Abstract
Nicotinamide phosphoribosyltransferase (NAMPT) was first reported as a pre-B-cell colony enhancing factor in 1994 with little notice, but it has received increasing attention in recent years due to accumulating evidence indicating that NAMPT is a pleiotropic protein such as a growth factor, a cytokine, an enzyme and a visfatin. Now, NAMPT has been accepted as an official name of this protein. Because of NAMPT’s multiple functions in a variety of physiological processes, their dysregulations have been implicated in the pathogenesis of a number of human diseases or conditions such as acute lung injury, aging, atherosclerosis, cancer, diabetes, rheumatoid arthritis and sepsis. This review will cover the current understanding of NAMPT’s structure and functions with an emphasis on recent progress of nicotinamide phosphoribosyltransferase’s pathological roles in various human diseases and conditions. Future directions on exploring its Terra incognita will be offered in the end.
Keywords: NAMPT, PBEF, Visfatin, Growth factor, Cytokine
Introduction
Nicotinamide phosphoribosyltransferase (NAMPT) was cloned by Samal and his colleagues in 1994 from activated human peripheral blood lymphocytes during their attempt to discover new factors for the earliest events in B-cell development [1]. The first NAMPT cDNA was screened out from those activated lymphocyte cDNA libraries using a degenerate oligonucleotide probe, which was designed on the basis of the similarity in the coding sequences of GM-CSF, IL-2, IL-1β, IL-6 and IL-13 at the signal peptidase processing site. NAMPT was initially named pre-B-cell colony enhancing factor (PBEF) after its function to promote pre-B-cell colony formation in the presence of stem cell factor plus interleukin 7. In the following ten years, PBEF received little notice. By 2004, only about 20 articles on PBEF were published in the literature. However, PBEF has garnered increasing attention in recent years due to accumulating evidence indicating that PBEF is a pleiotropic protein such as a growth factor, a cytokine, an enzyme and a visfatin. Until Dec 5, 2010, 712 publications have appeared in Pub Med when searched with key words: NAMPT OR pbef OR visfatin. To avoid the confusion, the name NAMPT will be used throughout this review since the NAMPT was approved as the official name of this gene by the Human Genome Organization Gene Nomenclature Committee. Because of NAMPT’s multiple functions in a variety of physiological processes, their dysregulations have been implicated in the pathogenesis of a number of human diseases or conditions such as acute lung injury, aging, atherosclerosis, cancer, diabetes, rheumatoid arthritis and sepsis. This review will cover the current understanding of NAMPT structure and functions with an emphasis on recent progress of NAMPT’s pathological roles in various human diseases and conditions. Future directions on exploring Terra incognita will be offered in the end.
NAMPT structure
This section covers the NAMPT structure in three parts: Gene, mRNA and Protein. The regulatory components in the NAMPT gene promoter, evolutionary conservation of the coding sequence and some featured single nucleotide polymorphisms [SNP] in the NAMPT gene are described in the first part. mRNA species, isoforms and tissue distributions are recounted in the second part. The characteristics of the primary, secondary and three dimensional structure of the NAMPT protein are detailed in the third part.
Gene
The human NAMPT gene spans a length of 36,908 bp on the position of chromosome 7:105, 675, 967–105,712, 874 according to the Human March 2006 Assembly on the University of California Santa Cruz Genome Browser. The NAMPT structural gene part is composed of 11 exons and 10 introns, which encodes 491 amino acids. Exon 1 encodes a short 5′-untranslated region and the signal peptide region, whereas exon 11 encodes the carboxyl end of the NAMPT protein and all of the 3′ untranslated region. The GT and AG consensus splice junction sequences were well conserved at all of the exon/intron boundaries. The analysis of the regulatory gene part at the 3.2 kb 5′-flanking region upstream of the transcription initiation site revealed two segments: the proximal 1.4 kb is more GC rich (60% GC), whereas the distal 1.6 kb has more AT bases (60% AT) [2]. Within the proximal region there is an extremely G+C-rich segment (72% GC) spanning the first 500 bp upstream of the major transcription initiation site, which lacks canonical TATA and CAAT boxes and contains several transcription initiation sites. In the distal region, however, several CAAT boxes and TATA-like and initiator sequences were found about 2 kb upstream from the transcription initiation site. This distal segment may act as a distal promoter. Several types of putative regulatory elements were identified. Multiple SP1 binding sites (GC-boxes) were found all along the 5′-flanking region; 12 of these were located at the predicted proximal promoter region. The binding sites for ubiquitous transcription factors such as the CCAAT/nuclear factor 1, AP-1 and AP-2 were also identified. The binding sites for NF-1 were concentrated in the putative distal promoter region, those for AP-2 were mainly in the proximal promoter region, and AP-1 sites were uniformly distributed. In addition, the hormonally and chemically responsive regulatory elements, which include the binding sites for the glucocorticoid receptor, corticotropin releasing factor, cAMP response element binding protein and the nuclear factors such as NF-IL6, were present in both proximal and distal promoter regions. NF-κB, another hormonally responsive element, was present only in the distal promoter region. Analysis of the introns showed the presence of the NF-κB binding site (GGGAGGCCC) localized in the third intron, whereas introns 5, 8, 9 and 10 had NF-κB -like elements (GGGAGGCXX) present. Another group of regulatory elements that bind tissue specific transcription factors, such as liver factor-1 and hepatic nuclear factors were also identified. However, the sequence motif ‘GPuGPuTTPyCAPy’ – which is well conserved in the 5′-flanking regions of hematopoetic cytokines including IL-2, IL-3, granulocyte-macrophage colony-stimulating factor and granulocyte-colony stimulating factor and is considered a diagnostic feature – was not present in the NAMPT gene.
The coding sequence of the NAMPT gene is highly conserved through evolution and the mammalian gene was found to be active in a prokaryotic host [3]. Significant sequence homology has been shared among prokaryotic organisms such as the bacterium Haemophilus ducreyi, primitive metazoan such as a marine sponge and humans [4]. Amino acid sequence alignment revealed that the canine NAMPT protein sequence is 96% identical to human and 94% identical to murine and rat counterparts [5]. This suggests that the gene is evolutionarily highly conserved and it must be important to life. Indeed, the homozygous Nampt gene knockout mouse is embryonically lethal [Ye et al., unpublished observation]. The pleiotropic functions of NAMPT in human physiology will be reviewed in the section: NAMPT Function.
The most common variations in genes are single nucleotide polymorphisms (SNPs), which are valuable to facilitate large-scale association genetics studies to associate sequence variations with heritable phenotypes. Therefore, there is a growing interest in SNP discovery and detection for each gene. As of December 3, 2010, dbSNP Build 132 [http://www.ncbi.nlm.nih.gov/SNP] reports that there occurs 411 SNPs in the human NAMPT gene. There is a G→A SNP which causes a coding synonymous mutation at Serine 301. No coding non-synonymous SNPs including nonsense, missense and frame shift mutations have been reported. Nearly half of references SNPs have been reported in the intron regions. There is an A→T SNP at the position of NAMPT mRNA 295 at the 5′untranslated region. There are numbers of SNPs at the 3′untranslated region. It should be pointed out that many of these SNPs in the dbSNP have not been validated with the population data. Functional consequences of most of these SNPs are currently unknown. Ye et al. [6] reported 11 SNPs in the human NAMPT gene proximal promoter by direct DNA sequencing from 36 people. They genotyped 2 SNPs in a Caucasian population and found that carriers of the haplotype GC from SNPs T-1001G and C-1535T (C-1535T was mislabeled as C1543T in the original publication) in the human NAMPT gene promoter had a 7.7-fold higher risk of acute respiratory distress syndrome(ARDS) while the haplotype TT represented a protective haplotype to ARDS. These observations were confirmed and extended by Bajwa et al. [7], who found that the NAMPT T-1001G variant allele and related haplotype are associated with increased odds of developing ARDS and increased hazard of intensive care unit mortality among at-risk patients, whereas the C-1535T variant allele and related haplotype are associated with decreased odds of ARDS among patients with septic shock and better outcomes among patients with ARDS. These works support that NAMPT is a genetic marker in ARDS. A few other studies also surveyed the association of some SNPs in the NAMPT gene with other human conditions or traits. Johansson et al. [8] found that the NAMPT G-948T gene polymorphism is associated with increased high-density lipoprotein cholesterol in obese subjects. Tokunaga et al. [9] reported that the −1535T/T genotype was associated with lower serum triglyceride levels and higher HDL-cholesterol levels in the non-diabetic Japanese subjects. Bailey et al. [10] in the Quebec Family Study found a significant association between two SNPs (rs9770242 and rs1319501), in perfect linkage disequilibrium, and fasting insulin and glucose levels. A more distal SNP (rs7789066) was significantly associated with the apolipoprotein B component of VLDL. Jian et al. [11] noticed significant associations between three SNP loci: −1535C/T, rs2058539 and rs10953502 and plasma glucose concentration at 0 and 120 min during oral glucose tolerance tests, the area under the response curve for plasma glucose, and triglyceride and total cholesterol levels in a Chinese population. Böttcher et al. [12] presented that the ratio of visceral/subcutaneous visfatin mRNA expression was associated with all three genetic polymorphisms (rs9770242, −948G-->T, rs4730153). Moreover, the −948G-->T variant was associated with 2-h plasma glucose and fasting insulin concentrations in nondiabetic Caucasian subjects in Germany.
mRNA
Three NAMPT mRNA species, about 4,000, 2,400 and 2,000 bp each, were detected in human tissues by northern blot analysis [1, 2]. According to Aceview [13], a comprehensive cDNA-supported gene and transcripts annotation [http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/], human NAMPT gene contains 19 different gt-ag introns. Transcription produces 19 different mRNAs, 14 alternatively spliced variants and 5 unspliced forms, which are supported by 719 cDNA clones. There are 5 probable alternative promotors, 6 nonoverlapping alternative last exons and 13 alternative polyadenylation sites. The mRNAs appear to differ by truncation of the 5′ end, truncation of the 3′ end, presence or absence of 2 cassette exons, overlapping exons with different boundaries, alternative splicing or retention of 4 introns. These NAMPT variants remains to be validated experimentally and their physiological relevances are currently unknown.
Chen et al. [14] detected 3 NAMPT transcripts in porcine. Variant 1 is the predominant form, encoding 491 amino acids. While the other two variants were predicted to encode two 3′ truncated proteins due to early termination. Nested PCR showed that variants 1 and 3 were ubiquitously expressed in porcine tissues and that variant 2 was expressed in most tissues examined with exception of testis and liver. Palin et al. [15] subsequently reported the cloning of six porcine NAMPT transcript variants, resulting from alternate polyadenylation or alternate splicing of exons. The discovery of NAMPT variants in porcine would be useful to the further investigation of the function of the NAMPT gene.
