrate that the link between flexibility and abstraction does not generalize to tasks that do not depend upon active and abstract representations. Thus, there was no link between flexibility and perceptual priming (Experiment 1 and 2) or gaze sensitivity (Experiment 2), consistent with the idea that more mature PFC function does not improve performance on all tasks. On these other measures, switchers and perseverators demonstrated comparable understanding of basic task instructions and comparable gaze sensitivity to targets in the abstraction task. Finally, performance on the spatial IQ task was not related to odd-one-out performance and only marginally related to switching; critically, the link between flexibility and abstraction was not mediated by spatial IQ performance.
It is of course not possible to assess all cognitive processes; therefore, a possibility remains that switchers’ advantage extends to processes that we were not able to measure. Future work should continue examining the boundaries of the specificity of the flexibility–abstraction link, by testing whether other cognitive measures are related to this link. Our specificity results are nonetheless consistent with findings that high working memory is actually associated with worse performance on tasks where the use of active and abstract representations might be counterproductive, such as implicit category learning (DeCaro et al., 2008).
Our findings are also consistent with accounts emphasizing the role of abstraction in developing cognitive flexibility (Vygotsky, 1962; Premack, 1984; Wallis et al., 2001; Gentner, 2003; Zelazo et al., 2003; Jacques and Zelazo, 2005; Rougier et al., 2005; Bunge and Zelazo, 2006). Our findings go beyond existing work in important ways. Namely, our work demonstrates a link between flexibility and abstraction when abstraction abilities are assessed outside of a flexibility task, allowing for the independent assessment of these processes and their relationship. In contrast, in some studies children were required to have abstraction abilities to succeed on the preswitch task, such that switching abilities could not be measured separately from abstraction abilities (as discussed in Footnote 2). Other studies have demonstrated that switching is easier when abstraction demands are reduced (Fisher, 2011; Honomichi and Chen, 2011), and that abstraction is easier when switching demands are reduced (Blaye and Jacques, 2009). These experiments demonstrate that both switching and abstraction are demanding, while our study demonstrates that these abilities go together early in development.
Our findings pose a challenge for other accounts of perseveration. For example, if the root of perseveration lies with the inability to disengage from a previously relevant dimension (Kirkham et al., 2003), or if perseverators’ difficulty lies with the inability to conceptually redescribe the stimulus in terms of a conflicting dimension (Perner and Lang, 2002; Kloo and Perner, 2003), it is not clear why perseveration would be related to performance on odd-one-out tasks, where inhibition of previously relevant dimensions, or the need to redescribe a stimulus in terms of a new dimension is not necessary. Similarly, if perseveration is due to the inability to activate a new rule after it has been ignored earlier in the task (Muller et al., 2006), it is unclear why perseveration would be related to performance on odd-one-out tasks, where the relevant dimensions has not just been ignored. Instead, what is common across the odd-one-out and generalization tasks is the need for abstract representations.
We speculate that the link between flexibility and abstraction in development reflects their common reliance on processes supported by PFC regions. One possibility is that both flexibility and abstraction depend on active neuronal firing in the PFC across delays and interference, which serves as a mechanism for maintaining information in working memory (Miller and Cohen, 2001). These active representations can support flexible switching by maintaining the currently relevant task information in working memory (Cohen and Servan-Schreiber, 1992; Morton and Munakata, 2002; Marcovitch et al., 2007; Towse et al., 2007; Blackwell et al., 2009), and could support abstraction both in the moment (while performing an abstraction task) and across development (while forming abstract representations). In the moment, the ability to maintain multiple items in working memory (e.g., two diagonal lines and one horizontal one) could support abstraction by allowing pair-wise comparisons to be made between stimuli to determine the relevant dimensional information (e.g., that the stimuli vary only in orientation, Speed, 2010; Anthony Wagner, personal communication, October 2009). Switchers’ more distributed pattern of gazes is consistent with this idea, that better working memory supports both their successful switching and their ability to gaze among stimuli for purposes of comparison in selecting the target. This perspective predicts that increasing the working memory demands in the odd-one-out task (e.g., by increasing the distance between stimuli, such that comparisons between stimuli require them to be strongly maintained in working memory) should yield an even greater advantage for switchers over perseverators.
