Abstract
The establishment of cadherin-dependent cell–cell contacts in human epidermal keratinocytes are known to be regulated by the Rac1 small GTP-binding protein, although the mechanisms by which Rac1 participates in the assembly or disruption of cell–cell adhesion are not well understood. In this study we utilized green fluorescent protein (GFP)-tagged Rac1 expression vectors to examine the subcellular distribution of Rac1 and its effects on E-cadherin–mediated cell–cell adhesion. Microinjection of keratinocytes with constitutively active Rac1 resulted in cell spreading and disruption of cell–cell contacts. The ability of Rac1 to disrupt cell–cell adhesion was dependent on colony size, with large established colonies being resistant to the effects of active Rac1. Disruption of cell–cell contacts in small preconfluent colonies was achieved through the selective recruitment of E-cadherin–catenin complexes to the perimeter of multiple large intracellular vesicles, which were bounded by GFP-tagged L61Rac1. Similar vesicles were observed in noninjected keratinocytes when cell–cell adhesion was disrupted by removal of extracellular calcium or with the use of an E-cadherin blocking antibody. Moreover, formation of these structures in noninjected keratinocytes was dependent on endogenous Rac1 activity. Expression of GFP-tagged effector mutants of Rac1 in keratinocytes demonstrated that reorganization of the actin cytoskeleton was important for vesicle formation. Characterization of these Rac1-induced vesicles revealed that they were endosomal in nature and tightly colocalized with the transferrin receptor, a marker for recycling endosomes. Expression of GFP-L61Rac1 inhibited uptake of transferrin-biotin, suggesting that the endocytosis of E-cadherin was a clathrin-independent mechanism. This was supported by the observation that caveolin, but not clathrin, localized around these structures. Furthermore, an inhibitory form of dynamin, known to inhibit internalization of caveolae, inhibited formation of cadherin vesicles. Our data suggest that Rac1 regulates adherens junctions via clathrin independent endocytosis of E-cadherin.
INTRODUCTION
The establishment of cell–cell contacts in epithelial cells is primarily mediated by the transmembrane Ca2+-dependent glycoprotein E-cadherin. The cadherins comprise a super-family of cell surface adhesion receptors that include the E-, P-, and N-cadherins. They display homophilic interactions between molecules expressed on adjacent cells through their extracellular domains. The conserved cytoplasmic tail interacts with the actin cytoskeleton through a complex of peripheral membrane proteins including α-, β-, and γ-catenins. Cadherin function has been shown to be important in many aspects of epithelial biogenesis. For example, spatial organization of most solid tissues in the body, maintenance of the differentiated phenotype, establishment of epithelial cell polarity, wound healing, and tumorigenesis are all dependent on cadherin-mediated cell–cell adhesion (reviewed in Gumbiner, 1996). During development, cadherin regulation has been shown to be important for tissue morphogenesis in mouse, Drosophila, and Xenopus embryos (Fleming and Johnson, 1988; Levine et al., 1994; Uemura et al., 1996). These morphogenic events involve the continual disruption and reformation of adhesive contacts that usually result in major changes in cell state or differentiation.
Several mechanisms have been implicated in the regulation of cadherin-mediated adhesiveness including association of the cytoplasmic domain of cadherins with catenins, tyrosine phosphorylation of the cadherin–catenin complex, and clustering of cadherins at the cell surface (reviewed in Gumbiner, 2000). Accumulating evidence indicates a role for Rho family GTPases in the regulation of cell–cell adhesion in epithelial cells. Rho GTPases are well established as key regulators of the actin cytoskeleton and studies in fibroblasts have demonstrated that Rho, Rac1, and Cdc42 induce distinct actin structures (Hall, 1998). In MDCK cells, expression of constitutively activated Rac1 results in increased accumulation of E-cadherin, β-catenin, and actin at sites of cell–cell contacts (Ridley et al., 1995; Takaishi et al., 1997). Similarly, in Drosophila epithelial cells, activation of Rac1 has been shown to recruit actin to sites of cell–cell contacts (Eaton et al., 1995; Harden et al., 1995). Tiam-1, an exchange factor for Rac1, has been shown to localize to cell–cell contacts and prevent HGF-induced cell scattering of MDCK cells, which is consistent with the observation that activated Rac1 increases E-cadherin–mediated cell adhesion (Hordijk et al., 1997). However, in a separate study involving MDCK cells, HGF-mediated disruption of adherens junctions was inhibited by dominant negative Rac1 (Potempa and Ridley, 1998). In human epidermal keratinocytes, a number of studies have revealed apparently complex roles for Rac1 in adherens junction assembly and disassembly, dependent on both junctional maturity and cellular context (Braga et al., 1997, 1999, Akhtar et al. 2000). It therefore appears that Rac1 can regulate both assembly and disassembly of adherens junctions. Dynamic rearrangement of adherens junctions is a critical step for various physiological processes, and it is tempting to speculate that Rac1 may modulate both disruption and assembly of cell contacts in the same cell type via different mechanisms. In support of this, a dominant active form of Rac1 when expressed in MDCK cells can either promote or prevent E-cadherin–mediated cell adhesion, which is dependent in an extracellular matrix–dependent manner (Sander et al., 1998).
Although Rac1 can regulate cell–cell adhesion through reorganization of the actin cytoskeleton, recent evidence also implicates a direct role for Rac1 and Cdc42 at sites of cell–cell contact. IQGAP1, a target of Cdc42 and Rac1, was shown to localize and interact with E-cadherin and β-catenin at sites of cell–cell contact. IQGAP1 effectively competes with α-catenin for binding to cadherin–catenin complexes, resulting in dissociation of α-catenin from the complex and decreased cell–cell adhesion. Rac1 and Cdc42 negatively regulate IQGAP1 function by inhibiting the interaction of IQGAP1 with β-catenin, thus rendering the α-catenin binding site free on cadherin–catenin complexes and thus stabilizing adhesion (Kuroda et al., 1998, Fukata et al., 1999). This model supports a positive role for Rac1 and Cdc42 in regulating cadherin–mediated cell–cell adhesion but does not serve to explain how activated Rac1 mediates cell–cell disruption in some epithelial cell lines.
A number of reports indicate that the Rho family GTPases are involved in regulating endocytic traffic (reviewed in Ellis and Mellor, 2000). Microinjection of constitutively activated forms of RhoA and Rac1 has been reported to block clathrin-mediated endocytosis of the transferrin receptor in fibroblasts (Lamaze et al., 1996). Activated Rac1 has also been shown to increase fluid-phase pinocytic activity (Ridley et al., 1992), and RhoA stimulates clathrin-independent endocytosis in Xenopus oocytes (Schmalzing et al., 1995). In addition, RhoD has been shown to regulate the trafficking of early and recycling endosomes and also localizes to these vesicles (Murphy et al., 1996). RhoB is associated with structures that resemble multivesicular bodies and has been shown to regulate EGF receptor trafficking (Robertson et al., 1995, Gampel et al., 1999). The mechanisms involved in intracellular trafficking of cadherins are as yet not clear, although a recent study reported that E-cadherin can be endocytosed and recycled back to the cell surface of MDCK cells and that this may occur via a clathrin-dependent mechanism (Le et al., 1999).