NAMPT is expressed in human heart, brain, placenta, lungs, liver, skeletal muscle, kidney and pancreases with the maximum amount of NAMPT mRNA in liver and the next highest amount in muscle tissue [1]. From analyzing tissue sources of 719 cDNA clones, it is found that NAMPT is expressed in nearly all organs, tissues, and cells examined. This indicates a ubiquitous tissue distribution of the NAMPT gene and suggests an omni-important function of this gene, as reviewed in the section: NAMPT Function.
Protein
The reference human NAMPT protein sequence (NP_005737) consists of 491 amino acids. Among them is a signal peptide from amino acids 1–26. It has a theoretic pI/Mw: 6.69/55.52 Kd. It contains 63 negatively charged residues (Asp + Glu) and 61 positively charged residues (Arg + Lys). Based on the N-terminal of the sequence as methionine, the estimated half-life of human NAMPT protein is 30 hours. The instability index [II] of human NAMPT is computed to be 35.48, and this classifies the human NAMPT as a stable protein (http://ca.expasy.org/cgi-bin/protparam).
Several groups have resolved three dimensional structure of mammalian NAMPT protein [16,17,18]. The 3D structure analysis of human and murine NAMPT by Khan et al. [16] showed that NAMPT has a notable overall structural similarity to quinolinic acid and nicotinic acid phosphoribosyltransferases, enzymes in two other NAD+ biosynthesis pathways, despite sharing little sequence similarity among them. The structure of the NAMPT monomer contains 22 β-strands and 15 α-helices and can be divided into three domains, A, B and C. Domain A consists of a seven-stranded fully antiparallel β-sheet with five helices on one face. Residues from both the N- and C-terminal regions of NAMPT (9–148, 391–427 and 459–484) belong to this domain. Domain B [residues 181–390] contains a seven-stranded β/α core. Helix α6, with nine turns (residues 149–180), connects domains A and B. Domain C (residues 428–458) contains a three stranded antiparallel b-sheet and covers the open face of the β-sheet in domain A.
The functional NAMPT forms a homodimer to catalyze the conversion of nicotinamide and phosphoribosyl-pyrophosphates to form nicotinoamide mononucleotide, a key step in the mammalian NAD synthetic salvage pathway. The structural and mutagenesis studies by Khan et al. [16] demonstrate that Asp219 is important in defining the substrate specificity of NAMPT. Wang et al. [17] showed that NAMPT has an autophosphorylation activity and hydrolyzes ATP. Autophosphorylation can increase its enzymatic activity. His247 is an important residue in this regard, which lies at the center of a conserved cluster of active site residues (a Ser280-His247-Asp313 triad). Mutations of the residue H247 either significantly decrease or abolish the NAMPT enzymatic activity. The H247E enzyme retains low enzymatic activity, whereas the H247A mutant has no detectable activity, suggesting a role for a negative charge at this residue in establishing maximal catalytic activity.
FK866, an inhibitor to NAMPT, is found to be bound in a tunnel at the interface of the NAMPT dimer, and mutations in this binding site can abolish the inhibition by FK866. Contrary to previous knowledge, the structures show that FK866 could compete directly with the nicotinamide substrate. Since the pleiotropic physiological functions of NAMPT and its dysregulation implicated in several human diseases, these structural studies provide valuable information for the potential development of new, better and more efficacious drugs to treat human diseases based on the NAMPT structure and functions.
NAMPT functions
This section deals with the three major functions of the NAMPT: Growth Factor, Cytokine and Nicotinamide phosphoribosyltransferase. Accumulating evidence suggests that NAMPT can function as a growth factor or a cytokine though the underlying molecular mechanisms remain to be established. It is beyond the dispute that NAMPT can function as a Nicotinamide phosphoribosyltransferase.
Growth factor
Growth factor generally refers to a naturally occurring protein capable of stimulating cellular growth, proliferation and cellular differentiation. Growth factors are important for regulating a variety of cellular processes. Several studies indicate that NAMPT may function as a growth factor. Samal et al. [1] first found that conditional medium from COS7 or PA317 cells transiently expressing human NAMPT can significantly enhance the number of pre-B-cell colonies derived from normal human or mouse bone marrow by at least 70% in the presence of both IL-7 and stem cell factor. Similar observation was obtained using the antibody purified NAMPT protein. Thus, the authors first named this protein as pre-B-cell colony enhancing factor. Van der Veer et al. [19] reported that NAMPT can promote vascular smooth muscle cell maturation. They found that knockdown of endogenous NAMPT increased smooth muscle cell apoptosis and reduced the capacity of synthetic smooth muscle cells to mature to a contractile state. On the other hand, human smooth muscle cells transduced with the NAMPT gene had enhanced survival, an elongated bipolar morphology, and increased levels of h-caldesmon, smoothelin-A, smoothelin-B, and metavinculin. Fukuhara and co-workers [20] proposed NAMPT as a Visfatin, an adipokine produced by visceral fat that can engage and activate the insulin receptor, however this publication was retracted because of questions regarding the reproducibility of the NAMPT/insulin receptor interaction from different preparations of recombinant NAMPT protein [21]. Xie et al. [22] found that NAMPT exerts an insulin-like activity as a growth factor for osteoblasts. They used the recombinant human NAMPT provided by Axxora Life Sciences (San Diego, CA, USA) in their experiments. They noticed that the effects of NAMPT such as glucose uptake, proliferation, and type I collagen enhancement in cultured human osteoblast-like cells bore a close resemblance to those of insulin and were inhibited by hydroxy-2-naphthalenylmethylphosphonic acid tris-acetoxymethyl ester, a specific inhibitor of IR tyrosine kinase activity. They also unexpectedly found that NAMPT downregulated osteocalcin secretion from human osteoblast-like cells. These data indicate that the regulation of glucose uptake, proliferation, and type I collagen production by NAMPT in human osteoblasts involves insulin receptor phosphorylation, the same signal transduction pathway used by insulin.
Cytokine
Cytokine is sometimes used interchangeably among scientists with the term Growth Factor. The term cytokine encompasses a large and diverse family of polypeptide regulators that are produced widely throughout the body by cells of diverse embryological origin. Their actions may be grouped as autocrine, paracrine and endocrine. Cytokines have been classified as lymphokines, interleukins, and chemokines. Now, these distinctions may not be valid due to the considerable redundancy and pleiotropism of many cytokines. NAMPT may be added to the list of cytokines. As stated above, the first NAMPT cDNA was screened out using a degenerate oligonucleotide probe designed on the basis of the similarity in the coding sequences of five different cytokines: GM-CSF, IL-2, IL-1β, IL-6 and IL-13, at the signal peptidase processing site [1] though the DNA or protein sequence of NAMPT bears no homology to other known cytokines. Ognjanovic et al. [2] reported that lipopolysaccharide, IL-1β, TNF α and IL-6 all significantly increased the expression of NAMPT in a 4 h treatment of the amniotic epithelial cell line, WISH cells. The addition of dexamethasone to IL-1β and TNFα significantly reduced the response to these cytokines. They concluded that NAMPT is a cytokine expressed in the normal fetal membranes and up-regulated when they are infected. NAMPT expression is up-regulated in a variety of acute and chronic inflammatory diseases including sepsis [23], acute lung injury [6], rheumatoid arthritis [24], inflammatory bowel disease [25], and myocardial infarction [26]. NAMPT plays a key role in the persistence of inflammation through its capacity to inhibit neutrophil apoptosis [23]. rhNAMPT treatment of WISH cells and fetal membrane explants significantly increased IL-6 and IL-8 gene expression [27]. We also found that an overexpression of NAMPT significantly augmented IL-8 secretion and mRNA expression in A549 cells, a human pulmonary carcinoma type II epithelial cell line and human pulmonary artery endothelial cells. It also significantly augmented IL-1β-mediated cell permeability. The opposite results were obtained with the knockdown of NAMPT expression. NAMPT expression also affected the expression of two other inflammatory cytokines (IL-16 and CCR3 genes) [28,29]. Hong et al. [30] demonstrated recombinant human NAMPT as a direct rat neutrophil chemotactic factor in in vitro studies. They also demonstrated a marked increase in bronchoalveolar lavage leukocytes after the intratracheal injection of rhNAMPT into C57BL/6J mice. Thus, NAMPT behaves like a chemokine.
Nicotinamide phosphoribosyl transferase
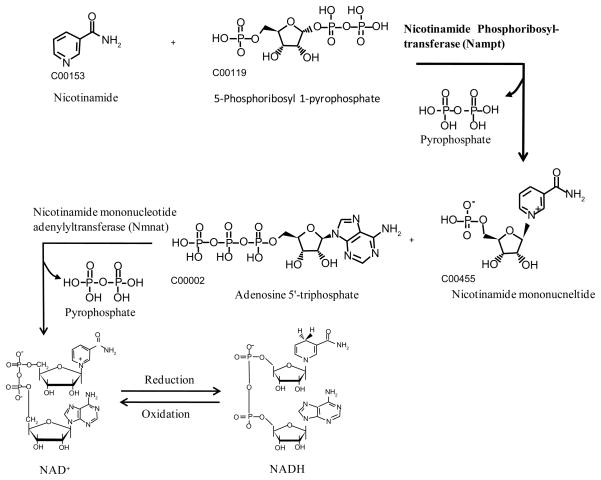
The clue that PBEF could be a nicotinamide phosphoribosyl transferase was first obtained by the work of Martin et al. [4] in 2001. They demonstrated that the presence of the nadV gene allowed A. pleuropneumoniae to utilize nicotinamide mononucleotide as a precursor for NAD biosynthesis, and indicate that the enzyme encoded by this gene is a novel NAMPT. They found that the sequence of nadV gene is homologous to that of human NAMPT, suggesting that mammalian PBEF may also function as a NAMPT. Rongvaux et al. [3] verified that similarly to its microbial counterpart, PBEF is a NAMPT, catalyzing the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to yield nicotinamide mononucleotide, an intermediate in the biosynthesis of NAD (Figure 1). The role of PBEF as a NAMPT was further confirmed by showing that the mouse gene was able to confer the ability to grow in the absence of NAD to a NAMPT-defective bacterial strain. Study by Revollo et al. [31] demonstrated that NAMPT catalyzes a rate-limiting step in a salvage pathway of the mammalian NAD biosynthesis. Van der Veer et al. [32] proved that it is due to the enhanced NAMPT activity of PBEF that cellular lifespan of human primary smooth muscle cells, human clonal smooth muscle cells, and fibroblasts derived from a patient with Hutchinson-Gilford progeria syndrome can be lengthened. Recent work by Revollo et al. [33] revealed that NAMPT regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme.
Figure 1. Mammalian salvage pathway of NAD+ synthesis mediated by nicotinamide phosphoribosyltransferase.