Across development, the ability to maintain information in working memory has been shown computationally to support the formation of abstract representations, by supporting the maintenance of a common higher-order representation (e.g., one that eventually codes for the dimension of color) across the sequential presentation of multiple exemplars (e.g., red, blue, and yellow; Rougier et al., 2005). Additionally, a separate factor such as language could mediate the simultaneous development of different facets of PFC-supported processes, such as flexibility and abstraction (Vygotsky, 1962; Spelke, 2003; Jacques and Zelazo, 2005), perhaps by supporting working memory processes (Adams and Gathercole, 1995; Miyake et al., 2004; Noble et al., 2005). Future work could test the role of working memory in supporting the link between flexibility and abstraction, by assessing participants’ working memory and testing whether it mediates this link.
One might ask how flexibility and abstraction relate to other aspects of executive function, and to other aspects of intelligence. The relationships between executive functions and intelligence, and the relationships among executive functions and among components of intelligence, are highly debated (e.g., Suß et al., 2002; Jurado and Rosselli, 2007; McGrew, 2009). Although spatial IQ did not explain the link between flexibility and abstraction in our study, some researchers have argued that using more active and abstract representations might constitute an important component of general intelligence (Conway et al., 2003; Gray et al., 2003; Wendelken et al., 2008). If so, the observed link between flexibility and abstraction might share variance with other executive functions, and with other measures of intelligence, such as Raven’s Progressive Matrices, which are more demanding on working memory and other executive processes (Crone et al., 2009). For example, the relationship between working memory and theory of mind disappears after controlling for more comprehensive IQ measures (Carlson et al., 2002), suggesting that active working memory processes and some IQ measures share much common variance. In contrast, other aspects of intelligence, such as crystallized intelligence, do not rely on the integrity of PFC regions (Duncan et al., 1995; Gray and Thompson, 2004), and therefore, can be less related to executive functioning measures. Similarly, other aspects of executive function, such as shifting mental sets, do not seem to be linked to intelligence (e.g., Friedman et al., 2006). We clearly cannot resolve here which parcellation of executive functions or intelligence is the best one, but we speculate that children who switch on the DCCS and do well on the odd-one-out tasks will show advantages on additional PFC-dependent cognitive measures, such as the Raven’s Progressive Matrices (Crone et al., 2009), given the computational benefits of active and abstract representations (e.g., Rougier et al., 2005; Pasnak et al., 2009). Future work testing such possibilities may inform not only an understanding of the developmental bases of abstract and flexible thought, but also ongoing debates about candidate components of executive function and intelligence.
The current work thus provides evidence for the emergence of an inherent link between flexibility and abstraction early in development, and suggests that representational factors contribute to developmental limitations in executive functions. Further work should elucidate the developmental trajectory of the relationship between flexible and abstract thinking across domains and across the lifespan (Craik and Bialystok, 2006), and the dissociation between categorical, abstract processes and those relying on more graded, stimulus-specific representations.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by grants from the National Institutes of Health (RO1 HD37163 and P50-MH079485). We thank Joedy Hulings for data collection in Experiment 1 and Eliana Colunga along with members of the Cognitive Development Center for useful discussions.
Footnotes
1We note that this finding was originally described (in Kharitonova et al., 2009) as a challenge to CCC theory due to the focus of this theory on children’s difficulty with switching between rules; however, several descriptions of this account indicate compatibility with these findings. CCC theory posits that perseverators are capable of following basic, lower-order rules (e.g., knowing where trucks go in the shape game), but lack the ability to navigate among the higher-order rules (e.g., knowing whether to follow the rules of the shape game vs. the color game; e.g., Zelazo et al., 1996). For example, “Although 3–4 year olds are capable of either description, they have difficulty switching flexibly between them” (Zelazo et al., 2003, p. 101). “Notice that when similar tasks only require reasoning within a single dimension, 3-year-olds perform well” (Zelazo et al., 2003, p. 103). Based on descriptions like these, Kharitonova et al. (2009) argued that according to CCC theory, it is not clear why perseverators who are capable of sorting by one rule (color or shape) would fail to continue this behavior with new stimuli, when no flexible switching to a new rule is required. However, CCC theory also posits that the ability to contrast different rules (as required for switching) is inherently linked to a concept of the underlying dimensions. For example, “Being able to reflect on color rules as color rules that contrast with shape rules (or rules from any other dimension) would seem to be required to comprehend the way in which different colors form a coherent category of variation (i.e., a dimension).” And “Indeed, according to CCC theory, the ability to formulate higher-order rules is necessary for the construction of the concept of dimensions, and for subsequent analytical processing of values on those dimensions. As Zelazo and Frye (1997) suggested, it is only by distancing themselves from discriminations within a dimension and considering two or more dimensions in contradistinction that children are able to conceptualize dimensions qua dimensions (see also Smith, 1989)” (Zelazo et al., 2003).