We recently reported that a constitutively active form of Rac1 induces formation of large intracellular vesicles around which Rac1 and E-cadherin tightly colocalize (Akhtar et al., 2000). In this article we sought to identify the nature of these vesicles and analyzed the role of Rac1 in endocytosis of E-cadherin. We demonstrate that Rac1 depletes levels of E-cadherin at sites of cell–cell contact by inducing clathrin-independent internalization of E-cadherin at the cell surface and discuss possible roles of Rac1 in the dynamic rearrangement of E-cadherin–mediated cell–cell adhesion.
MATERIALS AND METHODS
Cell Culture
SCC12f keratinocytes were cultured on a feeder layer of mitotically inactivated 3T3 fibroblasts in FAD medium (Imperial Labs, Andover, United Kingdom) supplemented with 10% fetal bovine serum (Imperial Labs) and 0.5 μg ml−1 hydrocortisone (Calbiochem, Nottingham, United Kingdom) as described previously (Rheinwald and Green, 1975; Hotchin et al., 1993). Cell–cell adhesion was disrupted by culturing keratinocytes in low (0.1 mM) calcium FAD supplemented with calcium-free fetal bovine serum for 4–12 h, depending on colony size. Alternatively, E-cadherin–mediated cell–cell adhesion was disrupted by exogenous addition of antibodies to E-cadherin (HECD-1, 10 μg ml−1) for 12 h. In some experiments protein synthesis was blocked using 10 μM cycloheximide treatment for 30 min before placing cells in low-calcium medium. In other experiments cells were treated with 10 μM cytochalasin D for 30 min to inhibit actin polymerization. SCC12f cells exhibit growth and differentiation characteristics similar to those observed in primary keratinocytes and were used in preference to primary keratinocytes because they are more amenable to nuclear microinjection (Rheinwald and Beckett, 1981; Akhtar et al., 2000).
Antibodies
Antibodies against myc (9E10), E-cadherin (HECD-1), α-catenin (VB-1), β-catenin (VB-2), and γ-catenin (VB-3) were gifts from Fiona Watt (ICRF, London). Antibodies to the sarcoplasmic and endoplasmic reticular calcium ATPase (Y1F4) and CD63 (VBM-5) were gifts from Francesco Michelangeli and Fedor Berditchevski (University of Birmingham, United Kingdom). Antibody to desmoplakin 1 (DP-1) was a gift from Tony McGee (NIMR, London). Commercial sources of antibodies were: Rac1, E-cadherin, EEA1, caveolin (Transduction Laboratories, Lexington, KY); transferrin receptor (Labvision Corporation, Fremont, CA); dynamin (Upstate Biotechnology, Lake Placid, NY). Primary antibodies were detected using Texas Red–conjugated goat anti-mouse, anti-rabbit IgG or donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) or horseradish peroxidase–conjugated goat anti-mouse, anti-rabbit IgG (Amersham, Amersham, United Kingdom). Filamentous actin was visualized using Texas Red–conjugated phalloidin (Molecular Probes, Eugene, OR).
Construction of GFP-tagged Rac1 Vectors
Construction of constitutively activated (L61) and dominant negative (N17) green fluorescent protein (GFP) Rac1 vectors has been described in detail elsewhere (Akhtar et al., 2000). In brief, pCDNA3-GFP-Rac1 expression vectors were constructed by linking GFP from pCdc2MmGFP (Zernicka-Goetz et al., 1996) to the amino terminus of N17 and L61 Rac1 cDNA (Ridley et al., 1992) with a -Gly-Gly-Gly-Ser- linker in between. Biological activity of the constructs was established by transfection of 293T cells and microinjection of Swiss 3T3 fibroblasts and MDA-MB-231 epithelial cells (Akhtar et al., 2000). Intracellular localization of GFP-Rac1 constructs in SCC12f keratinocytes was consistent with that seen with myc-tagged Rac1 fusion constructs (Robertson et al., 1995; Akhtar et al., 2000). GFP-tagged Rac1 effector mutants were constructed by subcloning L61A37 Rac1 and L61C40 Rac1 from pGEX2T (Lamarche et al., 1996) into pCDNA3-GFP-Rac1 using appropriate restriction sites. The sequence of each construct was verified by DNA sequencing.
Immunoblotting
Protein lysates were prepared from SCC12f keratinocytes cultured in normal calcium FAD or low-calcium FAD medium. Proteins were separated by SDS-PAGE, transferred to PVDF membrane (Millipore), immunoblotted with antibodies to E-cadherin or anti-myc, and detected by chemiluminescence as described previously (Hotchin and Hall, 1995).
Microinjection
The GFP-tagged Rac1 constructs were introduced into SCC12f keratinocytes by nuclear microinjection, as described previously (Akhtar et al., 2000). Cells were incubated for 16 h postinjection to allow expression. In some experiments cells were injected with a cDNA expressing an inhibitory (K44A) form of dynamin I (kindly provided by Harry Mellor, University of Bristol).
Immunohistocytochemistry
Expression and distribution of various proteins were visualized by indirect immunofluorescence. Keratinocytes cultured on glass coverslips were fixed for 10 min in PBS containing 4% (wt/vol) paraformaldehyde, washed with PBS, and permeabilized using PBS containing 0.2% (vol/vol) Triton X-100 (Sigma, St. Louis, MO). After permeabilization, the coverslips were incubated with primary and Texas Red–conjugated secondary antibodies as described previously (Akhtar et al., 2000). Immunostained cells were visualized using a Leica DMRB microscope (Deerfield, IL) and images processed using OpenLab software (Improvision, Coventry, United Kingdom).
Fluid-Phase and Receptor-mediated Uptake Assays
Fluid-phase uptake in SCC12F keratinocytes was assessed by addition of Texas Red–conjugated Dextran (Molecular Probes) to the cell culture medium (final concentration, 1 μg ml−1) after microinjection of cells and left for 16 h. Uptake of dye into Rac-induced vesicles was detected in live cells without fixation or permeabilization, whereas pinocytic uptake of fluid phase markers was detected in cells that were rinsed in PBS and fixed in 4% (wt/vol) paraformaldehyde. Uptake of transferrin was assessed by addition of transferrin-biotin (Sigma) to the cell culture medium containing 1% (wt/vol) bovine serum albumin (final concentration, 10 μg ml−1) for 2 h, followed by detection with Texas Red–conjugated streptavidin (Jackson ImmunoResearch Laboratories).