Nicotinamide, either derived from the degradation of NAD+ by NAD+ consuming enzymes or provided in the diet, is condensed with 5-phosphoribosyl-1-pyrophosphate to yield nicotinamide mononucleotide under the catalysis of nicotinamide phosphoribosyltransferase. Nicotinamide mononucleotide is then adenylylated to form NAD+, by Nicotinamide mononucleotide adenylyltransferase. NAD+ and NADH can be interconverted by a reduction and an oxidation reaction, respectively.
Because the salvage pathway of NAD synthesis is much more efficient and quicker one than that of de novo NAD synthesis, it is conceivable that NAMPT plays an important role in varieties of physiological processes in life via the synthesis of NAD. NAD is now regarded as a universal energy- and signal-carrying molecule [34, 35]. Recent research has unraveled an unexpectedly wide array of signaling pathways that involve nicotinamide adenine dinucleotide (NAD) and its phosphorylated form, NADP. NAD serves as substrate for protein modification including protein deacetylation and mono- and poly-ADP-ribosylation. Both NAD and NADP represent precursors of intracellular calcium-mobilizing molecules. It is now well accepted that NAD (P)-mediated signal transduction does not merely regulate metabolic pathways but might hold a key position in the control of fundamental cellular processes. In mammals, it has been shown recently that an NAD-dependent protein deacetylase, silent information regulator (SIR) T1/2, plays important roles in a variety of biological processes, such as stress and cytokine responses [36], by deacetylating transcriptional regulators. Endogenous mono-ADP-ribosylation in higher eukaryotes appears to modulate the immune response, cell adhesion, signal, and energy metabolism [37]. Recently, defensin-1, an antimicrobial arginine-rich protein secreted by immune cells, was demonstrated to lose its antimicrobial effect after its mono-ADP-ribosylation. Poly-ADP-ribosylation of proteins such as NFkB by poly-ADP-ribose polymerase can trigger the release of apoptosis-inducing factor from mitochondria and therefore effectively mediate apoptosis. Gerth et al. [38] demonstrated that NAD and ADP-ribose, generated from NAD by CD38, an NAD-glycohydrolase, induce the activation of a Ca2+ channel through a pathway that involves Ca2+ influx in human monocytes. Ca2+ ions play a critical role in variety of monocyte functions such as chemotaxis and production of cytokine (TNFα) [39]. Increased intracellular calcium in human monocyte-derived macrophages in vitro by loading with the basic calcium phosphate microcrystals was associated with secretion of proinflammatory cytokines (TNFα, IL-1β, and IL-8) capable of activating cultured endothelial cells and promoting capture of flowing leukocytes under shear flow [40].
NAMPT in human diseases
This section presents NAMPT in human diseases. The dysregulation of the NAMPT gene has been implicated in the susceptibility and pathogenesis of a number of human diseases and conditions because of its pleiotropic physiological functions as synopsized in the above section. This list is still growing. Due to the space limit, this section only summarizes and updates the current understanding of the possible pathological role(s) of NAMPT in acute lung injury, aging, atherosclerosis, cancer, diabetes, rheumatoid arthritis and sepsis.
Acute lung Injury and sepsis
Acute lung injury (ALI) is characterized by pulmonary inflammation, non-cardiogenic edema, and severe systemic hypoxemia. Acute respiratory distress syndrome (ARDS) is the severe form of ALI [41,42]. One of the earliest manifestations of ALI is a diffuse intense inflammatory process and damage to both endothelial and epithelial cell barriers, resulting in marked extravasation of vascular fluid into the alveolar airspace [43]. A number of inflammatory cytokines including tumor necrosis factor-alpha (TNFα) and interleukin 8 (IL-8) can induce or aggravate the inflammation of endothelial and epithelial cells, leading to these barrier dysfunctions [44]. The mortality and morbidity of ALI/ARDS remain high since the etiology and molecular pathogenesis are still not completely understood.
To identify novel candidate ALI genes, we employed a high-throughput functional genomics approach, with extensive microarray-based lung gene expression profiling in canine, murine and human ALI. In each of these experiments, we observed significant increases in the expression of NAMPT, a gene not previously associated with lung pathophysiology, and validated these results by quantitative real time PCR, western blotting, and immunohistochemistry. Increased NAMPT protein expression was also observed in branchoalveolar lavage (BAL) fluid and serum in both canine and murine models. These results suggest that NAMPT may be a potential biomarker in ALI [6]. Analysis of single nucleotide polymorphisms (SNPs) in the NAMPT gene proximal promoter region indicated that a GC haplotype had a higher risk (nearly 8 fold) of ALI, while a TT haplotype had a lower risk of ALI [6]. Our findings were confirmed and extended by Bajwa et al. [7], who showed that the NAMPT T-1001G variant allele and related haplotype are associated with increased odds of developing ARDS and increased hazard of intensive care unit mortality among at-risk patients. In contrast, the C-1535T variant allele and related haplotype are associated with decreased odds of ARDS among patients with septic shock and better outcomes among patients with ARDS. These findings support that NAMPT is a genetic marker in ALI.
To further investigate the role and molecular mechanism underlying NAMPT in the pathogenesis of ALI, we employed both in vitro cell and in vivo mouse models. We first observed that in human pulmonary artery endothelial cells, NAMPT expression can be augmented by both inflammatory cytokine such as IL-1β and mechanic stress such as cyclic-stretch [6], two hallmark risk factors critical to the pathogenesis of ALI. In the mouse model of ALI, we showed that heterozygous PBEF (+/−) mice were significantly protected [reduced BAL protein, BAL IL-6 levels, peak inspiratory pressures] when exposed to a model of severe ventilator associated lung injury (VALI) with 4 h, 40 ml/kg tidal volume and exhibited significantly reduced expression of VALI-associated gene expression modules. In addition, strategies to reduce NAMPT availability (neutralizing antibody) resulted in significant protection from VALI [30]. These results suggest that an overexpression of NAMPT may contribute to the initiation or aggravation of ALI pathogenesis. In a further mechanistic study, we found that the NAMPT-specific siRNA would attenuate thrombin-induced decreases in human lung endothelial cell barrier function, increased cytoskeletal rearrangement, and secretion of the proinflammatory cytokine IL-8 [45]. Overexpression of NAMPT significantly augmented IL-8 secretion and IL-8 mRNA expression in A549 cells and human pulmonary artery endothelial cells (HPAEC), respectively. It also significantly augmented IL-1β- or TNF-α mediated cell permeability in A549 cells and HPAEC. The knockdown of NAMPT expression significantly inhibited IL-1β- or TNFα-stimulated IL-8 secretion and mRNA level while the knockdown of NAMPT expression also significantly attenuated IL-1β- or TNFα-induced cell permeability in both cells. NAMPT expression also affected the expression of two other inflammatory cytokines (IL-16 and CCR3 genes) [28,29]. These results reveal that NAMPT overexpression, as occurs in ALI, may adversely affect the pulmonary cell barrier function, whose dysregulation is the well-recognized feature in the pathogenesis of ALI.
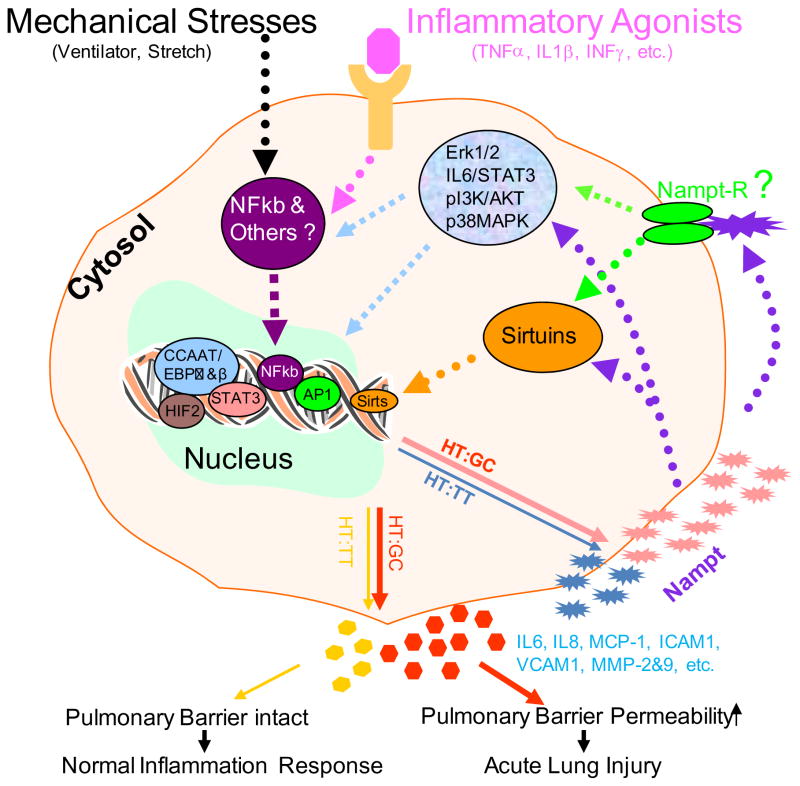
Based on the findings from our genomic, genetic, in vitro pulmonary cell culture and in vivo mechanistic exploration in animal models and other studies from different cells in literature, we presented a working molecular mechanistic schema underlying NAMPT in the susceptibility and pathogenesis of ALI in Figure 2. There has been well documented evidence supporting that mechanic stress such as those associated with ventilator [6, 30], cyclic or static stretch [6, 46] and inflammatory agonists such as TNFα (2, 29, 47), IL-1β (2, 28, 48) and interferon (IFN) [49], stimulate the expression of NAMPT. Kendal and Bryant-Greenwood demonstrated that NAMPT gene expression is modulated by NF-κB and AP-1 in human amniotic epithelial Cells [50]. Nowell et al. [51] discovered that IL-6 trans-signaling regulated NAMPT in a STAT-3-dependent manner. Hypoxemia is one of features in the pathogenesis of ALI. Hypoxic induction of human visfatin gene is directly mediated by hypoxia-inducible factor-1[52]. NAMPT functions as a nicotinamide phosphoribosyltransferase either intracellularly or extracellularly [31,33,53,54] and as a cytokine via an elusive receptor to affect the expression of other inflammatory cytokines or activators, which include CCR2, CCR3, Cox-2, IL-6, IL-8, IL-16, ICAM1, MCP-1, MMP-2, MMP-9, VCAM1, VEGF, etc. [2,28,29,55, 56,57,58,59,60]. A number of pathways have been implicated in the NAMPT-mediated augmentation of these molecules such as extracellular signal-regulated kinase 1/2 (ERK 1/2) [57,61,62], IL6/STAT3 [63], p38 mitogen-activated protein kinase (p38 MAPK) [57,60], phosphatiylinositol 3-kinase (PI3K)/Akt [55,57,60]. Recently, Skokowa et al. [53] reported that NAMPT is essential for inducing neutrophilic granulocyte differentiation. The molecular events triggered by NAMPT include NAD (+)-dependent sirtuin-1 activation, subsequent induction of CCAAT/enhancer binding protein-alpha and CCAAT/enhancer binding protein-beta, and, ultimately, upregulation of G-CSF synthesis and G-CSF receptor expression. G-CSF, in turn, further increases NAMPT levels. These results reveal a decisive role of the NAD (+) metabolic pathway in G-CSF-triggered myelopoiesis. Van Gool et al. [54] also found that NAMPT mediated intracellular NAD levels regulate TNF protein synthesis in a Sirt6-dependent manner. Taken together what we have learnt so far, we proposed the following possible molecular mechanism of NAMPT involved pathogenesis of ALI (Figure 2) in the case of lung insults such as those inducing direct or indirect lung injuries, mechanic and/or inflammatory stimuli via NF-kappa B or other pathways dramatically increase NAMPT expression from pulmonary alveolar epithelial cells, vascular endothelial cells, inflammatory cells and other cells in those patients carrying a susceptible haplotype GC(HT:GC) in their NAMPT gene promoters, which in turn mediates NAD dependent Sirtuins or other pathways intracellularly or extracellularly to markedly increase the production of other molecular activators. These lead to pulmonary barrier permeability increase, resulting in ALI. On the contrary, when patients, who carry a protective haplotype (HT:TT) in their NAMPT gene promoters, are subjected to those same insults, less NAMPT is expressed, less molecular activators produced, thus protecting against the pulmonary barrier dysfunction and reducing risk to ALI (6). It should be emphasized that many of these signal transduction pathways were drawn upon the evidence from studies in non-pulmonary cells or tissues. Thus, the schematic mechanisms presented in Figure 2 should be taken cum grano salis. More studies are warranted to validate these findings in pulmonary system, connect those molecular dots and answer the question marks in Figure 2.