2Abstraction has been measured before switching in the Flexible Item Selection Task as well (Jacques and Zelazo, 2001), in which children must select two out of three items that match on one dimension (a measure of abstraction), and then select a different two out of the items that match on another dimension (a measure of switching). However, because abstraction is a prerequisite for assessing switching in this paradigm, the two constructs could not be assessed independently.
3Our prediction was that odd-one-out-trials based on relatively broad categories (e.g., warm vs. cool colors) might make it easier for children to switch to that category (e.g., color) than odd-one-out trials based on relatively narrow categories (e.g., blue vs. green colors), even though the odd-one-out groupings across the conditions were chosen from an identical set of colors. Therefore, we exposed children to features from the postswitch dimension during the first odd-one-out.
4When designing the study, we were unaware of the recent finding showing that removing the target cards improves DCCS performance (Mack, 2007). It does not appear that removing the cards drastically improved switching in our study, as elaborated in the Section “Discussion.”
5We selected the stimuli for our 3-year-old participants based on lexical development norms (Dale and Fenson, 1996); all but one of the 20 chosen pictures (95%) were known by at least 93% of 30-month old children (the oldest age in the norms), and the remaining picture was known by 70% of them.
6In a pilot study in which the embedded odd-one-out task focused on an irrelevant dimension, size, a comparable number of children (5/12, or 42%; χ2(1) = 0.10, p = 0.7) switched on the color/shape flexibility task. Although 42–47% switch rates for our 40-month-olds (35–45 months) may seem high relative to some other studies (e.g., Carlson, 2005; Mack, 2007), they are compatible with our previous studies in this geographic location with participants of similar demographics (e.g., 65% switch rate with 45-month-olds in Kharitonova et al., 2009, Experiment 2, and 32% switch rate with 39-month-olds in Kharitonova et al., 2009, Experiment 1). These results suggest that neither removing the target cards nor using the postswitch dimension in the odd-one-out phase drastically improved switching in our study. Different switch rates are often observed across different labs (e.g., 38% switch rate in Mack (2007) baseline vs. 10% switch rate in Zelazo et al. (1996), despite having the same study design and virtually identical age ranges (36–41 vs. 36–42 months). Our primary analyses thus focus not on absolute switch rates, but on testing the relation between switching and abstraction.
7Another possibility is that being presented with the odd-one-out task after the switching task (in Experiment 1) led children to perform better on the odd-one-out task. Future work could test this possibility by manipulating the order of the tasks while equating all other aspects of the study.
References
- Adams A. M., Gathercole S. E. (1995). Phonological working memory and speech production in preschool children. J. Speech Hear. Res. 38, 403–414 [DOI] [PubMed] [Google Scholar]
- Atkinson J., Braddick O. (1992). Visual segmentation of oriented textures by infants. Behav. Brain Res. 49, 123–131 10.1016/S0166-4328(05)80202-5 [DOI] [PubMed] [Google Scholar]
- Badre D., Kayser A. S., D’Esposito M. (2010). Frontal cortex and the discovery of abstract action rules. Neuron 66, 315–326 10.1016/j.neuron.2010.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell K., Cepeda N., Munakata Y. (2009). When simple things are meaningful: working memory strength predicts children’s cognitive flexibility. J. Exp. Child Psychol. 103, 241–249 10.1016/j.jecp.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaye A., Jacques S. (2009). Categorical flexibility in preschoolers: contributions of conceptual knowledge and executive control. Dev. Sci. 12, 863–873 10.1111/j.1467-7687.2009.00832.x [DOI] [PubMed] [Google Scholar]