RESULTS
GFP-L61Rac1 Disrupts Cell–Cell Contacts by Recruitment of E-Cadherin–Catenin Complexes into Large Intracellular Vesicles
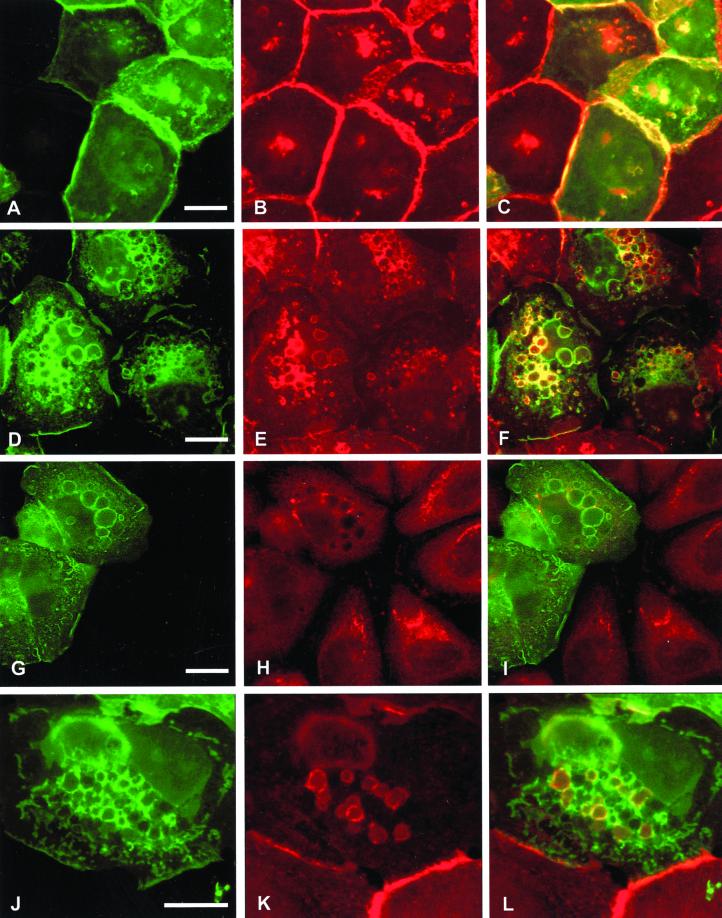
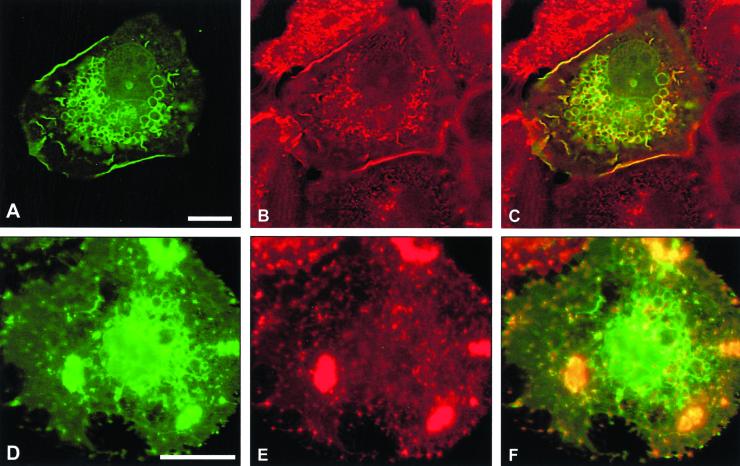
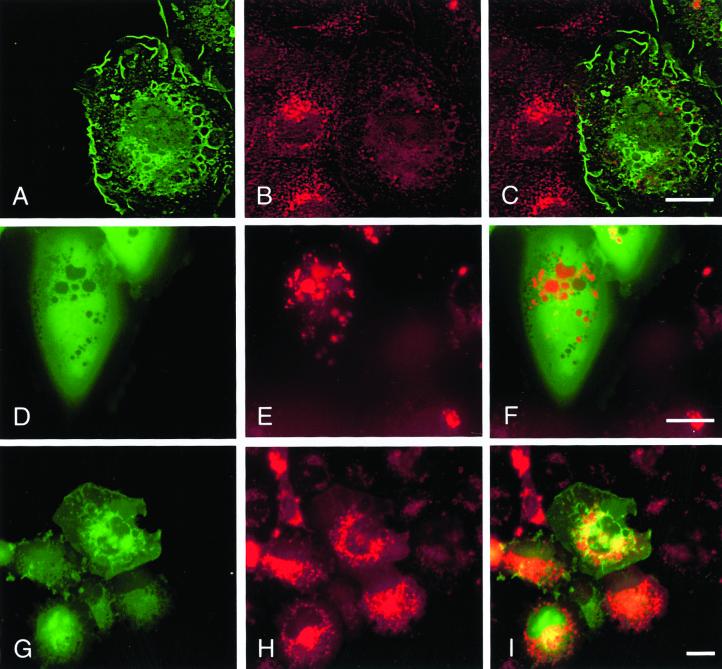
We have previously reported that microinjection of constitutively activated GFP-Rac1 induces cell spreading, membrane ruffles, and large intracellular vesicles in SCC12f keratinocytes and tightly colocalizes with E-cadherin at these sites (Akhtar et al., 2000). We therefore investigated whether recruitment of E-cadherin around intracellular vesicles by GFP-L61Rac1 affects levels of E-cadherin at sites of cell–cell contacts. Microinjection of GFP-L61Rac1 resulted in relocalization of E-cadherin to the perimeter of large intracellular vesicles as previously shown and a marked reduction in levels of E-cadherin at sites of cell–cell adhesion (Figure 1, D–F). Consistent with our previous study, levels of E-cadherin at cell junctions were not altered in cells microinjected with dominant negative GFP-Rac1 (Figure 1, A–C). Other cell adhesion molecules including CD44 (our unpublished results) and desmoplakin-1, a component of desmosomal junctions (Figure 1, G–I) did not colocalize with GFP-L61Rac1 in vesicles, indicating that this recruitment was specific to E-cadherin. To assess whether the E-cadherin–bounded vesicles were intracellular, we stained GFP-L61Rac1–injected cells for E-cadherin without permeabilization of the cells. Under these conditions E-cadherin was found to colocalize with GFP-L61Rac1 in a few cell surface vesicles (Figure 1, J–L), thus indicating that the remaining Rac1–bounded vesicles were intracellular. This was in comparison to cells permeabilized with Triton X-100, where E-cadherin was found to colocalize with GFP-L61Rac1 around most of the vesicles (Figure 1, D–F).
Figure 1.
Expression of constitutively active Rac1 disrupts cell–cell contacts by recruitment of E-cadherin into large intracellular vesicles. Keratinocytes cultured in normal growth medium were microinjected with cDNA constructs expressing GFP-tagged dominant negative Rac1 (GFP-N17Rac1; A–C) or GFP-tagged constitutively active Rac1 (GFP-L61Rac1; D–L). Cells were fixed and permeabilized (A–I) or fixed without permeabilization (J–L) 16 h after microinjection and stained for E-cadherin (B, E, and K) or desmoplakin-1 (H). Distribution of GFP-L61Rac1 and E-cadherin, or desmoplakin-1 was compared by merging images (C, F, I, and L). Images are from 0.4-μm sections taken 4.4 μm from basal surface of the cell. Keratinocyte colony size was <60 cells in all cases. Scale bar, 10 μm.
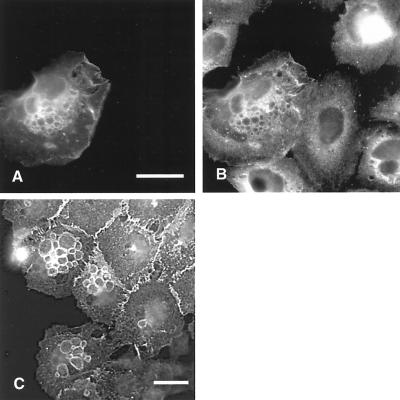
To address the possibility of vesicle formation occurring as a consequence of overexpression of GFP-L61Rac1, we microinjected keratinocytes with pCDNA3-GFP-L61Rac1 and compared expression of this construct to endogenous Rac1 expression by staining injected and noninjected cells with an antibody to Rac1 (Figure 2). Although some nonspecific staining was observed with this antibody, the level of Rac1 expression in cells injected with pCDNA3-GFP-L61Rac1 was not significantly higher than expression of endogenous Rac1 (Figure 2, A and B). Significantly, E-cadherin–positive vesicles are also observed in cells situated at the edge of large keratinocyte colonies (>200 cells), suggesting that these vesicles are a normal feature of keratinocytes (Figure 2C).
