Abstract
As prophylactic therapies and vaccines against viral infections continue to improve, drug resistant strains are continuing to arise; therefore it is imperative to develop new therapeutics against these diseases. For highly pathogenic viruses, such as Ebola and H5N1 influenza virus, the need for antivirals is even more urgent due to limited therapeutics against these viruses. Furthermore, the high pathogenicity of such viruses often makes it difficult to work with such agents. In this report, we describe a protocol called “One-stone-two-birds” which provides a safe and efficient screening system to identify anti-flu (entry) and anti-HIV (replication) activities. Using plant extracts as an example, we demonstrate the utility of this protocol in antiviral screening.
Keywords: Antiviral, Influenza, HIV, Ebola, Plant extract, Screening
Introduction
Prophylactic therapeutics and vaccines continue to be critical strategies to fight the spread of viral infections [1]. For example, vaccines are the primary method of controlling the spread of influenza A virus. Due to the ability of this virus to constantly acquire genetic changes [2–3], new vaccines are formulated and produced annually. These vaccines are based on the most widely spread strains from the previous year which are predicted to be most prevalent in the coming flu season. In addition to vaccines, several prophylactic therapies are available, such as Tamiflu and Relenza, which can be taken after infection to halt the spread of the virus [4,5]. These antivirals provide a treatment option post-infection, however, not all influenza A viruses respond to these treatments and some strains are resistant. Thus new antiviral therapies are urgently needed.
One impediment to developing antiviral therapeutics for highly pathogenic viruses, such as H5N1 influenza and Ebola, which require enhanced BSL-3 and 4 containment, respectively, is the safety concerns. To circumvent this problem, we have established a surrogate pseudotyping system [6], referred to as the One-stone-two-birds approach here, which allows us to study the antiviral activity targeted against both the HA-mediated entry mechanism of influenza virus and the replication process of HIV. In this study, we evaluated and identified plant extracts which demonstrated antiviral activities by this approach.
Several antimicrobial and medicinal therapies currently used have taken advantage of plants as a powerful resource. One such example is Cinchona ledgeriana, the rainforest plant which is a source of quinine and quinidine. Quinine was first discovered to have antimalaria activity. For many years, quinine was processed from the bark of the tree and put into pill form until it was discovered that the chemically synthesized form was active and that plant material was no longer needed. Another chemical in the tree, quinidine, was discovered to treat arrhythmia, however it was noted that the chemically synthesized form was not active and thus medicinal quinidine still requires extraction from the tree bark [7]. The cinchona tree is just one powerful example of how plants can be incredible sources of medicinal compounds [8–10].
In this study, plant extracts were evaluated using the One-stone-two-birds protocol and we have identified 17 lead antiviral extracts against influenza viral entry and HIV replication, demonstrating the power of this screening method which can be easily adapted for other viruses.
Materials and Methods
Plant materials
Plants were collected from the Cuc Phuong National park in Vietnam, as well as from the whole country of Laos P. D. R. Voucher specimens of the plants collected from Vietnam are in deposit at the Herbarium of Cuc Phuong National Park, and at the John G. Searle Herbarium of the Field Museum in Chicago. Voucher specimens of the plants collected from Laos are in deposit at the Herbarium of the Traditional Medicine Research Center, Vientiane Capital, Laos P. D. R., and at the John G. Searle Herbarium of the Field Museum in Chicago.
Preparation of plant extracts
Each dried and milled plant material (100 g) was extracted with methanol or dichloromethane or ethanol at room temperature to afford an extract (yield: 0.5–10 %). A 4–10 mg of the extract was then resuspended in DMSO to make a 4 mg/mL stock solution.
Production of HIV pseudovirions
Human embryonic kidney 293T cells were transiently transected with either 0.5µg VSV-G envelope expression plasmid or 0.5µg hemagglutinin envelope expression plasmid with 0.5µg neuraminidase expression plasmid and 2µg Env-deficient HIV vector (pNL4.3.Luc-R-E-) in 6 well plates via PEI (Invitrogen). Sixteen hours post-transfection, all media was replaced with fresh, complete DMEM. Forty-eight hours post-transfection, the supernatants were collected and filtered through a 0.45-µm-pore size filter (Nalgene) and the pseudovirions were directly used for infection.
