Abstract
Purpose
To validate, using physician review of abstracted medical chart data as a gold standard, a claims-based algorithm developed to identify gastrointestinal (GI) perforation cases among rheumatoid arthritis (RA) patients.
Methods
Patients with established RA, aged 18 years or older with hospital admissions between January 2004 and September 2009, were selected from a large US hospital-based database. An algorithm with ICD-9-CM diagnosis codes for GI perforation and combinations of GI-related diagnosis codes and CPT-4 procedure codes for relevant GI surgeries was used to identify potential GI perforation cases. Two senior experienced specialist physicians independently reviewed abstracted chart data and classified cases as “confirmed” or “unconfirmed” GI perforations. Positive predictive values (PPVs) to identify “confirmed” GI perforation were calculated and stratified by upper versus lower GI tract.
Results
Overall, 86 of 92 GI perforation cases were confirmed, yielding an overall PPV of 94% (95% CI: 86–98%). PPV was 100% (95% CI: 100–100%) for upper GI perforation (esophagus, stomach) and 91% (95% CI: 90–97%) for lower GI perforation (small intestine, PPV=100%; large intestine, PPV= 94%; unspecified lower GI, PPV=89%).
Conclusions
This algorithm, consisting of a combination of ICD-9-CM diagnosis and CPT-4 codes, could be used in future safety studies to evaluate GI perforation risk factors in RA patients.
Keywords: gastrointestinal perforation, validation, administrative claims, rheumatoid arthritis
INTRODUCTION
Gastrointestinal (GI) perforation is defined as a hole that passes through the wall of the esophagus, stomach, or small or large intestine.1 Following perforation, if contents of the GI tract remain confined to the surrounding tissues, the process may be self-limited, and the condition may escape detection without clinical consequence. However, GI perforations may result in release of enteric contents into the chest or peritoneal cavities. If left untreated, life-threatening peritonitis or septicemia may develop. The in-hospital mortality rate of patients hospitalized with GI perforation ranges from 9–30%, depending on the cause and location of the perforation, time to treatment, and presence of underlying comorbidities.2 Although not all GI perforations can be avoided, it is important to proactively treat conditions which are known risk factors for GI perforation such as ulcers, appendicitis, diverticulitis, cancer, and inflammatory bowel disease (IBD).1 Perforation of the GI tract can also result from barotrauma (Boerhaave’s), ingestion of foreign bodies or caustic substances, neoplastic or infectious fistulizing disease, and can be a rare complication of endoscopic procedures.
The incidence and prevalence of non-iatrogenic GI perforations is not well established. Although the incidence of non-steroidal anti-inflammatory drug (NSAID)-associated upper GI tract complications is well described in the literature,3 few studies have evaluated the incidence of lower GI tract complications.4 Furthermore, published work to date commonly evaluates multiple types of GI complications (e.g., bleeding, perforation, ulcers) together, making it difficult to establish the incidence of GI perforation alone.5
A systematic review of epidemiologic studies published between 1980 and 2000 (n=4) showed the pooled incidence rate estimate of upper GI perforations was 0.10 (95% CI: 0.04–0.23) per 1,000 person-years among all non-NSAID users in the general population.6 Rheumatoid arthritis (RA) patients may have a higher risk of GI perforation than the general population, however. A review of 13 clinical trials evaluating NSAIDs (n=11), disease-modifying antirheumatic drugs (n=1), and immunosuppressive drugs (n=1), performed in RA patients reported a cumulative incidence of GI perforations ranging from 0.0% to 7.1% during study follow-up.7–19 More direct and comparable evidence suggesting that RA patients may have a higher incidence of GI perforations than other patient populations was found from a U.S. study that observed that the rate of GI perforations was several times higher than in patients with psoriatic arthritis.20 GI perforations observed in RA patients may be attributable to concomitant medication use (e.g. glucocorticoids) and/or IBD, a known risk factor for GI perforation. Both RA and IBD share possible common pathological mechanisms, and having one condition may be associated with a heightened risk of developing the other.21–27
Given their size and easy accessibility, electronic administrative databases are a valuable data source for research to better understand the epidemiology of rare health events, such as GI perforations in RA patients. It is crucial, however, to assess the validity of using diagnosis codes contained within these healthcare databases to identify cases prior to utilizing them for epidemiological studies. While prior validation studies have examined the concordance between administrative claims databases and medical charts for upper GI perforations, strictures, ulcers, and obstructions,28–31 additional studies are needed to better characterize lower GI perforations. Therefore, the primary objective of this study was to validate an algorithm, to confirm a perforation in patients hospitalized for non-iatrogenic upper or lower GI perforations.
METHODS
Identification of GI Perforation Cases
Patients were selected from the Premier Perspective™ database, a database which contains service-level hospital reimbursement records for 600 U.S. hospitals, if they had one or more inpatient medical service claims with an associated diagnosis of RA (ICD-9-CM: 714.xx) between January 2004 and September 2009. GI perforation cases were further identified based on the presence of one or more GI perforation ICD-9-CM diagnosis codes, or the combination of a GI-related ICD-9-CM code with a CPT4 code for relevant GI surgery on billing claims for the hospitalization with RA diagnosis (Appendix). Both the diagnosis of RA and GI perforation could appear in any primary or non-primary position on the hospital claim. If patients had more than one hospitalization for GI perforation, only the first hospitalization was eligible for abstraction.