Figure 2.
Vesicle formation is not a consequence of microinjection or overexpression of GFP-L61Rac1 relative to endogenous Rac1. To compare expression levels of exogenous GFP-Rac1 relative to endogenous Rac1 keratinocytes were microinjected with a cDNA construct expressing GFP-L61Rac1 (A) and costained with an antibody that recognizes Rac1 (B). Large established colonies of noninjected keratinocytes (>200 cell size) were also fixed and stained with an antibody to E-cadherin. Cells at the colony edge were photographed to demonstrate the presence of E-cadherin–positive vesicles in noninjected keratinocytes (C). Scale bar, 10 μm.
We analyzed whether other components of cadherin junctions were also recruited around the Rac-induced vesicles. We demonstrated that α-, β-, and γ- catenins all colocalize with GFP-L61Rac1 around the perimeter of the vesicles (Figure 3). Thus our data suggest that components of cadherin–catenin complexes are specifically redistributed around intracellular vesicles in response to constitutive Rac activation, and this results in disruption of cell–cell contacts.
Figure 3.
Colocalization of GFP-L61Rac1 and catenins around vesicles. Keratinocytes cultured in normal growth medium were microinjected with a cDNA construct expressing GFP-L61Rac1. Sixteen hours after injection cells were fixed, permeabilized, and stained with antibodies to α-, β- or γ-catenin (B, D, and F, respectively). GFP-L61Rac1–expressing cells were detected by GFP expression (A, C, and E). Images are from 0.4-μm sections taken 4.4 μm from basal surface of the cell. Scale bar, 10 μm.
Ability of Rac1 to Induce Vesicles and Disrupt Cell–Cell Contacts Is Dependent on Stability of Cadherin Junctions
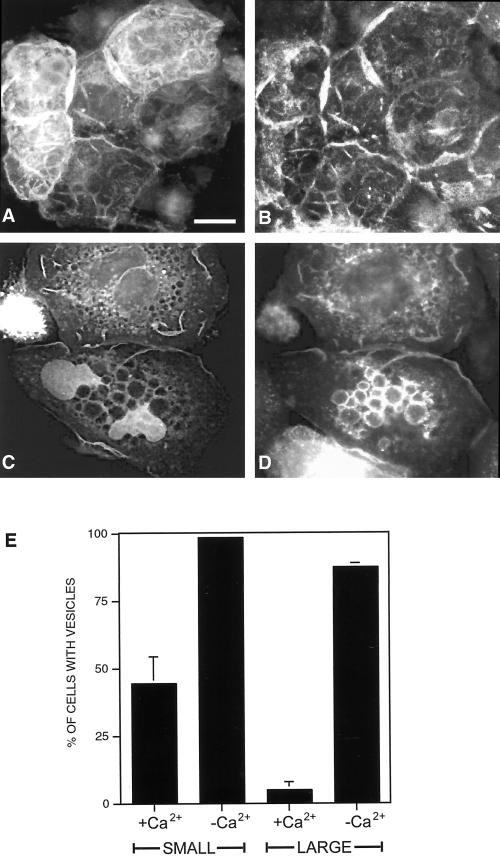
As keratinocyte colonies reach confluency, they generally display increased adhesiveness at sites of cell–cell contacts, which is associated with increased stabilization of cadherin complexes at the cell surface (Shore and Nelson, 1991). It has been previously reported that the ability of dominant negative Rac1 to disrupt cell–cell contacts in human epidermal keratinocytes is dependent on the maturation status of cadherin junctions (Braga et al., 1999). We therefore examined the effects of GFP-L61Rac1 on cell contacts in large colonies of SCC12f keratinocytes. Cells that were part of a large cell-sized colony (>200 cells) were microinjected with GFP-L61Rac1 in standard calcium-containing medium and 16 h later were costained for E-cadherin (Figure 4, A and B). Our results demonstrate that in large colonies, when cadherin junctions are stable, GFP-L61Rac1 is no longer capable of inducing vesicle formation, cell spreading, or disruption of cell–cell contacts as in smaller sized colonies (<60 cells). However, extensive membrane ruffling was observed indicating that the GFP-L61Rac1 was still active in these cells (Figure 4A). In our previous study we demonstrated that when cells are switched to low-calcium medium or incubated with the HECD-1 antibody to disrupt cadherin-dependent contacts, GFP-tagged Rac1 is redistributed away from sites of cell–cell adhesion (Akhtar et al., 2000). We therefore questioned whether the ability of Rac1 to induce vesicles was dependent on localization of Rac1 within the cell. Cells in confluent colonies were microinjected with pCDNA-GFP-L61Rac1, left for 16 h to allow expression, and then switched to low-calcium medium for 6 h to disrupt cadherin junctions. Under these conditions GFP-L61Rac1 relocalized from sites of cell–cell contacts and was now capable of inducing cell spreading and vesicle formation, with colocalization of GFP-L61Rac1 and E-cadherin observed in these vesicles (Figure 4, C and D). Cell counts revealed that when GFP-L61Rac1 was injected into cells that were part of smaller colonies (<60 cells), ∼40–50% of the injected cells contained vesicles, and this number was increased to almost 100% when injected cells were switched to low-calcium medium to disrupt cell contacts. In large colonies (>200 cells) cultured in standard medium, only 4–5% of cells injected with GFP-L61Rac1 contained vesicles, whereas ∼90% of the injected cells in large colonies contained vesicles when switched to low calcium (Figure 4E). Thus, our results demonstrate that GFP-L61Rac1 function in SCC12f is determined by the stability and maturity of cadherin junctions and that relocalization of Rac1 within the cell is linked to vesicle formation.
Figure 4.
Rac1 function is dependent on stability of cadherin-dependent contacts. Keratinocytes cultured in normal growth medium (>200 cells/colony) were microinjected with a cDNA construct expressing GFP-L61Rac1. Sixteen hours after injection cells were either maintained in normal growth medium (A and B) or switched to low-calcium medium for 6 h (C and D) and costained for E-cadherin (B and D). GFP-L61Rac1–expressing cells were visualized by GFP expression (A and C). Scale bar, 10 μm. To quantitate vesicle formation cell counts were performed on small (<60 cells/colony) or large (>200 cells/colony) colonies expressing GFP-L61Rac1 in normal growth medium (+Ca2+) or in low-calcium medium (−Ca2+). GFP-L61Rac1–expressing cells were scored positive if they contained a single large (>1 μm) vesicle. Data are mean + SD from three experiments, with a minimum of 50 injected cells counted per experiment.
Disruption of Cell–Cell Contacts Induces Vesicle Formation that Is Dependent on Endogenous Rac1 Activity
During the course of our experiments we observed that E-cadherin–containing vesicles are present in a small proportion of noninjected SCC12f keratinocytes (∼5%), suggesting that formation of these structures is a normal physiological response. These vesicles are usually evident in preconfluent cells and disappear as the colony size increases, although cells at the edge of larger colonies frequently have E-cadherin–positive vesicles (Figure 2C). We therefore analyzed whether formation of these vesicles was similarly dependent on the maturity of cadherin junctions. Cells (<60 cell colonies) were either grown in standard calcium medium (Figure 5A) or switched to low-calcium medium for 4 h to disrupt cell–cell contacts (Figure 5B) and stained for expression of E-cadherin. In colonies where cell–cell adhesion was disrupted by reducing extracellular calcium, ∼50% of the cells demonstrated formation of E-cadherin–positive vesicles (Figure 5B). Significantly, localization of endogenous Rac1 was also observed around these vesicles (Figure 5C, arrowed cell). To confirm that vesicle formation was a result of disrupting cadherin-dependent contacts, SCC12fs were incubated with the HECD-1 antibody to disrupt junctions in standard calcium medium. As with cells cultured in low-calcium medium, E-cadherin–positive vesicles formed in ∼50% of cells in which cell–cell adhesion was disrupted by addition of HECD-1 (Figure 5D).