- Bunge S. A., Kahn I., Wallis J. D., Miller E. K., Wagner A. D. (2003). Neural circuits subserving the retrieval and maintenance of abstract rules. J. Neurophysiol. 90, 3419–3428 10.1152/jn.00910.2002 [DOI] [PubMed] [Google Scholar]
- Bunge S. A., Zelazo P. D. (2006). A brain-based account of the development of rule use in childhood. Curr. Dir. Psychol. Sci. 15, 118–121 10.1111/j.0963-7214.2006.00419.x [DOI] [Google Scholar]
- Carlson S. M. (2005). Developmentally sensitive measures of executive function in preschool children. Dev. Neuropsychol. 28, 595–616 10.1207/s15326942dn2802_3 [DOI] [PubMed] [Google Scholar]
- Carlson S. M., Moses L. J., Breton C. (2002). How specific is the relation between executive function and theory of mind? Contributions of inhibitory control and working memory. Infant Child Dev. 11, 73–92 10.1002/icd.298 [DOI] [Google Scholar]
- Chalmers K. A., Halford G. S. (2003). Young children’s understanding of oddity: reducing complexity by simple oddity and “most different” strategies. Cogn. Dev. 18, 1–23 10.1016/S0885-2014(02)00140-5 [DOI] [Google Scholar]
- Chevalier N., Blaye A. (2008). Cognitive flexibility in preschoolers: the role of representation activation and maintenance. Dev. Sci. 11, 339–353 10.1111/j.1467-7687.2008.00679.x [DOI] [PubMed] [Google Scholar]
- Christie S., Gentner D. (2010). Where hypotheses come from: learning new relations by structural alignment. J. Cogn. Dev. 11, 356–373 10.1080/15248371003700015 [DOI] [Google Scholar]
- Cohen J. D., Servan-Schreiber D. (1992). Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol. Rev. 99, 45–77 10.1037/0033-295X.99.1.45 [DOI] [PubMed] [Google Scholar]
- Cole M. W., Schneider W. (2007). The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 37, 343–360 10.1016/j.neuroimage.2007.03.071 [DOI] [PubMed] [Google Scholar]
- Conway A. R. A., Kane M. J., Engle R. W. (2003). Working memory capacity and its relation to general intelligence. Trends Cogn. Sci. (Regul. Ed.) 7, 547–552 10.1016/j.tics.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Craik F. I. M., Bialystok E. (2006). Cognition through the lifespan: mechanisms of change. Trends Cogn. Sci. (Regul. Ed.) 10, 131–138 10.1016/j.tics.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Crone E. A., Somsen R. J. M., Zanolie K., Van der Molen M. W. (2006). A heart rate analysis of developmental change in feedback processing and rule shifting from childhood to early adulthood. J. Exp. Child. Psychol. 95, 99–116 10.1016/j.jecp.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Crone E. A., Wendelken C., van Leijenhorst L., Honomichl R. D., Christoff K., Bunge S. A. (2009). Neurocognitive development of relational reasoning. Dev. Sci. 12, 55–66 10.1111/j.1467-7687.2008.00743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz Y. M., Friedman D., Snodgrass J. G., Rothstein M. (2000). A developmental trajectory in implicit memory is revealed by picture fragment completion. Memory 8, 19–35 10.1080/096582100387687 [DOI] [PubMed] [Google Scholar]
- Dale P. S., Fenson L. (1996). Lexical development norms for young children. Behav. Res. Methods Instrum. Comput. 28, 125–127 10.3758/BF03203646 [DOI] [Google Scholar]
- DeCaro M., Thomas R., Beilock S. (2008). Individual differences in category learning: sometimes less working memory capacity is better than more. Cognition 107, 284–294 10.1016/j.cognition.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Duncan J., Burgess P., Emslie H. (1995). Fluid intelligence after frontal lobe lesions. Neuropsychologia 33, 261–268 10.1016/0028-3932(94)00124-8 [DOI] [PubMed] [Google Scholar]
- Fisher A. (2011). Automatic shifts of attention in the dimensional change card sort task: subtle changes in task materials lead to flexible switching. J. Exp. Child. Psychol. 108, 211–219 10.1016/j.jecp.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Friedman N. P., Miyake A., Corley R. P., Young S. E., DeFries J. C., Hewitt J. K. (2006). Not all executive functions are related to intelligence. Psychol. Sci. 17, 172–179 10.1111/j.1467-9280.2006.01681.x [DOI] [PubMed] [Google Scholar]