APPENDIX.
ICD-9-CM and CPT-4 Codes Used to Identify GI Perforations
| GI Site | Code Source and Number | Code Type and Description |
|---|---|---|
| Esophagus | ||
|
|
||
| ICD-9-CM | Diagnosis Code | |
|
|
||
| 530.4 | Perforation of esophagus | |
|
| ||
| Stomach | ||
|
|
||
| ICD-9-CM | Diagnosis Code | |
|
|
||
| 531.1 | Acute gastric ulcer with perforation | |
| 531.2 | Acute gastric ulcer with hemorrhage and perforation | |
| 531.5 | Chronic or unspecified gastric ulcer with perforation | |
| 531.6 | Chronic or unspecified gastric ulcer with hemorrhage and perforation | |
| 532.1 | Acute duodenal ulcer with perforation | |
| 532.2 | Acute duodenal ulcer with hemorrhage and perforation | |
| 532.5 | Chronic or unspecified duodenal ulcer with perforation | |
| 532.6 | Chronic or unspecified duodenal ulcer with hemorrhage and perforation | |
| 533.1 | Peptic ulcer, site unspecified: acute with perforation | |
| 533.2 | Peptic ulcer, site unspecified: acute with hemorrhage and perforation | |
| 533.5 | Peptic ulcer, chronic or unspecified with perforation | |
| 533.6 | Peptic ulcer, chronic or unspecified with hemorrhage and perforation | |
|
| ||
| Small intestine | ||
|
|
||
| ICD-9-CM | Diagnosis Code | |
|
|
||
| 534.1 | Acute gastrojejunal ulcer with perforation | |
| 534.2 | Acute gastrojejunal ulcer with hemorrhage and perforation | |
| 534.5 | Chronic or unspecified gastrojejunal ulcer with perforation | |
| 534.6 | Chronic or unspecified gastrojejunal ulcer with hemorrhage and perforation | |
| 557 | Acute ischemic colitis | |
| 557.1 | Chronic ischemic colitis | |
| 557.9 | Unspecific ischemic colitis | |
| 562 | Diverticulosis/diverticulitis of small intestine | |
|
|
||
| CPT-4 | Procedure Code | |
|
|
||
| 44602 | Suture of small intestine | |
| 44603 | Suture of small intestine | |
| 44120 | Resection of small intestine | |
| 44121 | Resection of small intestine | |
| 44125 | Resection of small intestine | |
| 44130 | Resection of small intestine | |
| 44202 | Resection of small intestine | |
| 44203 | Resection of small intestine | |
|
| ||
| Large Intestine | ||
|
|
||
| ICD-9-CM | Diagnosis Code | |
|
|
||
| 540 | Appendicitis, acute with perforation and generalized peritonitis | |
| 557 | Acute ischemic colitis | |
| 557.1 | Chronic ischemic colitis | |
| 557.9 | Unspecific ischemic colitis | |
| 562.1 | Diverticulosis/diverticulitis of large intestine | |
|
|
||
| CPT-4 | Procedure Code | |
|
|
||
| 44604 | Suture of large intestine | |
| 44605 | Suture of large intestine | |
| 44140 | Partial colectomy with anastomosis | |
| 44145 | Partial colectomy with anastomosis | |
| 44204 | Partial colectomy with anastomosis | |
| 44205 | Partial colectomy with anastomosis | |
|
| ||
| Unspecified lower GI | ||
|
|
||
| ICD-9-CM | Diagnosis Code | |
|
|
||
| 569.83 | Perforation of intestine | |
All patients included in the study were 18 years of age or older on the date of the GI perforation hospitalization. Since we were not interested in identifying and validating GI perforations related to “mechanical” etiologies, patients were excluded if they had an ICD-9-CM code for “accidental puncture or laceration during a procedure” (998.2x), “foreign body in digestive system” (938.xx), or “foreign body accidentally left during a procedure” (998.4x) in the primary position. Furthermore, patients with an ICD-9-CM code for neoplasm (140.xx-239.xx) in any position on the hospital claim were excluded due to the possibility that cancer patients might have additional risk factors for perforation that could obscure the association between RA-related risk factors and perforation.
Review of Hospital Medical Charts
A convenience sample was selected and medical records were requested from the hospitals with 5 or more potential GI perforation cases identified in the database. In an effort to oversample lower GI perforation cases, the distribution of upper versus lower GI perforations in the selected sample reflected an approximately 1:3 ratio.
Institutional Review Board approval was received from each hospital to conduct the medical chart review, and all data collected were HIPAA compliant. For each case, clinical information needed to validate the GI perforation was abstracted from the medical chart during the hospitalization. Data obtained from the medical chart review were used to validate GI perforation cases.