Figure 5.
Disruption of cell–cell contacts results in vesicle formation and redistribution of E-cadherin and endogenous Rac1 to the perimeter of these vesicles. Small keratinocyte colonies (<60 cells/colony) were cultured in normal medium (A and D) or in low-calcium medium to disrupt cell–cell adhesion (B and C). Alternatively cell–cell adhesion in cells cultured in normal medium was disrupted by addition of antibody to E-cadherin (HECD-1) for 12 h (D). Cells were stained with antibodies to E-cadherin (A, B, and D) or Rac1 (C). Images are from 0.4-μm sections taken 4.8 μm from basal surface of the cell. Scale bar, 10 μm.
To analyze whether vesicle formation in noninjected keratinocytes was a result of endogenous Rac1 activity, cells were microinjected with either pCDNA3-GFP as a control or with pCDNA3-GFP-N17Rac1 to block endogenous Rac activity. Cells were cultured in standard medium for 16 h to allow expression from the plasmid and then switched to low-calcium medium for 4 h to induce vesicles. Expression of GFP-N17Rac1 resulted in significant inhibition of vesicle formation in low calcium with only 10% of the injected cells containing vesicles (Figure 6, C–D), whereas 50% of the cells injected with the control GFP construct demonstrated the low-calcium–induced vesicles (Figure 6, A and B).
Figure 6.
Dominant negative Rac1 inhibits vesicle formation in cells cultured in medium containing low calcium. Keratinocytes cultured in normal growth medium were microinjected with DNA constructs expressing GFP alone (A and B) or GFP-tagged N17Rac1 (C and D). Sixteen hours after injection, cells were switched to low-calcium medium for 4 h and costained for E-cadherin (B and D). Images are from 0.4-μm sections taken 4.8 μm from basal surface of the cell. Scale bar, 10 μm.
Thus, the recruitment of E-cadherin around vesicles in response to junctional disruption is a normal physiological response in SCC12f keratinocytes and is controlled by endogenous Rac1 activity.
E-Cadherin Recruited around Vesicles Represents a Preexisting Stable Pool within the Cell
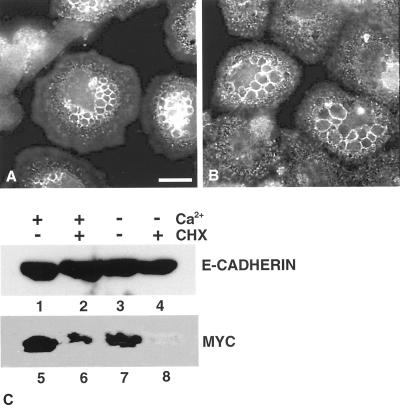
To determine whether de novo protein synthesis was required for recruitment of E-cadherin to the vesicles, we added cycloheximide 30 min before switching cells to low-calcium medium. Under these conditions levels of E-cadherin were unaffected, consistent with a known half-life of 5 h in epithelial cells (Shore and Nelson, 1991; Figure 7C, lanes 1–4). In control experiments, expression level of c-Myc, was found to rapidly decrease upon treatment of cells with cycloheximide, which is consistent with a known half-life of 30 min (Hann and Eisenman, 1984; Figure 7C, lanes 5–8). To induce vesicle formation, cells were switched to low-calcium medium, and localization of E-cadherin was visualized by indirect immunofluorescence (Figure 7). E-cadherin was recruited to the vesicles in cycloheximide-treated cells (Figure 7B), indicating that the source of E-cadherin was derived from a preexisting pool that was relocalized within the cell. Thus, our data indicate that recruitment of E-cadherin to the vesicles formed in response to junctional disruption does not require de novo protein synthesis.
Figure 7.
Cycloheximide treatment does not block localization of E-cadherin to intracellular vesicles. Keratinocytes cultured in normal growth medium were treated with cycloheximide to block protein synthesis (B) or left untreated (A) and switched to low-calcium medium for 4 h to induce vesicles. Cells were fixed and stained for E-cadherin expression. Images are from 0.4-μm sections taken 4.4 μm from basal surface of the cell. Scale bar, 10 μm. To control for activity of cycloheximide¤ keratinocyte lysates were prepared from cells treated with cycloheximide (+CHX) or left untreated (−CHX) and subsequently were maintained in normal growth medium (+Ca2+) or switched to low-calcium medium (−Ca2+). Fifteen micrograms of protein from each lane was resolved by SDS-PAGE, Western blotted, and immunoblotted with antibody to E-cadherin (C, lanes 1–4) or c-myc (C, lanes 5–8).
Actin Reorganization by Rac1 Is Important for Vesicle Formation
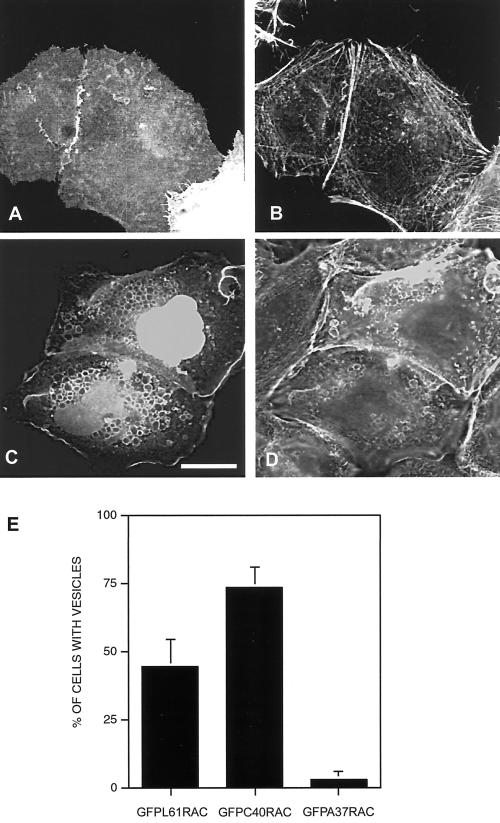
To analyze further how Rac1 regulates vesicle formation in keratinocytes, we asked whether modulation of the actin cytoskeleton was necessary for vesicle formation. In SCC12f cells microinjected with pCDNA3-GFP-L61Rac1 and costained with Texas Red–conjugated phalloidin, actin was found to colocalize with GFP-L61Rac1 around many, though not all, of the vesicles (Figure 8, A–C). Cytochalasin D treatment of cells expressing GFP-L61Rac1 resulted in a significant decrease in the numbers of vesicles present in cells (Figure 8, D–F). This suggests that actin reorganization may be essential for vesicle formation but not necessarily the subsequent maintenance of vesicles. To further assess the requirement of actin for vesicle formation, we used effector mutants of Rac1. In a previous study it was demonstrated that a F37A (A37) point mutation within the effector loop of L61Rac1 rendered it defective in its ability to induce cytoskeletal changes in fibroblasts (Lamarche et al., 1996). By contrast a Y40C (C40) point mutation within the effector loop resulted in a L61Rac mutant that was impaired in its signaling function to PAK and JNK but was still capable of reorganizing the actin cytoskeleton. We constructed GFP-tagged versions of these effector mutants of Rac1 and microinjected SCC12f keratinocytes with these constructs. We found that GFP-L61A37Rac1 does not induce vesicle formation when injected into SCC12f keratinocytes (Figure 9, A and B). Cell counts revealed that only 4–5% of the injected cells contained vesicles, compared with ∼50% of cells injected with GFP-L61Rac1 (Figure 9E). Cells injected with GFPL61C40Rac demonstrated extensive vesicle formation, with colocalization of the Rac effector mutant and actin around many, but not all, of the vesicles (Figure 9, C and D). Cell counts revealed that ∼80% of the injected cells contained vesicles (Figure 9E). This is higher than that seen for cells expressing GFP-L61Rac1 and might reflect the fact that more of the C40 mutant is available because it is able to bind only a limited number of effectors compared with L61Rac1. Thus, our results indicate that reorganization of the actin cytoskeleton by Rac1 is essential for vesicle formation.