- Gabrieli J. D. E., Fleischman D. A., Keane M. M., Reminger S. L., Morrell F. (1995). Double dissociation between memory systems underlying explicit and implicit memory in the human brain. Psychol. Sci. 6, 76–82 10.1111/j.1467-9280.1995.tb00310.x [DOI] [Google Scholar]
- Gentner D. (2003). “Why we’re so smart,” in Language in Mind: Advances in the Study of Language and Thought, eds Gentner D., Goldin-Meadow S. (Cambridge, MA: MIT Press; ), 195–235 [Google Scholar]
- Ghorashi S., Enns J. T., Klein R. M., Di Lollo V. (2010). Spatial selection and target identification are separable processes in visual search. J. Vis. 10, 1–12 10.1167/10.3.12 [DOI] [PubMed] [Google Scholar]
- Gray J. R., Chabris C. F., Braver T. S. (2003). Neural mechanisms of general fluid intelligence. Nat. Neurosci. 6, 316–322 10.1038/nn1014 [DOI] [PubMed] [Google Scholar]
- Gray J. R., Thompson P. M. (2004). Neurobiology of intelligence: science and ethics. Nat. Rev. Neurosci. 5, 471–482 10.1038/nrn1405 [DOI] [PubMed] [Google Scholar]
- Green A. E., Fugelsang J. A., Kraemer D. J. M., Shamosh N. A., Dunbar K. N. (2006). Frontopolar cortex mediates abstract integration in analogy. Brain Res. 1096, 125–137 10.1016/j.brainres.2006.04.024 [DOI] [PubMed] [Google Scholar]
- Hansen C. H., Hansen R. D. (1988). Finding the face in the crowd: an anger superiority effect. J. Pers. Soc. Psychol. 54, 917–924 10.1037/0022-3514.54.6.917 [DOI] [PubMed] [Google Scholar]
- Henry L. A., Bettenay C. (2010). The assessment of executive functioning in children. Child Adolesc. Mental Health 15, 110–119 10.1111/j.1475-3588.2010.00557.x [DOI] [PubMed] [Google Scholar]
- Henson R. N. A. (2003). Neuroimaging studies of priming. Prog. Neurobiol. 70, 53–81 10.1016/S0301-0082(03)00086-8 [DOI] [PubMed] [Google Scholar]
- Honomichi R. D., Chen Z. (2011). Relations as rules: the role of attention in the dimensional change card sort task. Dev. Psychol. 47, 50–60 10.1037/a0021025 [DOI] [PubMed] [Google Scholar]
- Jacques S., Zelazo P. D. (2001). The flexible item selection task (FIST): a measure of executive function in preschoolers. Dev. Neuropsychol. 20, 573–591 10.1207/875656401753549807 [DOI] [PubMed] [Google Scholar]
- Jacques S., Zelazo P. D. (2005). “On the possible roots of cognitive flexibility,” in The Development of Social Understanding and Communication, eds Homer B., Tamis-Lemonda C. (Mahwah, NJ: Erlbaum; ), 53–81 [Google Scholar]
- Jog M. S., Kubota Y., Graybiel A. M. (1999). Building neural representations of habits. Science 286, 1745. 10.1126/science.286.5445.1745 [DOI] [PubMed] [Google Scholar]
- Jurado M. B., Rosselli M. (2007). The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev. 17, 213–233 10.1007/s11065-007-9040-z [DOI] [PubMed] [Google Scholar]
- Kaufman A. S., Kaufman N. L. (2004). Kaufman Assessment Battery for Children, 2nd Edn Circle Pines, MN: AGS [Google Scholar]
- Kharitonova M., Chien S., Colunga E., Munakata Y. (2009). More than a matter of getting ‘unstuck’: flexible thinkers use more abstract representations than perseverators. Dev. Sci. 12, 662–669 10.1111/j.1467-7687.2008.00799.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham N. Z., Cruess L., Diamond A. (2003). Helping children apply their knowledge to their behavior on a dimension-switching task. Dev. Sci. 6, 449–476 10.1111/1467-7687.00303 [DOI] [Google Scholar]
- Kloo D., Perner J. (2003). Training transfer between card sorting and false belief understanding: helping children apply conflicting descriptions. Child Dev. 74, 1823–1839 10.1046/j.1467-8624.2003.00640.x [DOI] [PubMed] [Google Scholar]
- Kloo D., Perner J., Kerschhuber A., Dabernig S., andAichhorn M. (2008). Sorting between dimensions: conditions of cognitive flexibility in preschoolers. J. Exp. Child. Psychol. 100, 115–134 10.1016/j.jecp.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Lachter J., Forster K. I., Ruthruff E. (2004). Forty-five years after Broadbent (1958) : still no identification without attention. Psychol. Rev. 111, 880–913 10.1037/0033-295X.111.4.880 [DOI] [PubMed] [Google Scholar]