Figure 2. Working mechanisms underlying NAMPT in the susceptibility and pathogenesis of acute lung injury.
In the case of lung insults, mechanic and/or inflammatory stimuli via NF-kappa B or other pathways dramatically increase NAMPT expression from pulmonary alveolar epithelial cells, vascular endothelial cells, inflammatory cells and other cells in those patients carrying a susceptible haplotype GC[HT:GC] in their NAMPT gene promoters, which in turn mediates NAD dependent Sirtuins or other pathways intracellularly or extracellularly to markedly increase the production of other molecular activators. These lead to pulmonary barrier permeability increase, resulting in ALI. On the contrary, when patients, who carry a protective haplotype [HT:TT] in their NAMPT gene promoters, are subjected to those same insults, less NAMPT is expressed, less molecular activators produced, thus protecting against pulmonary barrier dysfunction and reducing risk to ALI. Those dashed lines indicate that more molecular dots need to be connected. The question mark denotes the unanswered question. NAMPT-R, NAMPT receptor. Refer to the text for the detail.
Sepsis, a life-threatening disorder characterized by a whole-body inflammatory state caused by infection, is a frequent cause of ALI/ARDS. Jia et al. [23] reported that NAMPT as a novel inflammatory cytokine that plays a requisite role in the delayed neutrophil apoptosis of clinical and experimental sepsis. They found that transcription of the NAMPT gene is increased in neutrophils from septic patients; prevention of NAMPT translation through the use of an antisense oligonucleotide largely restores the normal kinetics of apoptosis. Moreover, the incubation of quiescent neutrophils from healthy volunteers with recombinant NAMPT results in dose-dependent inhibition of apoptosis, and antisense NAMPT prevents the inhibition of apoptosis that results from exposure to LPS or to a variety of host-derived inflammatory cytokines [64]. They postulate that this prolonged survival of activated neutrophils may be lined to sustained inflammation and the organ injury of sepsis.
Aging
Aging is the accumulation of changes in an organism over time. It may be simply divided into “biological aging” (an organism’s physical state naturally declines as it ages) and “diseased aging” (a hastened degeneration such as progeria, a premature aging). Aging is a complex process, depending on both genetic compositions and environmental conditions.
Several evidences suggest that NAMPT may be an important regulator in aging. Axonal degeneration occurs in many neurodegenerative diseases. Sasaki et al. [65] found that NAMPT can delay axon degeneration in the presence of nicotinamide in an in vitro Wallerian degeneration assay. These results suggest that increased activity of the NAD biosynthetic pathway stemming from nicotinamide promotes axonal protection. Among several key enzymes involved in either de novo or salvage or Preiss-Handler independent pathways of mammalian NAD synthesis, the effectiveness against axonal degeneration by NAMPT is only next to nicotinamide mononucleotide adenylyltransferase 1. In addition, exogenous application of the NAD precursors can also delay axonal degeneration. These results indicate that stimulation of NAD biosynthetic pathways including NAMPT may be useful in preventing or delaying axonal degeneration. Van der Veer et al. [32] reported that NAMPT can extend the lifespan of human smooth muscle cells. They found that replicative senescence of smooth muscle cells was preceded by a marked decline in the expression and activity of NAMPT. Furthermore, reducing NAMPT activity with the antagonist FK866 induced premature senescence in smooth muscle cells, assessed by serial quantification of the proportion of cells with senescence-associated beta-galactosidase activity. In contrast, introducing the NAMPT gene into aging human smooth muscle cells delayed senescence and substantially lengthened cell lifespan, together with enhanced resistance to oxidative stress. NAMPT-mediated smooth muscle cells lifespan extension was associated with increased activity of the NAD+-dependent longevity enzyme SIRT1 and was abrogated in NAMPT-overexpressing cells transduced with a dominant-negative form of SIRT1 (H363Y). NAMPT overexpression also reduced the fraction of p53 that was acetylated on lysine 382, a target of SIRT1, suppressed an age-related increase in p53 expression, and increased the rate of p53 degradation. Moreover, add-back of p53 with recombinant adenovirus blocked the anti-aging effects of NAMPT. These data indicate that NAMPT is a longevity protein that can add stress-resistant life to human smooth muscle cells by optimizing SIRT1-mediated p53 degradation. Recently, Benigi et al. [66] noticed that the longevity phenotype in angiotension II type 1 receptor knockout mice was associated with an increased number of mitochondria and upregulation of the prosurvival genes NAMPT and sirtuin 3 (Sirt3) in the kidney. They postulated that disruption of angiotension II type 1 receptor promotes longevity in mice, possibly through the attenuation of oxidative stress and overexpression of prosurvival genes such as NAMPT and Sirt 3.
Other studies have shown that overexpression of NAMPT increases SIRT1 activity [32] and can protect cells from death due to PARP overexpression [67], which is consistent with the hypothesis that NAMPT is a functional equivalent of Pnc1 in mammals. Pnc1 is a stress- and calorie-responsive longevity gene that catalyzes the first and rate-limiting step in NAD+ biosynthesis from nicotinamide in yeast [68,69]. Pnc1 positively regulates SirT2, increased gene dosage or enhanced activity of which extends life span in S. cerevisiae, C. elegans, and D. melanogaster [70]. Yang et al. [68] identified that NAMPT as a stress- and nutrient-responsive gene that boosts mitochondrial NAD+ levels. NAMPT expression and mitochondrial NAD+ levels increase in vivo after fasting. Increased mitochondrial NAD+ promotes cell survival during genotoxic stress and that protection is provided by the mitochondrial sirtuins SIRT3 and SIRT4. These data show that NAMPT-regulated mitochondrial NAD+ levels dictate cell survival via SirT3 and SirT4. These insights into the importance of NAMPT mediated mitochondrial NAD+ and NAD related metabolism or signaling [71] will facilitate a new understanding of and the development of novel approaches to treating diseases of aging and other conditions.
Atherosclerosis
Atherosclerosis is a disease affecting arterial blood vessels. It is a progressive disease in which lipids, extracellular matrix, and activated vascular smooth muscle cells accumulate in the arterial wall, resulting in growth of an atherosclerotic plaque [72]. Its complex etiologies and pathogenesis remain to be fully elucidated.
In a microarray experiment, Dahl et al. [73] identified NAMPT expression was markedly enhanced in carotid plaques from symptomatic compared with plaques from asymptomatic individuals. This finding was confirmed by real-time reverse transcription polymerase chain reaction and immunohistochemistry analyses. The similar relationship between NAMPT and unstable lesions was also found in patients with coronary artery disease and a strong NAMPT immunostaining in lipid-rich regions was detected. Both oxidized low-density lipoprotein and tumor necrosis factor-alpha increased NAMPT expression in THP-1 monocytes, with a particularly enhancing effect when these stimuli were combined. Visfatin increased matrix metalloproteinase-9 activity in THP-1 monocytes and tumor necrosis factor-alpha and interleukin-8 levels in peripheral blood mononuclear cells. These findings suggest that NAMPT may be an inflammatory mediator, localized to foam cell macrophages within unstable atherosclerotic lesions, that potentially plays a role in plaque destabilization. Zhong et al. [74] also reported that serum NAMPT was increased in patients with carotid plaques.
Cheng et al. [75] noticed that NAMPT level in epicardial and abdominal adipose tissues were significantly higher in CAD patients relative to control subjects. In addition, significantly higher tissue NAMPT from abdominal fat depots were found compared to those from epicardial fat in CAD patients. It suggests that abdominal adiposity may play a more significant role than epicardial fat in the pathogenesis of coronary atherosclerosis.
More studies are warranted to firmly establish the relationship between NAMPT and atherosclerosis and to elucidate the role(s) and molecular mechanisms of NAMPT in the pathogenesis of atherosclerosis.
Cancer
Molecular screening, epidemiological survey and phamarcological studies have indicated that NAMPT may be an attractive diagnostic and drug target for cancer therapy.
Hufton et al. [76] first noticed that NAMPT expression was increased by 6 folds in primary colorectal cancer over the normal control using the suppression subtractive hybridization technique during their attempt to identify new candidate genes in cancer, which may provide novel points of therapeutic intervention. This result was confirmed at protein and tissue level by both western blotting and immunohistochemical analyses [77]. Using cDNA microarray based expression profiling of different grades of astrocytomas, Reddy et al. [78] identified several fold increased levels of PBEF1 transcripts in glioblastoma samples. Malignant astrocytomas comprise anaplastic astrocytoma and glioblastoma. Glioblastoma is the most malignant with a median survival of 10–12 months in patients. Their initial findings were validated using real time RT-qPCR and immunohistochemical staining on an independent set of tumor samples. Employing ELISA analysis of serum samples from astrocytoma patients to determine whether this protein levels in patients with astrocytoma correlate with the presence of tumor and tumor grade, they further found that in patients with astrocytoma, serum NAMPT levels correlate with tumor grade and is highest in glioblastoma. The authors suggest that NAMPT as a potential malignant astrocytoma serum marker and prognostic indicator in glioblastoma.