Inhibitory activity screening assay
Target A549 (human lung epithelial) cells were seeded at 0.5×105 cells per well (24-well plate) in complete DMEM. Crude plant extract (final concentration: 20 µg/mL) and 125 µL of the pseudovirus were incubated with target cells. Twenty-four hours post-infection, all media containing extract and virus was removed from target cells and replaced with fresh, complete DMEM. Forty-eight hours post-infection, cells were lysed and prepared for luciferase assay (Promega).
Results and Discussion
In this study, we developed a screening protocol, referred to as One-stone-two-birds, to identify potential inhibitory plant extracts against influenza (entry) and HIV replication (post-entry steps). Overall, 1,859 crude plant extracts were evaluated for potential antiviral activity. In collaboration with the International Biodiversity Group (ICBG) program at the University of Illinois Chicago (UIC), terrestrial plants were collected from the Cuc Phuong National Park in Vietnam as well as from the whole country of Laos. Plant materials were collected, dried, and methanol, dichloromethane, or ethanol extracts were produced and resuspended in DMSO. HIV-luciferase reporter pseudoviral particles (VSV-G and H5N1) were used to transduce human lung epithelial A549 target cells. We have previously established that A549 cells are susceptible to H5N1 pseudotyped viral entry. Plant extracts (20 µg/mL) were added simultaneously with viral supernatant. Twenty-four hours post infection, toxicity of the test extract was assessed in target cells visually under a light microscope. Extracts which induced a cytopathic effect (CPE), as indicated by cells rounding up off the plate and floating in the media, were noted and not further pursued. Forty-eight hours post-transduction, target cells were again assessed for CPE, then lysed and used to measure luciferase levels.
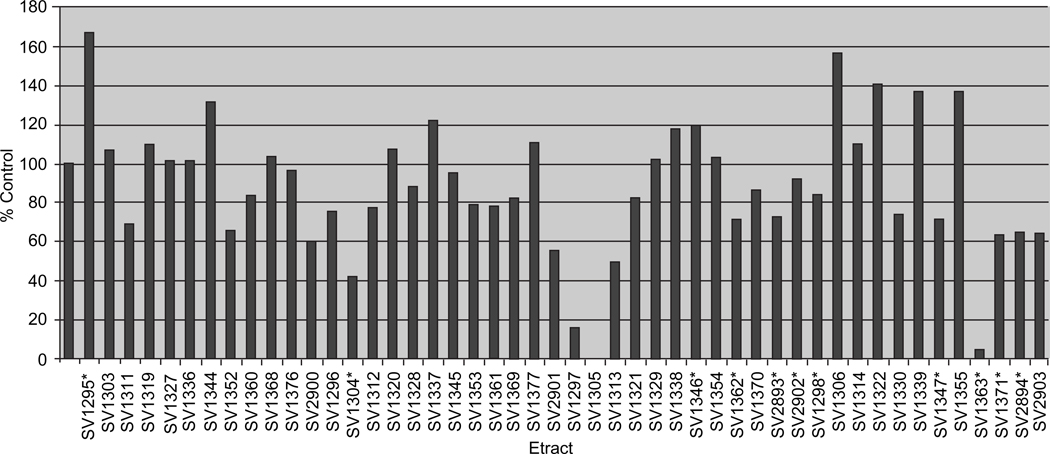
Plant extracts were evaluated based on their ability to reduce levels of luciferase in the infected target cells. A decrease in the level of luciferase activity indicated a potential inhibitory effect of the plant extract on either entry or a post-entry step. Those extracts which did not induce CPE and decreased luciferase levels when compared with the control sample suggested inhibition in viral replication. This comparison is represented as the percentage of the control (DMSO alone, Figure 1). Extracts which decreased infectivity by greater than 90% were identified as ‘hit’ extracts for further antiviral evaluation. While 90% inhibition is a stringent criterion, this high level of inhibitory activity is likely to lead to the isolation of antiviral compounds with the most potency. Of the extracts screened, 17 were identified as hits, with 11 having inhibition levels greater than 95%.
Figure 1.
Screening of crude plant extracts. Extracts were screened in A549 target cells and their anti-viral activity is represented as a percent control of the virus with DMSO alone. Those extracts which displayed cytotoxicity are noted with a *.