- Lieberman M. D., Jarcho J. M., Satpute A. B. (2004). Evidence-based and intuition-based self-knowledge: an fMRI study. J. Pers. Soc. Psychol. 87, 421–435 10.1037/0022-3514.87.4.421 [DOI] [PubMed] [Google Scholar]
- Mack W. (2007). Improving postswitch performance in the dimensional change card-sorting task: the importance of the switch and of pretraining by redescribing the test cards. J. Exp. Child. Psychol. 98, 243–251 10.1016/j.jecp.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Marcovitch S., Boseovski J. J., Knapp R. J. (2007). Use it or lose it: examining preschoolers’ difficulty in maintaining and executing a goal. Dev. Sci. 10, 559–564 10.1111/j.1467-7687.2007.00611.x [DOI] [PubMed] [Google Scholar]
- McClure S. M., Laibson D. I., Loewenstein G., Cohen J. D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science 306, 503–507 10.1126/science.1100907 [DOI] [PubMed] [Google Scholar]
- McGrew K. S. (2009). CHC theory and the human cognitive abilities project: standing on the shoulders of the giants of psychometric intelligence research. Intelligence 37, 1–10 10.1016/j.intell.2008.08.004 [DOI] [Google Scholar]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Miller E. K., Desimone R. (1994). Parallel neuronal mechanisms for short-term memory. Science 263, 520–522 10.1126/science.263.5154.1670 [DOI] [PubMed] [Google Scholar]
- Miyake A., Emerson M. J., Padilla F., Ahn J. (2004). Inner speech as a retrieval aid for task goals: the effects of cue type and articulatory suppression. Acta Psychol. 115, 123–142 10.1016/j.actpsy.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., Wager T. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Morton J. B., Bosma R., Ansari D. (2009). Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. Neuroimage 46, 249–256 10.1016/j.neuroimage.2009.01.037 [DOI] [PubMed] [Google Scholar]
- Morton J. B., Munakata Y. (2002). Active versus latent representations: a neural network model of perseveration, dissociation, and decalage in childhood. Dev. Psychobiol. 40, 255–265 10.1002/dev.10033 [DOI] [PubMed] [Google Scholar]
- Muller U., Dick A. S., Gela K., Overton W. F., Zelazo P. D. (2006). The role of negative priming in preschoolers’ flexible rule use on the dimensional change card sort task. Child Dev. 77, 395–412 10.1111/j.1467-8624.2006.00878.x [DOI] [PubMed] [Google Scholar]
- Munakata Y. (1998). Infant perseveration and implications for object permanence theories: a PDP model of the AB task. Dev. Sci. 1, 161–211 10.1111/1467-7687.00021 [DOI] [Google Scholar]
- Noble K. G., Norman M. F., Farah M. J. (2005). Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 8, 74–87 10.1111/j.1467-7687.2005.00394.x [DOI] [PubMed] [Google Scholar]
- O’Reilly R. C. (2010). The what and how of prefrontal cortical organization. Trends Neurosci. 33, 355–361 10.1016/j.tins.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasnak R., Kidd J. K., Gadzichowski M. K., Gallington D. A., Saracina R. P., Addison K. T. (2009). Promoting early abstraction to promote early literacy and numeracy. J. Appl. Dev. Psychol. 30, 239–240 10.1016/j.appdev.2008.12.006 [DOI] [Google Scholar]
- Perner J., Lang B. (2002). What causes 3-year-olds’ difficulty on the dimensional change card sorting task? Infant Child Dev. 11, 93–105 10.1002/icd.299 [DOI] [Google Scholar]
- Preacher K. J., Hayes A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40, 879–891 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Premack D. (1984). “Pedagogy and aesthetics as sources of culture,” in Cognitive Neuroscience, ed. Gazzaniga M. (New York, NY: Plenum Press; ), 15–35 [Google Scholar]
- Rieth C., Sireteanu R. (1994). Texture segmentation and ‘pop-out’ in infants and children: the effect of test field size. Spat. Vis. 8, 173–191 10.1163/156856894X00323 [DOI] [PubMed] [Google Scholar]