Figure 8.
Colocalization of actin and GFP-L61Rac1 around vesicles. Keratinocytes cultured in normal growth medium (<60 cells/colony) were microinjected with a DNA construct expressing GFP-tagged L61Rac1. Sixteen hours after injection, cells were treated with cytochalasin D for 30 min (D–F) or left untreated (A–C). Cells were fixed and stained for filamentous actin using Texas Red–conjugated phalloidin (B and E). Cells expressing the GFP-L61Rac1 construct were detected by GFP expression (A and D), and localization was compared by merging images (C and F). Images are from 0.4-μm sections taken 4.0 μm from basal surface of the cell. Scale bar, 10 μm.
Figure 9.
Vesicle formation is an actin-dependent process. Keratinocytes cultured in normal growth medium (<60 cells/colony) were microinjected with DNA constructs expressing GFP-L61A37Rac1 (A and B) or GFPL61C40Rac1 (C and D). Sixteen hours after injection, cells were fixed and stained with Texas Red–conjugated phalloidin (B and D). Expression of the Rac1 constructs was visualized by expression of GFP (A and C). Images are from 0.4-μm sections taken 4.4 μm from basal surface of the cell. Scale bar, 10 μm. Cells counts were performed on cells microinjected with DNA constructs expressing GFP-L61Rac1, GFP-L61C40Rac1, or GFPL61A37Rac1. Injected cells expressing each construct were scored positive if they contained one or more large (>1 μm) vesicles. Data are mean + SD from three experiments, with a minimum of 50 injected cells counted per experiment.
Rac-induced Vesicles Contain a Marker for Recycling Endosomes
To determine the origin of the large vesicular structures and to give an indication of the membrane trafficking pathway utilized by Rac1 to internalize E-cadherin, we analyzed whether markers for endoplasmic reticulum (ER), early/recycling endosomes, or late endosomes/lysosomes colocalized with GFP-L61Rac1 around the vesicles. We also analyzed uptake of fluid-phase markers and transferrin-biotin in GFP-L61Rac1–injected cells.
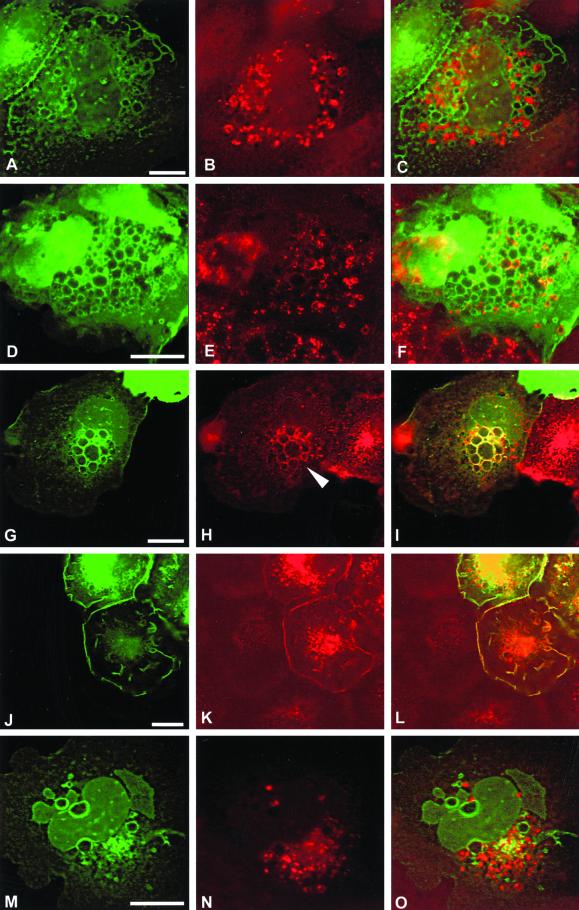
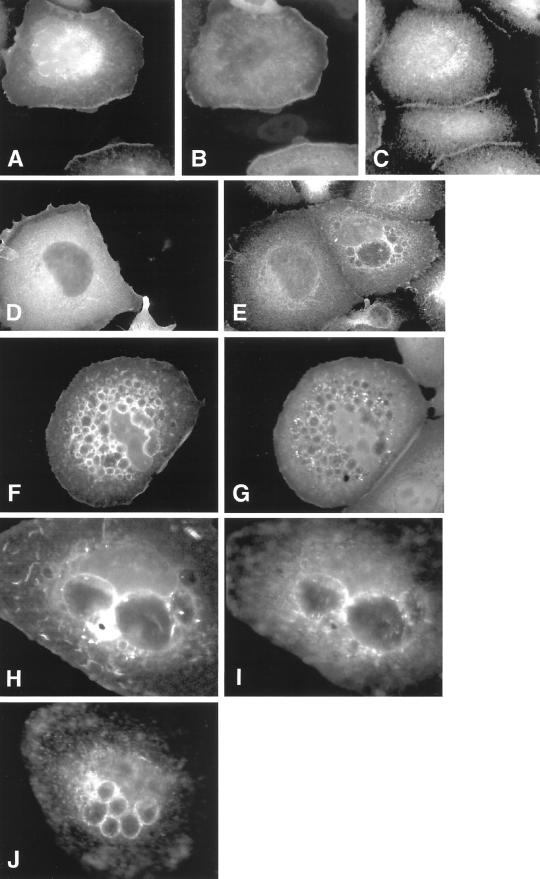
No colocalization of GFP-L61Rac1 with markers of ER (SERCA) or late endosomes (CD63) was observed (Figure 10, A–C and M–O). Colocalization of GFP-L61Rac1 and an early endosome marker (EEA1) was observed in some, though not all, of the vesicles (Figure 10, D–F). Strong colocalization of GFP-L61Rac1 and transferrin receptor, a marker of recycling endosomes, was observed around the majority of the Rac-induced vesicles (Figure 10, G–I). Studies in CHO cells and Hep2 cells have shown that 75–80% of the transferrin receptor is in perinuclear recycling endosomes at steady state (Mayor et al., 1993; Ghosh and Maxfield, 1995). We find that in noninjected SCC12f keratinocytes, the bulk of transferrin receptor was similarly localized to a perinuclear compartment (Figure 10H). However, in cells microinjected with GFP-L61Rac1, transferrin receptor was translocated to the periphery of the cell and was found concentrated at the perimeter of the large Rac-induced vesicles (Figure 10H, arrowhead). Colocalization of GFP-L61Rac1 and transferrin receptor was also observed in membrane ruffles at the cell surface (Figure 10, J–L). Vesicles induced by disrupting cell contacts in low-calcium medium also contained transferrin receptor (our unpublished results).