Three trained clinical research associates reviewed the medical charts for the hospitalizations and completed a standardized chart abstraction form that was developed specifically for this study. Information abstracted from each medical chart included the source of GI perforation documentation (endoscopy notes, radiology notes, surgery notes, autopsy notes, other), the type of GI perforation diagnosis (“confirmed/final,” “rule out,” “not sure”) from the treating physician, the location of the perforation (e.g., esophagus, stomach, duodenum), and the type of perforation (confined, free, unknown). In addition, details of in-hospital pharmacologic treatment (e.g., antibiotics), diagnostic and treatment procedures (e.g., abdominal/GI CT scan, laparoscopic/abdominal surgery), and hospitalization information (e.g., admission date, principal discharge diagnosis) were collected. Relevant patient medical histories (e.g., GI-related conditions, NSAID/steroid use, endoscopy procedures) were also abstracted when available.
Classification of GI Perforation Cases
Two senior internal medicine physicians with additional subspecialty training and board certification in gastroenterology (DJ) and rheumatology (JC) independently reviewed the abstracted chart data. Prior to viewing the abstracted data, both physicians agreed on a case definition for classifying a potential GI perforation case as a “confirmed” or “unconfirmed.” Criteria for a “confirmed” case included 1) evidence of a final treating physician diagnosis of “perforation” for a GI site, and 2) evidence from supporting diagnostic and/or treatment procedures. Without such evidence, a suspected GI perforation case was deemed “unconfirmed” by the physicians. Classification disagreements were discussed by the two reviewers and resolved through a consensus process; 100% concurrence was established. Both reviewers were blinded to the hospital claims profile of the suspected perforation cases.
Statistical Analysis
Patient demographics (age, sex, race, and type of perforation) were analyzed for all patients identified as having a GI perforation based on the coding algorithm in the database. Student t-tests and chi-square tests were used to assess differences between patients included in the chart review and those identified by the coding algorithm as having a GI perforation in the database but not selected for the chart review validation sample. For patients included in the chart review, information collected on the abstraction form regarding diagnostic and treatment procedures was reported descriptively.
The validity of the coding algorithm (Appendix) was described as the positive predictive value (PPV), defined as the proportion of patients with a positive test who actually have the condition.31 Several studies have utilized PPV to validate administrative records against medical chart records in gastroenterological diseases.26–29 In this study, PPV was calculated as the proportion of confirmed GI perforation cases based on the chart review (gold standard) among those identified in the administrative claims database as having a GI perforation as follows:
PPV was stratified by GI type and site (e.g., upper GI, lower GI, esophagus, stomach, small intestine, large intestine, unspecified lower GI). The 95% confidence intervals (CIs) of the PPVs were estimated based on a binomial distribution. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina), and findings with p-values <0.05 were considered statistically significant.
RESULTS
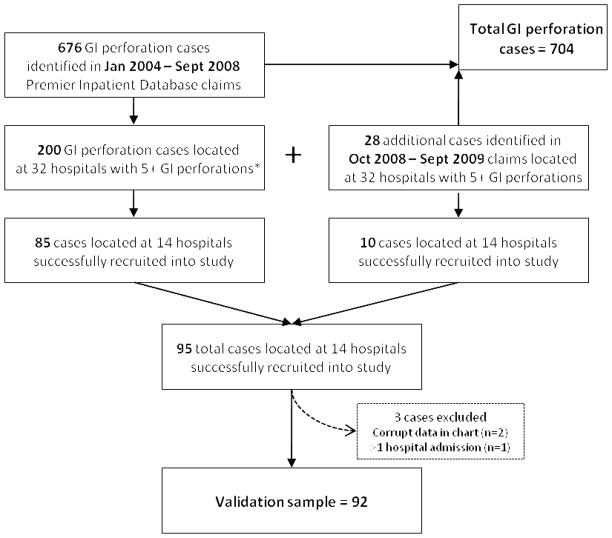
Based on the study’s inclusion and exclusion criteria, a total of 704 potential GI perforation cases were identified in the database. Among the 32 hospitals with the largest numbers of GI perforation cases, 228 cases were identified as eligible to be included in the medical chart review. Of these 228, data was abstracted for the 92 potential GI perforation cases that were located at the 14 participating hospitals (Figure 1). These cases occurred in hospitals located across four geographic regions in the United States (Northeast, Midwest, West, and South). In this final validation sample of 92 cases, 30% of cases had upper GI perforations and 70% had lower GI perforations.
Figure 1. Identification of GI Perforation Cases for Analysis and Selection of Validation Sample.
*Upper to lower GI perforations ratio monitored to ensure proportions in validation sample were similar to proportions in full analysis group.
Table 1 presents the characteristics of all 704 GI perforation cases identified from the database among RA patients. Overall, the mean age of GI perforation cases was 67 years, 73.7% of cases were female, and more than three-quarters of cases were white. The overall distribution of upper, lower, and both GI perforations were 33.5%, 65.3%, and 1.1%, respectively.
Table 1.