- Rougier N., Noelle D., Braver T. S., Cohen J., O’Reilly R. C. (2005). Prefrontal cortex and flexible cognitive control: rules without symbols. Proc. Natl. Acad. Sci. U.S.A. 102, 7338–7343 10.1073/pnas.0502455102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi D., Julesz B. (1985). Detection versus discrimination of visual orientation. Perception 14, 619–628 [DOI] [PubMed] [Google Scholar]
- Schacter D. L., Buckner R. L. (1998). Priming and the brain. Neuron 20, 185–195 10.1016/S0896-6273(00)80448-1 [DOI] [PubMed] [Google Scholar]
- Schneider W., Shiffrin R. M. (1977). Controlled and automatic human information processing: I. Detection, search, and attention. Psychol. Rev. 84, 1–66 10.1037/0033-295X.84.1.1 [DOI] [Google Scholar]
- Schott B. H., Richardson-Klavehn A., Henson R. N. A., Becker C., Heinze H. J., Duzel E. (2002). Neuroanatomical dissociation of encoding processes related to priming and explicit memory. J. Neurosci. 26, 792–800 10.1523/JNEUROSCI.2402-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. B. (1989). “From global similarities to kinds of similarities: the construction of dimensions in development,” in Similarity and Analogical Reasoning, eds Vosniadou S., Artony A. (Cambridge: Cambridge University Press; ), 146–178 [Google Scholar]
- Son J., Smith L. B., Goldstone R. (2010). Connecting instances to promote children’s relational reasoning. J. Exp. Child. Psychol. 108, 260–277 10.1016/j.jecp.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed A. (2010). Abstract relational categories, graded persistence, and prefrontal cortical representation. Cogn. Neurosci. 1, 126–152 10.1080/17588921003660728 [DOI] [PubMed] [Google Scholar]
- Spelke E. S. (2003). “What makes us smart? Core knowledge and natural language,” in Language in Mind: Advances in the Investigation of Language and Thought, eds Gentner D., Goldin-Meadow S. (Cambridge, MA: MIT Press; ), 277–310 [Google Scholar]
- Suß H. M., Oberauer C., Wittman W. W., Wilhelm O., Schulze R. (2002). Working-memory capacity explains reasoning ability – and a little bit more. Intelligence 30, 261–288 10.1016/S0160-2896(01)00100-3 [DOI] [Google Scholar]
- Tomasello M. (2000). The item-based nature of children’s early syntactic development. Trends Cogn. Sci. (Regul. Ed.) 4, 156–163 10.1016/S1364-6613(00)01462-5 [DOI] [PubMed] [Google Scholar]
- Towse J., Lewis C., Knowles M. (2007). When knowledge is not enough: the phenomenon of goal neglect in preschool children. J. Exp. Child. Psychol. 96, 320–332 10.1016/j.jecp.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Vygotsky L. (1962). Thought and language. Bull. Orton Soc. 14, 97–98 10.1007/BF02928399 [DOI] [Google Scholar]
- Wallis J. D., Anderson K. C., Miller E. K. (2001). Single neurons in prefrontal cortex encode abstract rules. Nature 411, 953–956 10.1038/35082081 [DOI] [PubMed] [Google Scholar]
- Wendelken C., Nakhabenko D., Donohue S. E., Carter C. S., Bunge S. A. (2008). ‘Brain is to thought as stomach is to…?’ – Specifying the role of rostrolateral prefrontal cortex in analogical reasoning. J. Cogn. Neurosci. 20, 682–693 10.1162/jocn.2008.20055 [DOI] [PubMed] [Google Scholar]
- Yerys B. E., Munakata Y. (2006). When labels hurt but novelty helps: children’s perseveration and flexibility in a card-sorting task. Child Dev. 77, 1589–1607 10.1111/j.1467-8624.2006.00961.x [DOI] [PubMed] [Google Scholar]
- Zelazo P. D., Frye D. (1997). “Cognitive complexity and control: a theory of the development of deliberate reasoning and intentional action,” in Language Structure, Discourse, and the Access to Consciousness, ed. Stamenov M. (Amsterdam: John Benjamins; ), 113–153 [Google Scholar]
- Zelazo P., Frye D., Rapus T. (1996). An age-related dissociation between knowing rules and using them. Cogn. Dev. 11, 37–63 10.1016/S0885-2014(96)90027-1 [DOI] [Google Scholar]
- Zelazo P. D., Muller U., Frye D., Marcovitch S. (2003). The Development of Executive Function in Early Childhood. Oxford: Blackwell Publishing; [DOI] [PubMed] [Google Scholar]