Figure 10.
Characterization of Rac1-induced vesicles. Keratinocytes cultured in normal growth medium were microinjected with a DNA construct expressing GFP-tagged L61Rac1. Sixteen hours after injection cells were fixed and stained for an ER marker (SERCA, B), an early endosome marker (EEA1, E), transferrin receptor (H and K), and a marker of late endosomes (CD63, N). Expression of injected constructs was visualized by expression of GFP (A, D, G, J, and M), and localization was compared by merging images (C, F, I, L, and O). Images are from 0.4-μm sections taken 4.4 μm from basal surface of the cell, except for J–L, which were taken 5.6 μm from basal surface of the cell. Scale bar, 10 μm.
To determine the endocytic mechanism employed by Rac, we monitored uptake of biotinylated transferrin after expression of GFP-L61Rac1. When keratinocytes were microinjected with pCDNA3-GFP-L61Rac1, we found that uptake of transferrin-biotin was markedly reduced in cells expressing GFP-L61Rac1 compared with noninjected cells, where transferrin-biotin localized to perinuclear vesicles (Figure 11, A–C). Transferrin is known to be internalized via a clathrin-dependent mechanism (reviewed in Mellman, 1996), and expression of constitutively active Rac1 has been reported to inhibit clathrin-mediated endocytosis in HeLa cells (Lamaze et al., 1996); therefore, our data point toward a clathrin-independent mechanism for Rac-mediated endocytosis of E-cadherin. By contrast, fluid phase uptake of Texas Red–conjugated dextran was not inhibited in cells expressing GFP-L61Rac1, and we observe increased pinocytosis in cells expressing GFP-L61Rac1 compared with noninjected cells (Figure 11, G–I). In addition, in cells imaged live without fixation or permeabilization, we observe incorporation of fluid phase dye in vesicles induced by expression of GFP-L61Rac1, indicating that the vesicles we observe arise from internalized cell surface membrane (Figure 11, D–F).
Figure 11.
Fluid phase uptake of transferrin and Texas Red dextran. Keratinocytes cultured in normal growth medium (<60 cells/colony) were microinjected with pCDNA-GFP-L61Rac1 (A, D, and G). Uptake of Texas Red dextran (E and H) and biotinylated transferrin (B) was assessed as described in MATERIALS AND METHODS. Incorporation of Texas Red into Rac-induced vesicles was monitored in live cells without fixation or permeabilization (D–F). Merged images show distribution of GFP-L61Rac1 (in green) and transferrin biotin or Texas Red biotin (in red). Scale bar, 10 μm
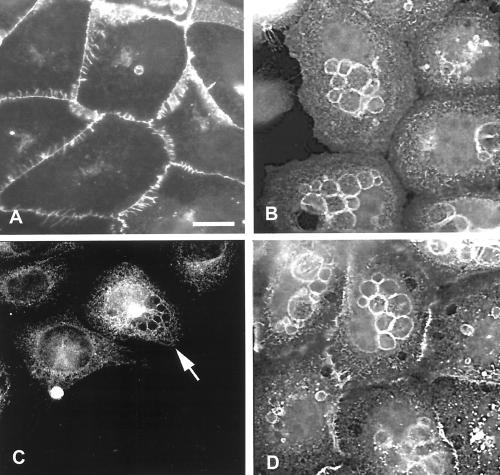
To analyze further the origin of the Rac1-dependent vesicles in keratinocytes, we coinjected keratinocytes (colony size < 60 cells) with cDNAs expressing GFP-L61Rac1 and a dominant negative form (K44A) of dynamin. Unlike the situation in cells expressing GFP-L61Rac1 alone, coexpression of GFP-L61Rac1 and dominant negative dynamin did not lead to disruption of junctions, as determined by staining with a pan-cadherin antibody (Figure 12, A–C). In a separate experiment, cells cultured in low-calcium medium were microinjected with a cDNA expressing a dominant negative form (K44A) of dynamin. In noninjected cells, vesicle formation was observed in ∼50% of cells after switching of cells to low-calcium medium. In cells expressing the dominant negative form of dynamin, we observe a marked decrease in numbers of cells forming cadherin-positive vesicles (10%; Figure 12, D and E). Thus, expression of dominant negative dynamin inhibits Rac1-mediated disruption of cell junctions, indicating that the disruption of junctions and the internalization of cadherin are not separate events.
Figure 12.
Rac1–induced vesicle formation is a clathrin-independent mechanism. Keratinocytes cultured in normal growth medium (<60 cells/colony) were coinjected with pCDNA-GFP-L61Rac1 (A) and a cDNA expressing a dominant negative form of dynamin I (K44A; B) and left for 16 h before fixation. Expression of injected constructs was visualized by expression of GFP (A) and staining for dynamin (B). Distribution of E-cadherin was visualized using a polyclonal pan-cadherin antibody (C). In a separate experiment, keratinocytes were microinjected with a DNA construct expressing dominant negative form of dynamin I (K44A) and cultured overnight in normal growth medium to allow expression, before being switched to low-calcium medium for 4 h. Cells were fixed and stained for dynamin to detect expression of the dominant negative dynamin construct (D) and E-cadherin using a polyclonal pan-cadherin antibody (E). To analyze distribution of clathrin and caveolin, keratinocytes microinjected with pCDNA-GFP-L61Rac1 were stained with antibodies to clathrin (F) and caveolin (H). Expression of GFP-L61Rac1 was visualized by GFP expression (G and I). Keratinocytes (<60 cells/colony) were also cultured in low-calcium medium for 4 h to induce vesicle formation, fixed, and stained with an antibody to caveolin (J).
In addition to its well-documented role in clathrin-mediated endocytosis, dynamin has more recently been implicated in internalization of caveolae (Henley et al., 1998; Dessy et al., 2000). When cells expressing GFP-L61Rac1 were stained with antibodies to clathrin and caveolin, colocalization of caveolin, but not clathrin, and GFP-L61Rac1 was observed around vesicles (Figure 12, F–I). Similarly, when vesicle formation was induced by culturing keratinocytes (colony size < 60) in low-calcium medium, we observed strong localization of caveolin around these vesicles (Figure 12J). Taken together, these results argue for a caveolin-dependent, clathrin-independent mechanism of E-cadherin endocytosis.
DISCUSSION
In this article we have studied the role of Rac1 in redistribution of E-cadherin from adherens junctions in human keratinocytes. We have demonstrated that activation of Rac1 regulates cell–cell junctions in SCC12f keratinocytes through internalization of E-cadherin–catenin complexes. As a consequence, levels of E-cadherin at the cell surface are depleted, resulting in destabilization of cell junctions. This is consistent with the observation that levels of E-cadherin on the cell surface regulate adhesiveness at sites of cell–cell contacts (Yap et al., 1997). Reduction of extracellular levels of calcium is known to disrupt cell–cell adhesion, and we observe similar E-cadherin–containing vesicles in these cells, the formation of which is dependent on endogenous Rac activity. Regulation of E-cadherin endocytosis by small GTPases has previously been examined in MDCK cells where, in contrast to our data, HGF-induced coendocytosis of E-cadherin and c-Met was inhibited in cells expressing dominant active forms of RhoA or Rac1 (Kamei et al., 1999). This discrepancy is not surprising because the role of Rac1 in regulating cell–cell adhesion has been shown to be dependent on both cell type and cell context (Hordijk et al., 1997; Takaishi et al., 1997; Potempa et al., 1998; Braga et al., 1999). In our study, the increased internalization of cell surface E-cadherin in response to constitutively active Rac1 is consistent with its effects on down-regulation of cell–cell adhesion in SCC12f cells.