Through a chemical screen to find new antitumor drugs, Hasmann and Schemainda [79] identified the first low molecular weight compound, designated FK866 {the chemical name: (E)-N-[4-(1-benzoylpiperidin-4-yl) butyl]-3-(pyridin-3-yl) acrylamide}, which induces apoptosis by highly specific and potent inhibition of nicotinamide phosphoribosyltransferase in HepG2 human liver carcinoma cells. FK866 has no primary effect on cellular energy metabolism and thus has no direct and immediate cytotoxicity but gradually depletes the cells of a vital factor, NAD, by inhibiting NAMPT, which eventually triggers apoptosis. The authors proposed that FK866 may be used for treatment of diseases implicating deregulated apoptosis such as cancer for immunosuppression or as a sensitizer for genotoxic agents. Furthermore, it may provide an important tool for investigation of the molecular triggers of the mitochondrial pathway leading to apoptosis through enabling temporal separation of NAD+ decrease from ATP breakdown and apoptosis. Using1H-decoupled phosphorus (31P) magnetic resonance spectroscopy, Muruganandham et al. [80] observed that FK866 increased cell death (apoptosis) and subsequent radiation sensitivity in the mammary carcinoma. FK866 has been shown to have anti-tumor, anti-metastatic and anti-angiogenic activities in a murine renal cell carcinoma model [81]. The three dimensional structural analysis of the NAMPT–FK866 complex by three groups reveals that the FK866 compound binds at the nicotinamide-binding site of NAMPT to competitively compete directly with the nicotinamide substrate to inhibit its activity [16–18]. This structural analysis provided a molecular basis for the inhibition of FK866 on NAMPT and a starting point for the development of new anticancer agents.
Accumulated evidence indicates that at least three molecular mechanisms may implicate NAMPT in the pathogenesis of cancer. One of the hallmarks of human cancers is the intrinsic or acquired resistance to apoptosis. Evasion of apoptosis may contribute to carcinogenesis, tumor progression and also to treatment resistance [82]. NAMPT inhibits apoptosis of tumor cells via its role as a key enzyme in NAD biosynthetic salvage pathway. NAD may affect apoptosis through several potential mechanisms [83]: first, NAD mediates cellular energy metabolism that is a critical factor determining cell death modes; second, the NADH/NAD+ ratio is a major index of cellular reducing power that affects MPT-a mediator of apoptosis under many conditions; third, NAD+ levels mediate the activity of caspase-dependent endonuclease DFF40–-an executioner of DNA fragmentation in certain apoptotic cascades; and fourth, NAD+-dependent sirtuins may mediate apoptosis. Future studies on this topic are critical for our comprehensive understanding about the roles of NAMPT in cell death via the NAD synthesis.
Li et al. [63] reported that NAMPT protects macrophages from ER stress-induced apoptosis by activating an IL-6/STAT3 signaling pathway via a non-enzymatic mechanism. The mechanism involves a two-step sequential process: rapid induction of interleukin 6 (IL-6) secretion, followed by IL-6-mediated autocrine/paracrine activation of the prosurvival signal transducer STAT3. The ability of NAMPT to trigger this IL-6/STAT3 cell survival pathway did not depend on the presence of the NAMPT enzymatic substrate nicotinamide in the medium, could not be mimicked by the NAMPT enzymatic product nicotinamide mononucleotide (NMN), was not blocked by the NAMPT enzyme inhibitor FK866, and showed no correlation with enzyme activity in a series of site-directed mutant NAMPT proteins.
The growth of cancers is dependent upon the formation of new blood vessels, a process including angiogenesis. Angiogenesis is a dominant process supporting tumor growth. Neovascularization by angiogenesis is the prerequisite for the expansion of tumor [84]. Kim et al. [58] found that NAMPT potently stimulates in vivo neovascularization in chick chorioallantoic membrane and mouse Matrigel plug. They also demonstrated that NAMPT activates migration, invasion, and tube formation in human umbilical vein endothelial cells (HUVECs). Moreover, NAMPT evokes activation of the ERK1/2 in endothelial cells, which are closely linked to angiogenesis. Inhibition of ERK activation markedly decreases NAMPT-induced tube formation of HUVECs and NAMPT-stimulated endothelial cell sprouting from rat aortic rings. Taken together, these results demonstrate that NAMPT promotes angiogenesis via activation of mitogen-activated protein kinase ERK-dependent pathway and suggest that NAMPT may play important roles in various pathophysiological angiogenesis. Bae et al. [62] further reported that NAMPT-induced angiogenesis is mediated by endothelial fibroblast growth factor-2 (FGF-2). They proposed that NAMPT-induced endothelial angiogenesis was composed largely of two sequential steps: the induction of Erk1/2-dependent FGF-2 gene expression by NAMPT and the subsequent FGF-2-induced angiogenesis. These data suggest an integral role for NAMPT-FGF-2 signaling axis in modulating endothelial angiogenesis. Adya et al. [60] also reported that NAMPT induced dose- and time-dependent proliferation and capillary-like tube formation of human umbilical vein endothelial cells, perhaps by stimulating vascular endothelial growth factor via the MAPK and PI3K/Akt signaling pathways.
Diabetes mellitus
Diabetes mellitus, simply referred to as diabetes, is a syndrome of disordered metabolism with hyperglycemia as a hallmark phenotype. It is usually divided into Type 1 and Type 2 diabetes. Type 1 diabetes is characterized by loss of the insulin-producing β cells of the islets of Langerhans in the pancreas, leading to a deficiency of insulin. Type 2 diabetes is characterized differently due to insulin resistance or reduced insulin sensitivity, combined with a reduced insulin secretion. Diabetes is a complex disease. Its long- and extensively-sought hereditary and environmental causes are not fully known. Initial attention brought to the relationship between NAMPT and type 2 diabetes was due to the work by Fukuhara et al. [20] in 2005.
Seeking to identify new candidate genes for metabolic syndrome, Fukuhara et al. [20] discovered NAMPT as one of the more abundantly expressed genes in visceral fat than in subcutaneous fat using a differential display method to compare 8800 polymerase chain reaction (PCR) products from paired samples of subcutaneous fat and visceral fat. They found that in 101 male and female human subjects, plasma NAMPT concentrations correlated strongly with the amount of visceral fat but only weakly with the amount of subcutaneous fat. They also noticed that during the obese-becoming period between 6 and 12 week old KKAy mice, a model for obese type 2 diabetes, and high fat diet-fed c57BL/6J mice, plasma NAMPT levels were significantly increased with the parallel change of their mRNA levels in visceral fat, not in subcutaneous fat. These results suggest that NAMPT is a secreted factor produced abundantly by visceral fat; the author dubbed NAMPT as visfatin. To explore the physiological function of visfatin/NAMPT, they prepared NAMPT gene knockout mice. Homozygous NAMPT gene knockout mice were embryonically lethal. The authors found that plasma NAMPT levels in NAMPT gene heterozygous knockout mice were only 2/3 of those in wild type mice but their plasma glucose level were significantly higher. This suggests that like insulin, NAMPT may have a physiological role in lowering plasma glucose levels. They further found that added NAMPT, injected intravenously or expressed endogenously by adenovirus vector, significantly lowers plasma glucose level in both KKAy and c57BL/6J mice. In vitro NAMPT treatment enhanced glucose uptake in 3T3-L1 adipocytes, L6 myocytes, and suppressed glucose release in H4IIEC3 hepatocytes. Furthermore, NAMPT stimulated the accumulation of triacylglycerols in pre-adipocytes. These effects were similar to those of insulin. Mechanistically, they found that NAMPT bound to insulin receptor but not to the same site as insulin binds. NAMPT could stimulate insulin signaling such as inducing tyrosine phosphorylation of the insulin receptor (IR), insulin receptor substrate–1 (IRS-1), and IRS-2 in the liver similarly as insulin. Taken together, the authors consider NAMPT as an insulin-mimetic. Although this paper was withdrawn from Science due to the variation of different batches of recombinant NAMPT for their adipogenic and insulin-mimetic activities, this report has immediately and continuously drawn increased interest among biomedical researchers to the roles and mechanisms underpinning NAMPT in the pathogenesis of diabetes, obesity, insulin resistance, and metabolic syndrome.
Among epidemiological surveys following the report of Fukuhara et al. [20] on the relationship between plasma NAMPT level or visceral fat NAMPT mRNA amount and Type 2 diabetes in patients, a number of groups reported that plasma NAMPT level or visceral fat NAMPT mRNA amount were significantly increased in or positively associated with Type 2 diabetes or obesity or insulin resistance or metabolic syndrome in patients under the baseline without any intervention of medication, surgery, and other reagents [85–98]. These seem to be consistent with the original findings of Fukuhara et al. [20]. Patients with Type 1 diabetes also had higher NAMPT concentrations than controls [99,100]. However, other groups have obtained opposite findings [101–103] or no changes of plasma NAMPT level in Type 2 diabetes [104–109]. These conflicting findings on the correlation of plasma NAMPT level with diabetes may be stemmed from four major reasons: small sample size, variable phenotyping criteria, different populations and ethnicities and different assays. Indeed, most above-cited reports had sample size around or below 50. The small sample size not only limits the statistic power to detect the true association but also sometimes yield misleading conclusions. Heterogeneous patient populations of different age, sex, ethnicity, diagnosis criteria could confound the comparison among the different reports. Differences in the qualitative and quantitative assays of NAMPT used in different groups [110] may also in part explain the conflicting observations in the relation of circulating NAMPT to human obesity or insulin resistance.