Characteristics of RA Patients Included and Not Included in the Chart Review
| Total Identified Cases Based on Claims Database | Included in Chart Review Validation | Not Included in Chart Review Validation | P-valuea | |
|---|---|---|---|---|
| Number of cases | 704 | 92 | 612 | |
| Proportion of patients (%) | 100 | 13.1 | 86.9 | |
| Age: mean (SD) | 66.9 (14.0) | 66.9 (12.8) | 66.9 (14.2) | 0.973 |
| Range | 18–89 | 24–89 | 18–89 | |
| Gender (%) | 0.220 | |||
| Female | 73.7 | 68.5 | 74.5 | |
| Male | 26.3 | 31.5 | 25.5 | |
| Race (%) | 0.027 | |||
| White | 75.6 | 82.6 | 74.5 | |
| Black | 7.1 | 1.1 | 8.0 | |
| Hispanic | 2.4 | 5.4 | 2.0 | |
| American Indian | 0.6 | 1.1 | 0.5 | |
| Asian/Pacific Islander | 1.0 | 0.0 | 1.1 | |
| Other | 13.4 | 9.8 | 13.9 | |
| Type of GI Perforation (%) | 0.331 | |||
| Upper | 33.5 | 29.4 | 34.2 | |
| Lower | 65.3 | 70.6 | 64.5 | |
| Both | 1.1 | 0.0 | 1.3 |
SD=standard deviation
P-value reported compares included and not included in the chart review groups.
Note: patients were identified from Premier inpatient database based on algorithm identified in Table 1, and lower GI perforation cases were oversampled.
The age (mean 66.9 years in both groups, range 24–89 vs. 18–89, p=0.973) and sex distribution (68.5% vs. 74.5% female, p=0.22) were similar between patients included and not included in the validation analysis. The race distribution of cases included in the validation analysis was significantly different than of those not included (p=0.027), with the proportion of white patients being higher in the validated cases (82.6%) than non-validated cases (74.5%). The ratio between upper and lower GI perforation cases (approximately 3:7) was similar in both groups (p=0.331).
Table 2 shows the individual codes or coding combinations that were used to identify GI perforation cases in the database. Eight of the 10 coding combinations evaluated produced PPVs of 100%. One of 17 cases (5.9%) identified by a combination ICD-9-CM diagnosis and CPT-4 procedure code in the large intestine and 5 of 47 (10.6%) of cases identified as by the “unspecified lower GI” ICD-9-CM diagnosis code (569.83) were not confirmed GI perforations.
Table 2.
Predefined Algorithm to Identify Hospitalized GI Perforation Cases
| Total Identified GI Perforation Cases in Claims Dataa (n = 704) | GI Perforation Cases Included in Validation Sampleb (n = 92) | PPV | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Confirmed | Unconfirmed | ||||||
| GI Site | ICD-9-CM Diagnosis Code | CPT-4 Procedure Code | n | n | n | n | % | |
| Esophagus | 530.4 | 28 | 5 | 5 | 0 | 100 | ||
|
| ||||||||
| Stomach | 531.1 | 22 | 2 | 2 | 0 | 100 | ||
| 531.2 | 1 | 0 | 0 | 0 | N/A | |||
| 531.5 | 78 | 8 | 8 | 0 | 100 | |||
| 531.6 | 7 | 1 | 1 | 0 | 100 | |||
| 532.1 | 23 | 2 | 2 | 0 | 100 | |||
| 532.2 | 2 | 0 | 0 | 0 | N/A | |||
| 532.5 | 70 | 8 | 8 | 0 | 100 | |||
| 532.6 | 9 | 0 | 0 | 0 | N/A | |||
| 533.1 | 1 | 0 | 0 | 0 | N/A | |||
| 533.2 | 2 | 0 | 0 | 0 | N/A | |||
| 533.5 | 7 | 1 | 1 | 0 | 100 | |||
| 533.6 | 0 | 0 | 0 | 0 | N/A | |||
|
| ||||||||
| Small Intestine | 534.1 | 0 | 0 | 0 | 0 | N/A | ||
| 534.2 | 0 | 0 | 0 | 0 | N/A | |||
| 534.5 | 5 | 1 | 1 | 0 | 100 | |||
| 534.6 | 0 | 0 | 0 | 0 | N/A | |||
| 557.0 or 557.1 or 557.9 or 562.0 | A | 44602 or 44603 or 44120 or 44121 or 44125 or 44130 or 44202 or 44203 | 0 | 0 | 0 | 0 | N/A | |
| N | ||||||||
| D | ||||||||
|
| ||||||||
| Large Intestine | 540 | 130 | 17 | 16 | 1 | 94.1 | ||
| 557.0 or 557.1 or 557.9 or 562.1 | A | 44604 or 44605 or 44140 or 44145 or 44204 or 44205 | 15 | 0 | 0 | 0 | N/A | |
| N | ||||||||
| D | ||||||||
|
| ||||||||
| Unspecified Lower GI | 569.83 | 325 | 47 | 42 | 5 | 89.4 | ||
PPV=Positive Predictive Value
GI perforation cases identified from the Premier Inpatient Database and based on the coding algorithm in Appendix.