The data presented in Figure 1 is an example of the first round extract evaluation. The extracts listed on the X-axis were screened against the H5N1/HIV pseudovirus and the relative level of infectivity is represented as the percentage of the control (pseudovirus plus DMSO only, as 100%). In this representative extract screen, 2 of the 48 extracts tested have reduced luciferase levels greater than 90%. As indicated by the ‘*’ notation, SV1363 induced CPE in target cells. The low luciferase level for this sample was likely due to cytotoxicity. Thus, SV1363 and the other likely toxic extracts were not further pursued. The other extract which reduced luciferase levels by greater than 90%, SV1305, had the greatest inhibitory effect among the 48 extracts tested in this representative first round evaluation and did not induce CPE in target cells. SV1305, along with other inhibitory extracts during the primary round of screening, were chosen for further investigation to determine their antiviral specificity.
To confirm inhibitory activity and determine specific antiviral activity, hit extracts were assayed using the H5N1 influenza pseudovirus as well as a VSV-G pseudovirus. Each hit extract was added simultaneously with either H5N1 or VSV-G pseudovirus to A549 target cells. Cytotoxicity was again assessed visually. Forty-eight hours postinfection, luciferase levels were measured. After this second round of testing, hit extracts were classified as either inhibitory against H5N1 influenza alone or against both pseudoviruses. Extracts which were effective against a single virus type suggest inhibitory activity at the entry level, as the difference between the two infecting pseudoviruses is their surface glycoprotein which mediates entry. Those extracts which were effective against both pseudoviruses suggest that the extract targets either a post-entry step (on HIV) or that it targets a host factor in the A549 target cells. Eleven extracts were classified as having dual inhibition, suggesting HIV or a host factor as a target, while 6 were characterized as specifically inhibiting the H5N1 pseudotyped virus. A complete list of plant extract activity and their assigned specificity is shown in Table 1.
Table 1.
Identification of hit plant extracts.
| Extract | % Inhibition of VSV-Gpp | % Inhibition of H5N1pp |
Inhibitory Classification |
|---|---|---|---|
| 47 | 95.57% | 95.62% | HIV |
| 73 | 97.6% | 97.78% | HIV |
| 87 | 41.8% | 98.26% | Influenza |
| 1305 | 97.93% | 99.91% | HIV |
| 2888 | 76.75% | 98.20% | Influenza |
| 5508 | 99.97% | 99.83% | HIV |
| 5510 | 63.91% | 96.16% | HIV |
| 5542 | −66.29% | 94.60% | Influenza |
| 5544 | −46.94% | 91.83% | Influenza |
| 5614 | 99.99% | 99.69% | HIV |
| 5642 | 73.1% | 97.74% | HIV |
| 5762 | 84.72% | 92.96% | HIV |
| 5774 | 88.71% | 92.53% | HIV |
| 5780 | 97.19% | 97.96% | HIV |
| 5781 | 64.3% | 87.7% | HIV |
| 8016 | −28.94% | 96.44% | Influenza |
| 8025 | 17.24% | 89.76% | Influenza |
Novel antiviral therapeutics are urgently needed as resistance continues to increase to several current prophylactic treatments available, such as with the influenza drugs Relenza and Tamiflu. In this study, we have developed a novel One-stone-two-birds protocol to safely and efficiently screen extracts for antiviral activity against highly pathogenic viruses. This approach eliminates the requirement of BSL-3 to BSL-4 safety containment when screening for antivirals against infectious agents such as HIV, Ebola, and H5N1 influenza. By alleviating the strict safety requirements, antiviral therapeutic development can be hastened at the initial screening stage. This safe and sensitive screening protocol should lead to more lead antiviral targets which will facilitate the development of novel therapeutics.
Specifically, we have examined the anti-flu and anti-HIV potential of plant extracts for further therapeutic development. This potential is illustrated by the initial screening results of terrestrial plant extracts from Vietnam and Laos. During the primary round of screening, 17 hit extracts were identified. It is foreseeable to increase the potential of this approach by adapting this assay for high throughput screening. This adaptation will increase the number of extracts which can be assayed quickly; likely increasing the number of hits identified which may, in turn, increase the number of novel antiviral treatments available. Coupled with high throughput screening is the ability to adapt this protocol for several different highly pathogenic viruses. By using an alternate surface glycoprotein and/or viral core, the potential antiviral activity against other viruses can be assessed, making this approach a powerful resource for antiviral therapeutic development.