Insulin-like effects of visfatin was observed in human osteoblasts by Xie et al. [111]. They found that NAMPT, purchased from Axxora Life Sciences (San Diego, CA, USA), induced tyrosine phosphorylation of IR, IRS-1, and IRS-2. Moreover, the effects of NAMPT - glucose uptake, proliferation, and type I collagen enhancement of cultured human osteoblast-like cells - bore a close resemblance to those of insulin and were inhibited by hydroxy-2-naphthalenylmethylphosphonic acid tris-acetoxymethyl ester, a specific inhibitor of IR tyrosine kinase activity. They also unexpectedly found that NAMPT downregulated osteocalcin secretion from human osteoblast-like cells. These data indicate that the regulation of glucose uptake, proliferation, and type I collagen production by NAMPT in human osteoblasts involves IR phosphorylation, the same signal-transduction pathway used by insulin. This study finding is concordia cum veritate with the insulin mimetic effect of NAMPT reported initially by Fukuhara et al. [20] though some studies failed to reproduce these results [33, 71]. Revollo et al. [33, 112] have recently demonstrated that NAD biosynthesis mediated by intracellular NAMPT and extracellular NAMPT plays a critical role in the regulation of glucose stimulated insulin secretion in pancreatic β cells. They found that FK866, a potent NAMPT inhibitor, significantly inhibits NAD biosynthesis and glucose stimulated insulin secretion in isolated wild-type primary pancreatic islets, and administration of NMN restores normal NAD biosynthesis and glucose stimulated insulin secretion in FK866-treated wild-type primary pancreatic islets. In addition, they found that NAMPT heterozygous [NAMPT+/−] female mice with about 50% plasma NAMPT level of the wild type mice show also reduced plasma NMN level and impaired glucose tolerance due to a defect in glucose stimulated insulin secretion, which can be completely ameliorated by administration of NMN, suggesting that the observed defects in glucose stimulated insulin secretion are due to a lack of NAMPT-mediated NAD biosynthesis. These findings strongly suggest that NAMPT-mediated NAD biosynthesis plays an important role in the regulation of glucose homeostasis and also that the maintenance of high NMN levels by eNAMPT in blood circulation is critical for normal β cell function, probably because pancreatic islets have very low levels of iNAMPT compared to other tissues.
Although the biological role of NAMPT related to diabetes remains to be fully elucidated, there are some studies that have investigated the regulation of NAMPT expression in various environment cues. Brema et al. [113] found that plasma NAMPT concentration was significantly reduced by approximately 80 % after 12 weeks of aerobic exercise training in severely obese young subjects with Type 2 diabetes. The same group also demonstrated a reduction of NAMPT following exercise in subjects with type 1 diabetes mellitus [113]. Storka et al. [114] found that the release of NAMPT from adipocytes, skeletal muscle cells and human umbilical vein endothelial cells was significantly increased after treatment with angiotensin converting enzyme inhibitors and angiotensin receptor blockers(lisinopril, telmisartan and valsartan). This may in part explain their beneficial antidiabetic effects in patients with type 2 diabetes independent of their blood pressure-lowering effects. Choi et al. [115] reported that serum glucose and insulin concentrations significantly decreased in rosiglitazone or fenofibrate-treated Otsuka Long-Evans Tokushima fatty (OLETF) rats compared to untreated OLETF rats. Rosiglitazone significantly increased serum adiponectin concentration from 20 to 40 weeks of age, whereas fenofibrate reduced TNF-alpha concentration. The expression of NAMPT and adiponectin mRNA in visceral fat deposits was elevated by rosiglitazone or fenofibrate treatments when compared to untreated OLETF rats, whereas, TNFα mRNA was down-regulated by these drugs. These results suggest that rosiglitazone and fenofibrate may prevent Type 2 diabetes by regulating adipocytokines including NAMPT, adiponectin, and TNFα. PPAR-delta agonist (L-165041) significantly increased NAMPT mRNA expression in rat visceral fat depot and 3T3-L1 adipocytes [116]. Zhou et al. [117] demonstrated that macrostemonoside A, a tigogenin steroidal saponin isolated from the bulbs of Allium macrostemon Bung, could stimulate NAMPT expression in 3T3-L1 adipocytes at the transcriptional level via p38 MAPK signaling pathway. Its regulation on NAMPT in adipocytes may constitute an important element in its improvement of insulin resistance and diabetes. However, other studies failed to find the regulatory effect on NAMPT by thiazolidinediones [118], rosiglitazone [119], pioglitazone and metformin [120]. It appears a déjà vu that the same pitfalls as explained above with epidemiological surveys also occur in these studies.
The role of genetic variation in the NAMPT gene in the pathophysiology of Type 2 diabetes and related diseases or conditions has been explored. Three SNPs (rs9770242, −948G-->T, rs4730153) in the human NAMPT gene was associated with the ratio of visceral/subcutaneous NAMPT mRNA expression. The −948G-->T variant was also associated with 2-h plasma glucose and fasting insulin concentrations in non-diabetic subjects [10]. A significant association was found between two SNPs (rs9770242 and rs1319501) and fasting insulin or glucose levels [10]. A significant association was observed at −948C-->A, with minor allele frequencies of 0.157 in Type 2 diabetes cases and 0.119 in non-diabetic controls. In a non-diabetic population, the same −948 allele that conferred increased risk of Type 2 diabetes was significantly associated with higher plasma levels of fibrinogen and C-reactive protein. These findings suggest that the NAMPT gene may play a role in determining Type 2 diabetes susceptibility, possibly by modulating chronic, low-grade inflammatory responses [121]. A SNP (rs7789066) was significantly associated with the apolipoprotein B component of VLDL [10]. The −1535T/T genotype was associated with lower serum triglyceride levels and higher HDL-cholesterol levels in the non-diabetic Japanese subjects [9]. A similar association between the haplotype containing SNP −1535 and triglyceride levels were also observed in a Chinese population [11]. Sun et al. [122] reported that fasting serum NAMPT correlated with triacylglycerols. These results suggest that NAMPT may play a regulatory role in lipid metabolism.
Rheumatoid arthritis
Rheumatoid arthritis [RA] is a chronic, systemic autoimmune disorder that most commonly causes inflammation and tissue damage in joints. Despite that the first recognized description of rheumatoid arthritis was made in 1800 by Dr. Augustin Jacob Landré-Beauvais in Paris [123], its pathogenesis remains incompletely understood.
Otero et al. [124] first reported that patients with rheumatoid arthritis showed considerably higher plasma levels of NAMPT, leptin, and adiponectin than healthy controls, implicating NAMPT as one of the biomarkers in RA. This observation was confirmed by Nowell et al. [51]. Brentano et al. [125] reported that increased levels of NAMPT in serum and synovial fluid correlated with the degree of inflammation and clinical disease activity in patients with RA. NAMPT itself activated the transcription factors NF-kB and activator protein 1 and induced IL-6, IL-8, MMP-1, and MMP-3 in RA synovial fibroblasts as well as IL-6 and TNFα in monocytes. NAMPT knockdown in RA synovial fibroblasts significantly inhibited basal and TLR ligand-induced production of IL-6, IL-8, MMP-1, and MMP-3. These findings support that NAMPT is a proinflammatory and destructive mediator of joint inflammation in RA and NAMPT may be a potential therapeutic target. This notion was further corroborated by Busso et al. [126], who found that NAMPT is a key player in inflammatory arthritis. Increased expression of NAMPT was confirmed in mice with collagen-induced arthritis, both in serum and in the arthritic paw. A specific competitive inhibitor of NAMPT, FK866 (also known as APO866 and WK175) effectively reduced arthritis severity with comparable activity to etanercept, a recombinant-DNA drug linking human soluble TNF receptor to the Fc component of human immunoglobulin G1 (IgG1) and acts as a TNF inhibitor, and decreased pro-inflammatory cytokine secretion in affected joints. Moreover, NAMPT inhibition reduced intracellular NAD concentration in inflammatory cells and circulating TNFα levels during endotoxemia in mice. In vitro pharmacological inhibition of NAMPT reduced the intracellular concentration of NAD and pro-inflammatory cytokine secretion by inflammatory cells. Thus, NAMPT links NAD metabolism to inflammatory cytokine secretion by leukocytes, and its inhibition might therefore have therapeutic value in treating RA.
Concluding Remarks
NAMPT has drawn an ever-increasing attention in biomedical fields because of its presumed pleiotropic physiological functions and its dysregulation implicated in a number of human diseases and conditions. Recent progress has provided new insights into the molecular basis of NAMPT specific inhibitor FK866, both enzymatic and non-enzymatic functions of NAMPT, and a novel role of NAMPT in glucose stimulated insulin secretion from pancreatic β cells. However, we are still far from fully understanding the biology and pathophysiology of NAMPT at molecular and cellular levels. Many in vitro and in vivo studies on the functions and disease associations of NAMPT have yielded contradictory results. A number of Terra incognita in NAMPT research remains to be explored.
At the gene level, only a couple of experiments characterized a few regulatory elements in the NAMPT gene promoter. There have been only a handful of reports on the functional consequences and disease associations of several SNPs out of 411 SNPs currently known in NAMPT. Epigenetic regulation of the NAMPT gene is still a virgin area. At the mRNA level, only one out of predicted 19 NAMPT isoforms has been well characterized. We have not investigated whether these isoforms are due to alternative splicing or alternative promoters. At the protein level, there are a number of unanswered questions. For example, how is NAMPT without a known signal peptide secreted from cells? How is NAMPT transported from cytoplasma to nucleus during the different phase of cell cycle? What are the functional and pathological roles of the posttranslational modifications of NAMPT as predicted by computer programs?
Functionally, it is generally recognized that NAMPT as a nicotinamide phosphoribosyltransferase. NAMPT-mediated NAD biosynthesis of a salvage pathway plays a critical role in life and disease in mammalians. Imai recently proposed a new concept “NAD World” with NAMPT as a driver and Sirt 1as a mediator [112]. This NAD connection may explain major physiological functions of NAMPT. However, past findings [1] and recent evidence from others [63] and our group [127] indicate that NAMPT may also have non-enzymatic functions such as growth factor or cytokine or other functions. It is of importance to know what the authentic receptor is for NAMPT, if any, via which extracellular NAMPT interacts with cells to initiate NAMPT-mediated signal transduction for its biological effect. More studies may be needed to clarify the controversial issue of NAMPT’s insulin-mimetics function.
The list of tantalizing clues on the association of NAMPT with different human diseases and conditions is growing fast. Well-conducted epidemiological surveys or clinical studies in large patient population size with standardized phenotyping and quantitative blood NAMPT assays are needed to firmly substantiate the association of NAMPT with any disease or condition of interest or establish the regulatory effect on NAMPT expression by some drugs or other treatments. It would be advisable to carry out well-controlled prospective studies to determine the potential diagnostic and/or prognostic value of circulating NAMPT in the prediction of any associate disease or condition as well as therapeutic effect of NAMPT regulation. Certainly, more detailed and in-depth in vivo and in vitro studies are necessary to elucidate molecular and cellular mechanisms underpinning NAMPT’s role in the associated diseases or conditions.