GI perforation cases validated using chart review data based on physicians’ independent review.
Table 3 displays the calculated PPVs of GI perforations, by site. The overall calculated PPV was 93.5% (95% CI: 86.3%–97.6%). Among the 27 cases identified with an upper GI perforation (5 esophagus, 22 stomach), the PPV was 100%. The PPV for lower GI perforation cases was 90.8% (95% CI: 90.0%–96.5%). The PPVs for small intestine, large intestine, and unspecified lower GI were 100%, 94.1% (95% CI: 71.3%–100%), and 89.4% (95% CI: 76.9%–96.5%), respectively. The PPV of the perforation algorithm for cases occurring in higher volume hospitals (> 6 cases in our sample) was similar (96%) to the PPV (90%) in lower volume hospitals (<= 6 perforation cases in our sample).
Table 3.
Overall Positive Predictive Value for GI Perforation
| Total Identified Cases Based on Claims Database* | Cases Included in Chart Review Validation Study | Positive Predictive Value | ||||
|---|---|---|---|---|---|---|
| Total | Confirmed | Unconfirmed | ||||
| N | N | N | N | % | (95% Confidence Interval)^ | |
| GI site | ||||||
| Upper | 244 | 27 | 27 | 0 | 100.0% | (100% – 100%) |
| Esophagus | 28 | 5 | 5 | 0 | 100.0% | (100% – 100%) |
| Stomach | 216 | 22 | 22 | 0 | 100.0% | (100% – 100%) |
| Lower | 468 | 65 | 59 | 6 | 90.8% | (90.0% – 96.5%) |
| Small intestine | 5 | 1 | 1 | 0 | 100.0% | (100% – 100%) |
| Large intestine | 145 | 17 | 16 | 1 | 94.1% | (71.3% – 100%) |
| Unspecified | ||||||
| lower GI | 325 | 47 | 42 | 5 | 89.4% | (76.9% – 96.5%) |
| Overall | 704 | 92 | 86 | 6 | 93.5% | (86.3% – 97.6%) |
Identified cases: GI perforation cases identified from Premier inpatient database based on algorithm identified in Table 1
Cases included in the validation study: GI perforation cases being validated using chart review data based on physicians’ independent review
Sum of individual codes may exceed count in broader categories as patients may have multiple codes
95% confidence intervals were approximated using the binomial distribution
Table 4 describes characteristics of the 86 confirmed GI perforation cases. The majority of confirmed GI perforation cases (65.1%) had lower GI perforations, 28 cases had upper GI perforations (32.6%), and 2 had a GI perforation of an undetermined location (2.3%). As perforation cases reported within each location were not mutually exclusive, some cases reported having perforations at adjacent locations, such as both the stomach and duodenum. The majority of cases had a free perforation (n=73, 84.9%), while 12 cases had a confined perforation (14.0%); in one case the type of perforation could not be determined from the medical chart (1.1%). Although all patients selected from the administrative claims database had an RA diagnosis, only 96.5% (n=83) patients had this documented in the medical chart during the hospitalization.
Table 4.
Treatments and Medical History of Patients with Confirmed GI Perforations
| Cases with Confirmed GI Perforation
|
||
|---|---|---|
| N | % | |
| Number of patients | 86 | |
| Any use of antibiotics (%) | 84 | (97.7%) |
| Any diagnostic procedures performed* (%) | 78 | (90.7%) |
| Abdominal/GI CT scan | 73 | (84.9%) |
| Abdominal/GI CT ultrasonography | 8 | (9.3%) |
| Abdominal/GI CT MRI | 0 | (0.0%) |
| Abdominal/GI CT X-ray | 39 | (45.3%) |
| Any treatment procedures performed* (%) | 77 | (89.5%) |
| Laparoscopic surgery | 20 | (23.3%) |
| Abdominal surgery | 63 | (73.3%) |
| Length of stay: mean (SD) | 15.9 (12.9) | |
| Discharge status (%) | ||
| Subject discharged home | 46 | (53.5%) |
| Subject discharged to another institution | 22 | (25.6%) |
| Subject expired | 17 | (19.8%) |
| Other | 1 | (1.1%) |
| Patient history | ||
| GI endoscopy within 1 week | ||
| Yes | 3 | (3.5%) |
| No | 73 | (84.9%) |
| Unknown | 9 | (10.5%) |
| Missing | 1 | (1.2%) |
| Any NSAIDs use in the last 12 months | ||
| Yes | 27 | (31.4%) |
| No | 34 | (39.5%) |
| Unknown | 25 | (29.1%) |
| Any steroid use in the last 12 months | ||
| Yes | 51 | (59.3%) |
| No | 26 | (30.2%) |
| Unknown | 9 | (10.5%) |
| Medication history based on NSAIDs and steroid use | ||
| No NSAIDs or steroid use | 13 | (15.1%) |
| Only NSAIDs but no steroid use | 9 | (10.5%) |
| Only steroid but not NSAIDs use | 20 | (23.3%) |
| Both NSAIDs and steroid use | 15 | (17.4%) |
| Either NSAIDs or steroid use was unknown | 29 | (33.7%) |
Table 4 also presents treatment, medical procedures, hospital discharge information, and patient history of confirmed GI perforation cases. Nearly all patients with confirmed GI perforations received antibiotics (n=84, 97.9%). Seventy-eight cases received diagnostic imaging (90.7%), with the most commonly used type of imaging being a CT scan (n=73). Seventy-seven cases (89.5%) underwent either laparoscopic surgery (n=20, 23.3%) or abdominal surgery (n=63, 73.3%) to repair the perforation. The mean length of hospitalization was 16 days (range=1–93, SD=12.9). Discharge locations for patients included: 53% to home (n=46), 25.5% to another institution (n=22), and 19.8% died in the hospital (n=22). Among patients with confirmed GI perforations, 3 cases had an endoscopy procedure within one week prior to hospitalization (3.5%). Among patients who had medication histories reported in the medical chart (n=57), 77% had used an NSAID or steroid, and 15 patients (26%) had used both NSAIDs and steroids in the year prior to hospitalization.