In the current screen, each of the 17 active extracts identified was further characterized to determine its specific antiviral activity. To determine the specific activity, VSV-G/HIV core pseudovirus and H5N1/HIV core pseudovirus were used. This dual challenge of hit extracts led to the classification of 6 extracts having specific antiflu activity while 11 were identified as potential anti-HIV extracts. As entry is mediated by the HA protein of influenza, the hit extracts identified which specifically inhibit the H5N1 pseudovirus can be presumed to block entry, either acting on HA directly or by blocking a required entry factor on the host cell. After the initial identification of 17 hit extracts, testing with H5N1 and VSV-G pseudoviruses to confirm activity and specificity led to the conclusion that extracts with dual pseudovirus inhibition were acting on post-entry/replication steps of HIV. This finding adds to the potential of the One-stone-two-birds system in identifying extracts for antiviral therapeutic development. Not only can entry inhibitors for highly pathogenic viruses such as H5N1 influenza, Ebola, and SARS be screened, but the broader activity of extracts against more than one pseudovirus allows HIV post-entry/ replication steps to be targeted as well. While these observations are based on initial infection assays, the definitive classification cannot be determined until the target of inhibition is identified. This target could be specific to either a viral factor or a host factor. Furthermore, while this screening approach serves as a powerful starting platform for antiviral therapeutic development, active extracts must be evaluated using the appropriate infectious virus system.
In addition to primary screening, this One-stone-two-birds approach can be used to direct isolation and identification of the active compound(s) from the plant extracts of interest. While the crude hit extracts tested here were able to block infectivity by greater than 90%, it is important to note that the active component(s) is likely a small fraction of the extract and thus, may have much greater inhibitory activity at a lower concentration once isolated and purified. In combination with several fractionation and separation techniques, the One-stone-two-birds approach has the capacity to identify the active compound(s) responsible for the overall inhibitory activity of the extract. As illustrated, this One-stone-two-birds system has tremendous potential to identify highly active lead extracts for antiviral therapeutic development.
Acknowledgements
All work involving plant sample collection described in this paper was carried out under NIH grants 1UO1-TW01015-01, 1UO1-TW-01015-10S1 and 1UO1-TW-01015-10S2 administered by the Fogarty International Center through funds from NIH, NSF, and USDA Foreign Agricultural Service, as part of an International Cooperative Biodiversity Groups (ICBG) program.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Burke JD, Fish EN. Antiviral Strategies: The Present and Beyond. Curr Mol Pharmacol. 2009;2:32–39. doi: 10.2174/1874467210902010032. [DOI] [PubMed] [Google Scholar]
- 2.Wilson IA, Cox NJ. Structural Basis of Immune Recognition of Influenza Virus Hemagglutinin. Annu Rev Immunol. 1990;8:737–787. doi: 10.1146/annurev.iy.08.040190.003513. [DOI] [PubMed] [Google Scholar]
- 3.Cox N, Bender C. The molecular epidemiology of influenza viruses. Semin Virol. 1995;6:359–370. [Google Scholar]
- 4.von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 5.Kim CU, Lew W, Williams MA, Liu H, Zhang L, et al. Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Rumschlag-Booms E, Wang J, Xiao H, Yu J, et al. Analysis of hemagglutinin-mediated entry tropism of H5N1 avian influenza. Virol J. 2009;6:39. doi: 10.1186/1743-422X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang F, Hanon S, Lam P, Schweitzer P. Quinidine Revisited. Am J Med. 2009;122:317–321. doi: 10.1016/j.amjmed.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Tagboto S, Townson S. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasitol. 2001;20:199–295. doi: 10.1016/s0065-308x(01)50032-9. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay D, Khan MT. Ethnomedicines and ethnomedicinal phytophores against herpesviruses. Biotechnol Annu Rev. 2008:297–348. doi: 10.1016/S1387-2656(08)00012-4. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT. A natural history of botanical therapeutics. Metabolism. 2008:S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]