Acknowledgments
We thank all the colleagues who contributed to this work and apologize to those authors whose works are not directly cited due to the theme of this review and space limitations. Some of the cited works from the Ye lab have been partly supported by NHLBI/NIH Grant HL080042 (Ye, S.Q.) and the University of Missouri start-up fund (Ye, S.Q.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Samal B, Sun Y, Stearns G, Xie C, Suggs S, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, et al. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 3.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Martin PR, Shea RJ, Mulks MH. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J Bacteriol. 2001;183:1168–1174. doi: 10.1128/JB.183.4.1168-1174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlothlin JR, Gao L, Lavoie T, Simon BA, Easley RB, et al. Molecular cloning and characterization of canine pre-B-cell colony-enhancing factor. Biochem Genet. 2005;43:127–141. doi: 10.1007/s10528-005-1505-2. [DOI] [PubMed] [Google Scholar]
- 6.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171:361–370. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 7.Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC. Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med. 2007;35:1290–1295. doi: 10.1097/01.CCM.0000260243.22758.4F. [DOI] [PubMed] [Google Scholar]
- 8.Johansson LM, Johansson LE, Ridderstråle M. The visfatin (PBEF1) G-948T gene polymorphism is associated with increased high-density lipoprotein cholesterol in obese subjects. Metabolism. 2008;57:1558–1562. doi: 10.1016/j.metabol.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Tokunaga A, Miura A, Okauchi Y, Segawa K, Fukuhara A, et al. The -1535 promoter variant of the visfatin gene is associated with serum triglyceride and HDL-cholesterol levels in Japanese subjects. Endocr J. 2008;55:205–212. doi: 10.1507/endocrj.k07e-039. [DOI] [PubMed] [Google Scholar]
- 10.Bailey SD, Loredo-Osti JC, Lepage P, Faith J, Fontaine J, et al. Common polymorphisms in the promoter of the visfatin gene (PBEF1) influence plasma insulin levels in a French-Canadian population. Diabetes. 2006;55:2896–2902. doi: 10.2337/db06-0189. [DOI] [PubMed] [Google Scholar]
- 11.Jian WX, Luo TH, Gu YY, Zhang HL, Zheng S, et al. The visfatin gene is associated with glucose and lipid metabolism in a Chinese population. Diabet Med. 2006;23:967–973. doi: 10.1111/j.1464-5491.2006.01909.x. [DOI] [PubMed] [Google Scholar]
- 12.Böttcher Y, Teupser D, Enigk B, Berndt J, Klöting N, et al. Genetic variation in the visfatin gene (PBEF1) and its relation to glucose metabolism and fat-depot-specific messenger ribonucleic acid expression in humans. J Cli Endocrinol Metab. 2006;91:2725–2731. doi: 10.1210/jc.2006-0149. [DOI] [PubMed] [Google Scholar]
- 13.Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7:S12. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Xia T, Zhou L, Chen X, Gan L, et al. Gene organization, alternate splicing and expression pattern of porcine visfatin gene. Domest Anim Endocrinol. 2007;32:235–245. doi: 10.1016/j.domaniend.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Palin MF, Labrecque B, Beaudry D, Mayhue M, Bordignon V, et al. Visfatin expression is not associated with adipose tissue abundance in the porcine model. Domest Anim Endocrinol. 2008;35:58–73. doi: 10.1016/j.domaniend.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Khan JA, Tao X, Tong L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat Struct Mol Biol. 2006;13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Zhang X, Bheda P, Revollo JR, Imai S, et al. Structure of NAMPT/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- 18.Kim MK, Lee JH, Kim H, Park SJ, Kim SH, et al. Crystal structure of visfatin/pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866. J Mol Biol. 2006;362:66–77. doi: 10.1016/j.jmb.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 19.van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, et al. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 20.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 21.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, et al. Retraction. Science. 2007;318:565. doi: 10.1126/science.318.5850.565b. [DOI] [PubMed] [Google Scholar]
- 22.Xie H, Tang SY, Luo XH, Huang J, Cui RR, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 23.Jia SH, Li Y, Parodo J, Kapus A, Fan L, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentano F, Schorr O, Ospelt C, Stanczyk J, Gay RE, et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007;56:2829–2839. doi: 10.1002/art.22833. [DOI] [PubMed] [Google Scholar]
- 25.Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci(Lond) 2008;114:275–288. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- 26.Dahl TB, Yndestad A, Skjelland M, Øie E, Dahl A, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 27.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 28.Liu P, Li H, Cepeda J, Zhang LQ, Cui X, et al. Critical role of PBEF expression in pulmonary cell inflammation and permeability. Cell Bio Int. 2009;33:19–30. doi: 10.1016/j.cellbi.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Liu P, Cepeda J, Fang D, Easley RB, et al. Augmentation of Pulmonary Epithelial Cell IL-8 Expression and Permeability by Pre-B-cell Colony Enhancing Factor. J Inflamm (Lond) 2008;5:15. doi: 10.1186/1476-9255-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008;178:605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 32.van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- 33.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, et al. NAMPT/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger F, Ramirez-Hernandez MH, Ziegler M. The new life of a centenarian: signalling functions of NAD[P] Trends Biochem Sci. 2004;29:111–118. doi: 10.1016/j.tibs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267:1550–1564. doi: 10.1046/j.1432-1327.2000.01187.x. [DOI] [PubMed] [Google Scholar]
- 36.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corda D, Di Girolamo M. Functional aspects of protein mono-ADP-ribosylation. EMBO J. 2003;22:1953–1958. doi: 10.1093/emboj/cdg209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerth A, Nieber K, Oppenheimer NJ, Hauschildt S. Extracellular NAD+ regulates intracellular free calcium concentration in human monocytes. Biochem J. 2004;382:849–856. doi: 10.1042/BJ20040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacIntyre JP, Pope BL. The involvement of protein kinase C, calcium, and 5-lipoxygenase in the production of tumor necrosis factor by a cloned interleukin-3 dependent cell line with natural cytotoxic activity. Int J Immunopharmacol. 1991;13:175–184. doi: 10.1016/0192-0561(91)90096-p. [DOI] [PubMed] [Google Scholar]
- 40.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways. A vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;9572:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 42.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 43.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 44.Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest. 2006;130:1906–1914. doi: 10.1378/chest.130.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye SQ, Zhang LQ, Adyshev D, Usatyuk PV, Garcia AN, et al. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc Res. 2005;70:142–151. doi: 10.1016/j.mvr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta. 2008;29:255–265. doi: 10.1016/j.placenta.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, et al. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39:250–255. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- 48.Williams MR, Kataoka N, Sakurai Y, Powers CM, Eskin SG, et al. Gene expression of endothelial cells due to interleukin-1 beta stimulation and neutrophil transmigration. Endothelium. 2008;15:73–165. doi: 10.1080/10623320802092443. [DOI] [PubMed] [Google Scholar]
- 49.Patrone L, Damore MA, Lee MB, Malone CS, Wall R. Genes expressed during the IFN gamma-induced maturation of pre-B cells. Mol Immunol. 2002;38:597–606. doi: 10.1016/s0161-5890(01)00097-9. [DOI] [PubMed] [Google Scholar]
- 50.Kendal CE, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor [PBEF/Visfatin] gene expression is modulated by NF-kappa B and AP-1 in human amniotic epithelial cells. Placenta. 2007;28:305–14. doi: 10.1016/j.placenta.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–2095. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 52.Bae SK, Kim SR, Kim JG, Kim JY, Koo TH, et al. Hypoxic induction of human visfatin gene is directly mediated by hypoxia-inducible factor-1. FEBS Lett. 2006;580:4105–4113. doi: 10.1016/j.febslet.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 53.Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD (+)-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- 54.Van Gool F, Gallí M, Gueydan C, Kruys V, Prevot PP, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med. 2009;15:206–210. doi: 10.1038/nm.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adya R, Tan BK, Chen J, Randeva HS. Pre-B cell colony enhancing factor [PBEF]/visfatin induces secretion of MCP-1 in human endothelial cells: role in visfatin-induced angiogenesis. Atherosclerosis. 2008;205:113–119. doi: 10.1016/j.atherosclerosis.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 56.Shen CJ, Tsai EM, Lee JN, Chen YL, Lee CH, et al. The concentrations of visfatin in the follicular fluidsof women undergoing controlled ovarian stimulation are correlated to the number of oocytes retrieved. Ferti l Steril. 2010;93:1844–1850. doi: 10.1016/j.fertnstert.2008.12.090. [DOI] [PubMed] [Google Scholar]
- 57.Liu SW, Qiao SB, Yuan JS, Liu DQ. Visfatin Stimulates Production of Monocyte Chemotactic Protein-1 and Interleukin-6 in Human Vein Umbilical Endothelial Cells. Horm Metab Res. 2009;41:281–286. doi: 10.1055/s-0028-1102914. [DOI] [PubMed] [Google Scholar]
- 58.Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH, et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim Biophys Acta. 2008;1783:886–895. doi: 10.1016/j.bbamcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Adya R, Tan BK, Chen J, Randeva HS. Nuclear factor-kappaB induction by visfatin in human vascular endothelial cells: its role in MMP-2/9 production and activation. Diabetes Care. 2008;31:758–760. doi: 10.2337/dc07-1544. [DOI] [PubMed] [Google Scholar]
- 60.Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78:356–365. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- 61.Kim SR, Bae SK, Choi KS, Park SY, Jun HO, et al. Visfatin promotes angiogenesis by activation of extracellular signal-regulated kinase 1/2. Biochem Biophys Res Commun. 2007;357:150–156. doi: 10.1016/j.bbrc.2007.03.105. [DOI] [PubMed] [Google Scholar]
- 62.Bae YH, Bae MK, Kim SR, Lee JH, Wee HJ, et al. Upregulation of fibroblast growth factor-2 by visfatin that promotes endothelial angiogenesis. Biochem Biophys Res Commun. 2009;379:206–211. doi: 10.1016/j.bbrc.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, et al. Extracellular NAMPT promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008;283:34833–34843. doi: 10.1074/jbc.M805866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor [PBEF]/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 65.Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribos) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 68.Yang H, Yang T, Baur JA, Perez E, Matsui T, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang H, Lavu S, Sinclair DA. NAMPT/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin Ther Targets. 2007;11:695–705. doi: 10.1517/14728222.11.5.695. [DOI] [PubMed] [Google Scholar]