DISCUSSION
To our knowledge, this study was the first to systematically validate administrative claims data against medical chart data for the identification of GI perforation events among hospitalized RA patients. The objective of this study was to determine whether the coding algorithm we developed to identify GI perforation cases within the inpatient claims database accurately captured the presence of GI perforations. This was accomplished by calculating the PPV of the coding algorithm. Results of this study showed an overall PPV of 93.5% for the coding algorithm, with PPVs for individual perforation types ranging from 89.4% to 100%. The high PPV suggests the proposed coding algorithm can be used identify patients with GI perforations in administrative claims with a high level of accuracy.
Our PPVs for GI perforations—ranging from 89.4%–100%–compares favorably to PPVs in other validation studies for GI-related complications reported in the literature which range from 27% to 97% and vary depending on the type, severity, and location of complications evaluated. 28–31 For example, a U.S.-based study using a database of commercially-insured patients reported PPVs of 56.5% for upper GI bleeding and 87.8% for severe upper GI bleeding.31 A study using the Saskatchewan database in Canada showed PPVs ranging from 70–90% for upper GI bleeding and perforation,30 and a study using a regional database in Italy reported PPVs for peptic ulcer ranging from 59–97%, with more site-specific codes increasing the PPVs.29
Definitions of “confirmed” or “unconfirmed” GI perforations were used in the review process, rather than “positive” and “negative” cases, because this characterization is more useful from an epidemiologic perspective. The PPVs we calculated may be viewed as conservative estimates because all cases with insufficient information to confirm or refute the diagnosis of a GI perforation were classified as “unconfirmed.”
Three cases that were identified in the administrative database as having an RA diagnosis and a GI perforation lacked evidence of RA in their charts—while this may have been due to a neglected or miscoded diagnosis for RA, it is more likely that it was due to not having outpatient claims data for these patients. We do not expect our results for GI perforation cases among the RA sub-population to be biased or differ from results for GI perforation cases in the population without RA, as it seems unlikely that an RA diagnosis would influence how a GI perforation case is documented. Therefore, we suggest that performance of the algorithm is likely to be similar for patients with other forms of inflammatory arthritis (e.g. psoriatic arthritis). However, as a potential limitation, performance of the algorithm for patients with other rheumatic diseases that can have GI manifestations (e.g. systemic lupus erythematosus), or for patients with cancer, may be different.
Although efficient, selection of hospitals with the most cases for chart review may affect the PPVs that we observed results. High volume hospitals may be larger, have a higher prevalence of cases, and/or are more prone to code GI perforations. In order to assess the impact of case prevalence on PPV, we stratified cases by hospital volume (>6 cases vs. ≤6 cases). There was no significant difference in the PPVs for each code in the high vs. low volume hospitals.
Another limitation is that we were unable to validate all of the coding combinations included in the coding algorithm used to identify GI perforation cases (Table 2). Seven of the 11 coding combinations were not assessed for PPV because cases identified by those particular combinations were treated at non-participating hospitals. Five additional codes were not validated because no patients were identified in the administrative database by those coding combinations. In particular, the perforation definition that combined GI conditions (e.g., diverticulosis, diverticulitis) and GI procedures (e.g., suture or resection) were not commonly observed in this study. This might be related to the nature of our data source, as these procedures are commonly billed by surgeons outside of the hospital-based data source used for our analysis. For that reason, these less specific diagnosis and GI procedure codes should be used with caution as they were not represented within our validation sample.
Nearly half of all potential GI perforation cases both included and excluded from the validation sample were identified from the database with the ICD-9-CM code for “perforation of intestine” (ICD-9-CM 569.83). As an obvious limitation to this ICD-9-CM code, cases identified with this code could not be uniquely assigned to perforations of the small or the large intestine. Thus, while the validity is high to identify a confirmed perforation (PPV=89.4%), the specific site of perforation in the intestine could not be readily determined from the claims data.
Finally, this study evaluated the ability of the coding algorithm to correctly identify GI perforation events among patients with RA as described by the PPV. We were not able to characterize the sensitivity of the algorithm, or the negative predictive value (NPV); determining sensitivity (required for accurate estimates of incidence) and the NPV are more difficult (because they require an independent mechanism for case finding) and this was not within the scope of our study.
CONCLUSION
This validation study found that the proposed GI perforation algorithm had an overall PPV of 93.5% for identifying confirmed cases, with PPVs for individual types of GI perforations ranging from 89.4%–100%. The true PPV of the proposed algorithm may be even higher given this study’s conservative approach to classifying confirmed GI perforation cases. Further studies are needed to elucidate GI perforation rates and risk factors among RA patients. This validation study demonstrated that GI perforation-related diagnosis codes and the proposed algorithm may be used in future observational studies to assess the association between risk factors and GI perforation in this patient population.
Supplementary Material
Key points.
Using the gold standard of patient medical record review, this study evaluated an administrative claims-based algorithm to identify rheumatoid arthritis patients who were hospitalized for upper or lower GI perforations
This validation study found that the proposed algorithm has an overall PPV of 93.5% for identifying confirmed GI perforation cases, with PPVs for individual types of GI perforations ranging from 89.4%–100%.
The use of GI perforation-related diagnosis codes and the proposed algorithm may be useful in future observational studies to evaluate risk factors for GI perforation events.
Acknowledgments
The authors thank Michael L. Ganz for his contribution to the study and manuscript review and Kristin A. Hanson, PharmD, MS and Jyoti S. Nandi, MD, PhD who provided medical writing services on behalf of United BioSource Corporation, Bethesda, Maryland, USA. This study was funded by F. Hoffmann-La Roche Ltd. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Sponsor: This work was funded by Genentech, a member of the Roche group
Footnotes
Conflict of Interest: Dr. Curtis receives support from the NIH (AR053351) and AHRQ (R01HS018517). JRC and DAJ served as consultants for Genentech, SYC is employed by United BioSource Corporation which was contracted with Genentech to conduct the analyses, and AJ is employed by Genentech.
References
- 1.Gastrointestinal perforation. [Accessed November 2008];MedlinePlus Medical Encyclopedia. http://www.nlm.nih.gov/MEDLINEPLUS/ency/article/000235.htm.
- 2.Lanas A, Perez-Aisa MA, Feu F, Ponce J, Saperas E, Santolaria S, Rodrigo L, Balanzo J, Bajador E, Almela P, Navarro JM, Carballo F, Castro M, Quintero E. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am J Gastroenterol. 2005 Aug;100:1685–93. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 3.Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Review: adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010 Apr;24(2):121–32. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Laine L, Smith R, Min K, Chen C, Dubois RW. Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2006;24:751–67. doi: 10.1111/j.1365-2036.2006.03043.x. [DOI] [PubMed] [Google Scholar]
- 5.Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Muñoz M, Rodrigo L, Calvet X, Del-Pino D, Garcia S. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104(7):1633–41. doi: 10.1038/ajg.2009.164. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Díaz S, Rodríguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol. 2002 Feb;55(2):157–63. doi: 10.1016/s0895-4356(01)00461-9. [DOI] [PubMed] [Google Scholar]
- 7.Bevis PJ, Bird HA, Lapham G. An open study to assess the safety and tolerability of meloxicam 15 mg in subjects with rheumatic disease and mild renal impairment. Br J Rheumatol. 1996 Apr;35(Suppl 1):56–60. doi: 10.1093/rheumatology/35.suppl_1.56. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn WD, Jr, Prupas HM, Silverfield JC, Poiley JE, Caldwell JR, Collins RL, Miller MJ, Sikes DH, Kaplan H, Fleischmann R. Tenidap in rheumatoid arthritis. A 24-week double-blind comparison with hydroxychloroquine-plus-piroxicam, and piroxicam alone. Arthritis Rheum. 1995 Oct;38(10):1447–56. doi: 10.1002/art.1780381011. [DOI] [PubMed] [Google Scholar]
- 9.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000 Nov 23;343(21):1520–8. doi: 10.1056/NEJM200011233432103. 2 p following 1528. [DOI] [PubMed] [Google Scholar]
- 10.Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008 Nov;67(11):1516–23. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furst DE, Kolba KS, Fleischmann R, Silverfield J, Greenwald M, Roth S, Hall DB, Roszko PJ Meloxicam Rheumatoid Arthritis Investigators. Dose response and safety study of meloxicam up to 22.5 mg daily in rheumatoid arthritis: a 12 week multicenter, double blind, dose response study versus placebo and diclofenac. J Rheumatol. 2002 Mar;29(3):436–46. [PubMed] [Google Scholar]