- 72.Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 73.Dahl TB, Yndestad A, Skjelland M, Øie E, Dahl A, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 74.Zhong M, Tan HW, Gong HP, Wang SF, Zhang Y, et al. Increased serum visfatin in patients with metabolic syndrome and carotid atherosclerosis. Clin Endocrinol (Oxf) 2008;69:878–884. doi: 10.1111/j.1365-2265.2008.03248.x. [DOI] [PubMed] [Google Scholar]
- 75.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 76.Hufton SE, Moerkerk PT, Brandwijk R, de Bruïne AP, Arends JW, et al. A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett. 1999;463:77–82. doi: 10.1016/s0014-5793(99)01578-1. [DOI] [PubMed] [Google Scholar]
- 77.Van Beijnum JR, Moerkerk PT, Gerbers AJ, De Bruïne AP, Arends JW, et al. Target validation for genomics using peptide-specific phage antibodies: a study of five gene products overexpressed in colorectal cancer. Int J Cancer. 2002;101:118–127. doi: 10.1002/ijc.10584. [DOI] [PubMed] [Google Scholar]
- 78.Reddy PS, Umesh S, Thota B, Tandon A, Pandey P, et al. PBEF1/NAmPRTase/Visfatin: a potential malignant astrocytoma/glioblastoma serum marker with prognostic value. Cancer Biol Ther. 2008;7:663–668. doi: 10.4161/cbt.7.5.5663. [DOI] [PubMed] [Google Scholar]
- 79.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 80.Muruganandham M, Alfieri AA, Matei C, Chen Y, Sukenick G, et al. Metabolic signatures associated with a NAD synthesis inhibitor-induced tumor apoptosis identified by 1H-decoupled-31P magnetic resonance spectroscopy. Clin Cancer Res. 2005;11:3503–3513. doi: 10.1158/1078-0432.CCR-04-1399. [DOI] [PubMed] [Google Scholar]
- 81.Drevs J, Loser R, Rattel B, Esser N. Antiangiogenic potency of FK866/K22.175, a new inhibitor of intracellular NAD biosynthesis, in murine renal cell carcinoma. Anticancer Re. 2003;23:4853–4858. [PubMed] [Google Scholar]
- 82.Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124:511–515. doi: 10.1002/ijc.24064. [DOI] [PubMed] [Google Scholar]
- 83.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 84.Chamberlain MC. Antiangiogenesis: biology and utility in the treatment of gliomas. Expert Rev Neurother. 2008;8:1419–1423. doi: 10.1586/14737175.8.10.1419. [DOI] [PubMed] [Google Scholar]
- 85.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, et al. Visfatin in human pregnancy: maternal gestational diabetes vis-à-vis neonatal birthweight. J Perinat Med. 2008;37:218–231. doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hallschmid M, Randeva H, Tan BK, Kern W, Lehnert H. Relationship of Cerebrospinal Fluid Visfatin [PBEF/NAMPT] Levels to Adiposity in Humans. Diabetes. 2008;58:637–640. doi: 10.2337/db08-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang Y, Xu XM, Wang HS, Wang PW. Correlation between the expression of gastrocolic omentum visfatin mRNA and gestational diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi. 2008;43:824–827. [PubMed] [Google Scholar]
- 88.Ziegelmeier M, Bachmann A, Seeger J, Lossner U, Kratzsch J, et al. Adipokines influencing metabolic and cardiovascular disease are differentially regulated in maintenance hemodialysis. Metabolism. 2008;57:1414–1421. doi: 10.1016/j.metabol.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 89.Retnakaran R, Youn BS, Liu Y, Hanley AJ, Lee NS, et al. Correlation of circulating full-length visfatin (PBEF/NAMPT) with metabolic parameters in subjects with and without diabetes: a cross-sectional study. Clin Endocrinol (Oxf) 2008;69:885–893. doi: 10.1111/j.1365-2265.2008.03264.x. [DOI] [PubMed] [Google Scholar]
- 90.Botella-Carretero JI, Luque-Ramírez M, Alvarez-Blasco F, Peromingo R, San Millán JL, et al. The increase in serum visfatin after bariatric surgery in morbidly obese women is modulated by weight loss, waist circumference, and presence or absence of diabetes before surgery. Obes Surg. 2008;18:1000–1006. doi: 10.1007/s11695-007-9369-7. [DOI] [PubMed] [Google Scholar]
- 91.Alghasham AA, Barakat YA. Serum visfatin and its relation to insulin resistance and inflammation in type 2 diabetic patients with and without macroangiopathy. Saudi Med J. 2008;29:185–192. [PubMed] [Google Scholar]
- 92.Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:565–570. doi: 10.1016/j.metabol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Lewandowski KC, Stojanovic N, Press M, Tuck SM, Szosland K, et al. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia. 2007;50:1033–1037. doi: 10.1007/s00125-007-0610-7. [DOI] [PubMed] [Google Scholar]
- 94.Fernández-Real JM, Moreno JM, Chico B, López-Bermejo A, Ricart W. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30:616–621. doi: 10.2337/dc06-1581. [DOI] [PubMed] [Google Scholar]
- 95.Dogru T, Sonmez A, Tasci I, Bozoglu E, Yilmaz MI, et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76:24–29. doi: 10.1016/j.diabres.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 96.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 97.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 98.Berndt J, Klöting N, Kralisch S, Kovacs P, Fasshauer M, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 99.Haider DG, Pleiner J, Francesconi M, Wiesinger GF, Muller M, et al. Exercise training lowers plasma visfatin concentrations in patients with type 1 diabetes. J Clin Endocrinol Metab. 2006;91:4702–4704. doi: 10.1210/jc.2006-1013. [DOI] [PubMed] [Google Scholar]
- 100.Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, Recasens M, Esteve E, et al. Serum visfatin increases with progressive β-cell deterioration. Diabetes. 2006;55:2871–2875. doi: 10.2337/db06-0259. [DOI] [PubMed] [Google Scholar]
- 101.Akturk M, Altinova AE, Mert I, Buyukkagnici U, Sargin A, et al. Visfatin concentration is decreased in women with gestational diabetes mellitus in the third trimester. J Endocrinol Invest. 2008;31:610–613. doi: 10.1007/BF03345611. [DOI] [PubMed] [Google Scholar]
- 102.Kato A, Odamaki M, Ishida J, Hishida A. Relationship between serum pre-B cell colony-enhancing factor/visfatin and atherosclerotic parameters in chronic hemodialysis patients. Am J Nephrol. 2009;29:31–35. doi: 10.1159/000148648. [DOI] [PubMed] [Google Scholar]
- 103.Jian WX, Luo TH, Gu YY, Zhang HL, Zheng S, et al. The visfatin gene is associated with glucose and lipid metabolism in a Chinese population. Diabet Med. 2006;23:967–973. doi: 10.1111/j.1464-5491.2006.01909.x. [DOI] [PubMed] [Google Scholar]
- 104.Palin MF, Labrecque B, Beaudry D, Mayhue M, Bordignon V, et al. Visfatin expression is not associated with adipose tissue abundance in the porcine model. Domest Anim Endocrinol. 2008;35:58–73. doi: 10.1016/j.domaniend.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 105.Tsiotra PC, Tsigos C, Yfanti E, Anastasiou E, Vikentiou M, et al. Visfatin, TNF-alpha and IL-6 mRNA expression is increased in mononuclear cells from type 2 diabetic women. Horm Metab Res. 2007;39:758–763. doi: 10.1055/s-2007-990288. [DOI] [PubMed] [Google Scholar]
- 106.Körner A, Garten A, Blüher M, Tauscher R, Kratzsch J, et al. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92:4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- 107.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus. Metabolism. 2007;56:451–458. doi: 10.1016/j.metabol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 108.Chan TF, Chen YL, Lee CH, Chou FH, Wu LC, et al. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J Soc Gynecol Investig. 2006;13:364–367. doi: 10.1016/j.jsgi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 109.Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006;91:3165–3170. doi: 10.1210/jc.2006-0361. [DOI] [PubMed] [Google Scholar]
- 110.Körner A, Garten A, Blüher M, Tauscher R, Kratzsch J, et al. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92:4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- 111.Xie H, Tang SY, Luo XH, Huang J, Cui RR, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 112.Imai SI. The NAD World: A New Systemic Regulatory Network for Metabolism and Aging-Sirt1, Systemic NAD Biosynthesis, and Their Importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brema I, Hatunic M, Finucane F, Burns N, Nolan JJ, et al. Plasma visfatin is reduced after aerobic exercise in early onset type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:600–602. doi: 10.1111/j.1463-1326.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 114.Storka A, Vojtassakova E, Mueller M, Kapiotis S, Haider DG, et al. Angiotensin inhibition stimulates PPARgamma and the release of visfatin. Eur J Clin Invest. 2008;38:820–826. doi: 10.1111/j.1365-2362.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 115.Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun. 2005;336:747–753. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- 116.Choi KC, Lee SY, Yoo HJ, Ryu OH, Lee KW, et al. Effect of PPAR-delta agonist on the expression of visfatin, adiponectin, and resistin in rat adipose tissue and 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;357:62–67. doi: 10.1016/j.bbrc.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 117.Zhou H, Yang X, Wang NL, Zhang YO, Cai GP. Macrostemonoside A promotes visfatin expression in 3T3-L1 cells. Biol Pharm Bull. 2007;30:279–283. doi: 10.1248/bpb.30.279. [DOI] [PubMed] [Google Scholar]
- 118.Hammarstedt A, Pihlajamäki J, Rotter Sopasakis V, Gogg S, Jansson PA, et al. Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab. 2006;91:1181–1184. doi: 10.1210/jc.2005-1395. [DOI] [PubMed] [Google Scholar]
- 119.Pfützner A, Marx N, Walcher D, Löbig M, Seidel D, et al. Impact of rosiglitazone on visfatin and adiponectin plasma concentrations in patients with type 2 diabetes and coronary artery disease. Clin Lab. 2008;54:237–241. [PubMed] [Google Scholar]
- 120.Erdem G, Dogrum T, Tasci I, Bozoglu E, Muhsiroglu O, et al. The effects of pioglitazone and metformin on plasma visfatin levels in patients with treatment naive type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;82:214–218. doi: 10.1016/j.diabres.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 121.Zhang YY, Gottardo L, Thompson R, Powers C, Nolan D, et al. A visfatin promoter polymorphism is associated with low-grade inflammation and type 2 diabetes. Obesity (Silver Spring) 2006;14:2119–2126. doi: 10.1038/oby.2006.247. [DOI] [PubMed] [Google Scholar]
- 122.Sun G, Bishop J, Khalili S, Vasdev S, Gill V, et al. Serum visfatin concentrations are positively correlated with serum triacylglycerols and down-regulated by overfeeding in healthy young men. Am J Clin Nutr. 2007;85:399–404. doi: 10.1093/ajcn/85.2.399. [DOI] [PubMed] [Google Scholar]
- 123.Landré-Beauvais AJ. The first description of rheumatoid arthritis. Unabridged text of the doctoral dissertation presented in 1800. Joint Bone Spine. 2001;68:130–143. doi: 10.1016/s1297-319x(00)00247-5. [DOI] [PubMed] [Google Scholar]
- 124.Otero M, Lago R, Gomez R, Lago F, Dieguez C, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brentano F, Schorr O, Ospelt C, Stanczyk J, Gay RE, et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007;56:2829–2839. doi: 10.1002/art.22833. [DOI] [PubMed] [Google Scholar]
- 126.Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS ONE. 2008;3:e2267. doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu P, Li H, Cepeda J, Xia Y, Kempf JA, et al. Regulation of inflammatory cytokine expression in pulmonary epithelial cells by pre-B-cell colony-enhancing factor via a nonenzymatic and AP-1-dependent mechanism. J Biol Chem. 2009;284:27344–27351. doi: 10.1074/jbc.M109.002519. [DOI] [PMC free article] [PubMed] [Google Scholar]