- 12.Huskisson EC, Ghozlan R, Kurthen R, Degner FL, Bluhmki E. A long-term study to evaluate the safety and efficacy of meloxicam therapy in patients with rheumatoid arthritis. Br J Rheumatol. 1996 Apr;35(Suppl 1):29–34. doi: 10.1093/rheumatology/35.suppl_1.29. [DOI] [PubMed] [Google Scholar]
- 13.Kawai S, Tanaka K, Ohno I, Utsunomiya K, Seino Y. Safety of long-term tacrolimus therapy for rheumatoid arthritis: an open-label, uncontrolled study in non-elderly patients. Mod Rheumatol. 2008;18(4):345–53. doi: 10.1007/s10165-008-0058-8. [DOI] [PubMed] [Google Scholar]
- 14.Laine L, Connors LG, Reicin A, Hawkey CJ, Burgos-Vargas R, Schnitzer TJ, Yu Q, Bombardier C. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003 Feb;124(2):288–92. doi: 10.1053/gast.2003.50054. [DOI] [PubMed] [Google Scholar]
- 15.Neustadt DH. Double blind evaluation of the long-term effects of etodolac versus ibuprofen in patients with rheumatoid arthritis. J Rheumatol Suppl. 1997 Feb;47:17–22. [PubMed] [Google Scholar]
- 16.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000 Sept 13;284(10):1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, Geis GS. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995 Aug 15;123(4):241–49. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Simon LS, Hatoum HT, Bittman RM, Archambault WT, Polisson RP. Risk factors for serious nonsteroidal-induced gastrointestinal complications: regression analysis of the MUCOSA trial. Fam Med Mar. 1996;28(3):204–210. [PubMed] [Google Scholar]
- 19.Wojtulewski JA, Schattenkirchner M, Barceló P, Le Loët X, Bevis PJ, Bluhmki E, Distel M. A six-month double-blind trial to compare the efficacy and safety of meloxicam 7.5 mg daily and naproxen 750 mg daily in patients with rheumatoid arthritis. Br J Rheumatol. 1996 Apr;35(Suppl 1):22–8. doi: 10.1093/rheumatology/35.suppl_1.22. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JR, Xie F, Chen L, Spettell C, McMahan RM, Fernandes J, Delzell E. The incidence of gastrointestinal perforations among rheumatoid arthritis patients. Arthritis Rheum. 2011 Feb;63(2):346–51. doi: 10.1002/art.30107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen R, Robinson D, Jr, Paramore C, Fraeman K, Renahan K, Bala M. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001–2002. Inflamm Bowel Dis. 2008 Jun;14(6):738–43. doi: 10.1002/ibd.20406. [DOI] [PubMed] [Google Scholar]
- 22.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008 Jan;214(2):149–60. doi: 10.1002/path.2287. Review. [DOI] [PubMed] [Google Scholar]
- 23.Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev. 2007 Aug;6(7):503–9. doi: 10.1016/j.autrev.2007.03.008. Review. [DOI] [PubMed] [Google Scholar]
- 24.Barcellos LF, Kamdar BB, Ramsay PP, DeLoa C, Lincoln RR, Caillier S, Schmidt S, Haines JL, Pericak-Vance MA, Oksenberg JR, Hauser SL. Clustering of autoimmune diseases in families with a high-risk for multiple sclerosis: a descriptive study. Lancet Neurol. 2006 Nov;5(11):924–31. doi: 10.1016/S1474-4422(06)70552-X. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes JM, Collins P. Lessons for inflammatory bowel disease from rheumatology. Dig Liver Dis. 2006 Mar;38(3):157–62. doi: 10.1016/j.dld.2005.09.020. Review. [DOI] [PubMed] [Google Scholar]
- 26.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, Moser KL, Begovich AB, Carlton VE, Li W, Lee AT, Ortmann W, Behrens TW, Gregersen PK. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005 Apr;76(4):561–71. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witowski J, Ksiazek K, Jörres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci. 2004 Mar;61(5):567–79. doi: 10.1007/s00018-003-3228-z. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham NS, Cohen DC, Rivers B, Richardson P. Validation of administrative data used for the diagnosis of upper gastrointestinal events following nonsteroidal anti-inflammatory drug prescription. Aliment Pharmacol & Ther. 2006;24:299–306. doi: 10.1111/j.1365-2036.2006.02985.x. [DOI] [PubMed] [Google Scholar]
- 29.Cattaruzzi C, Troncon MG, Agostinis L, García Rodríguez LA. Postivie predictive value of ICD-9th codes for upper gastrointestinal bleeding and perforation in the SISR database. J Clin Epidemiol. 1999;52(6):499–502. doi: 10.1016/s0895-4356(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 30.Raiford DS, Perez GS, García Rodríguez LA. Positive predictive value of ICD-9 codes in the identification of cases of complicated peptic ulcer disease in the Saskatchewan Hospital automated database. Epidemiology. 1996;7:101–4. doi: 10.1097/00001648-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Wahl PM, Rodgers K, Schneeweiss L, Gage BF, Butler J, Wilmer C, Nash M, Esper G, Gitlin N, Osborn N, Short LJ, Bohn RL. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf. 2010 Jun;19(6):596–603. doi: 10.1002/pds.1924. [DOI] [PubMed] [Google Scholar]
- 32.Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ. 1994 Jul;309(6947):102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.