Characterization of the Rac1-induced vesicles revealed that they are endosomal in nature with colocalization of GFP-L61Rac1 and EEA1, an early endosome marker, around some of the vesicles. The absence of markers specific for late endosomes and lysosomes suggests that these vesicles are not fated for degradation, and this is supported by the observation that transferrin receptor, a marker for recycling endosomes, tightly colocalized with GFP-L61Rac1 around the majority of the vesicles. Consistent with this, Le et al. (1999) demonstrated that a surface pool of E-cadherin is constantly endocytosed and recycled back to the plasma membrane in MDCK cells.
We have shown that activated Rac1 colocalizes with actin around some, though not all of the vesicles, and some of the Rac-induced vesicles were unaffected in cytochalasin D–treated cells. In addition, the Rac effector mutant defective in actin reorganization was incapable of vesicle formation. Taken together these data suggest that only the initial steps involved in the process of vesicle formation are dependent on actin. The precise mechanism by which Rac1 exerts its effects on the actin cytoskeleton to induce vesicle formation remains unclear. However, our data with the C40 Rac effector mutant suggests that CRIB domain effectors for Rac1 are not involved in this process.
We suggest that these vesicles arise from a non–clathrin-dependent mechanism of internalization. Internalization of transferrin-biotin, which is regulated by a clathrin-dependent mechanism, is down-regulated in response to expression of GFP-L61Rac1, consistent with a previously published report in which Rac1 was shown to down-regulate clathrin-dependent, receptor-mediated endocytosis in HeLa cells (Lamaze et al., 1996). It is known that clathrin-independent mechanisms of internalization can be stimulated when clathrin-dependent endocytosis is down-regulated (Damke et al., 1995), and our results suggest an alternative mechanism of endocytosis. The colocalization of GFP-L61Rac1 and caveolin around these vesicles suggests that caveolae might be involved in the internalization of E-cadherin. This is supported by our observation that an inhibitory form of dynamin inhibits vesicle formation in our cells. Dynamin has a well-established role in clathrin-mediated endocytosis but more recently has also been implicated in internalization of caveolae (Henley et al., 1998; Schmid et al., 1998; Dessy et al., 2000). Taken together, this suggests a role for caveolae in the Rac-mediated uptake of E-cadherin and would be consistent with recent evidence demonstrating that Rac1 localizes to caveolae (Michaely et al., 1999). The mechanism by which E-cadherin endocytosed through a clathrin-independent mechanism enters a transferrin receptor–positive compartment remains to be resolved.
Our findings suggest that, in preconfluent cells, Rac1 regulates E-cadherin function but that as cadherin junctions progressively mature on contact with neighboring cells, this level of regulation is decreased. This would be consistent with the cell context–dependent activity of Rac1 reported by others (Braga et al., 1999). Although we cannot exclude the possibility that Rac1 activity may be down-regulated in cells with stable cadherin contacts, this seems unlikely as these cells display numerous membrane ruffles, to which Rac1 is localized. Small, preconfluent keratinocyte colonies are very dynamic structures undergoing a rapid expansion in cell numbers, with accompanying cell migration as confluency is reached. By contrast, cells in large colonies are less mobile because of restrictions in space and the mechanical forces imposed by adjacent cells. There is evidence to indicate that lamellae grow by addition of recycled membrane, and exocytosis of internalized membrane has been shown to be necessary for the formation of new membrane ruffles (Hopkins et al., 1994; Bretscher and Aguado-Velasco, 1998). Thus, one reason why Rac-mediated vesicles occur in small but not large colonies might be related to the turnover of cell membrane required in the rapidly expanding colonies. This would also explain why we observe extensive vesicle formation when we disrupt cell–cell junctions by reducing extracellular calcium or by addition of antibodies to E-cadherin. This idea of contact-dependent Rac1 activity would also explain the observation of E-cadherin–containing vesicles in cells at the free edges of large colonies. Contact-dependent Rac1 function has potential implications in a physiological context, for example, during wound healing. In cells that are part of an intact tissue containing stable adhesive contacts, rapid remodeling of junctions would not be required. However, during wound healing when cells need to migrate to close the wound, Rac1 could then have a dual function in down-regulating cell surface cadherins through endocytosis and remodeling the membrane at free edges to produce lamellipodia. Because we propose that cadherins endocytosed in this manner are not fated for degradation, once contact is made with adjacent cells, adhesion could then be rapidly established by recycling E-cadherin to the cell surface. Supporting this is the observation that in MDCK cells Rac1 promotes either cell migration or cell–cell adhesion, depending on the extracellular matrix substrate (Sander et al., 1998).
In summary, our findings have identified that Rac1 regulates adherens junctions through endocytosis of E-cadherin in an actin-dependent manner. The Rac1-induced endocytosis of E-cadherin occurs via a clathrin-independent mechanism that may involve caveolae. In addition, our data suggest that E-cadherin endocytosed in such a manner is not fated for degradation but has the potential to be recycled. This has implications for tissue remodeling events, such as a wound healing, where dynamic regulation of E-cadherin by Rac1 would allow the continual disruption and reformation of cell–cell contacts.
ACKNOWLEDGMENTS
We are grateful to Mark Marsh and Harry Mellor for advice and critical reading of an earlier version of this manuscript, Mark McNiven for helpful discussion, and colleagues (acknowledged in text) for antibodies and reagents. This work was supported by a Medical Research Council Project grant G9537491 to N.A.H.
REFERENCES
- Akhtar N, Hudson KR, Hotchin NA. Co-localization of Rac1 and E-cadherin in human epidermal keratinocytes. Cell Adh Comm. 2000;7:465–476. doi: 10.3109/15419060009040304. [DOI] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr Biol. 1998;8:721–724. doi: 10.1016/s0960-9822(98)70281-7. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessy C, Kelly RA, Balligand JL, Feron O. Dynamin mediates caveolar sequestration of muscarine cholinergic receptors and alterations in NO signaling. EMBO J. 2000;19:4272–4280. doi: 10.1093/emboj/19.16.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S, Auvinen P, Luo L, Jan YN, Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Mellor H. Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 2000;10:85–88. doi: 10.1016/s0962-8924(99)01710-9. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Johnson MH. From egg to epithelium. Annu Rev Cell Biol. 1988;4:459–485. doi: 10.1146/annurev.cb.04.110188.002331. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem. 1999;274:26044–26050. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- Gampel A, Parker PJ, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase rhoB. Curr Biol. 1999;9:955–958. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- Ghosh RN, Maxfield FR. Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEp2 cells. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hann SR, Eisenman RN. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984;4:2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden N, Loh HY, Chia W, Lim L. A dominant inhibitory version of the small GTP-binding protein Rac disrupts cytoskeletal structures and inhibits developmental cell shape changes in Drosophila. Development. 1995;121:903–914. doi: 10.1242/dev.121.3.903. [DOI] [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin NA, Kovach NL, Watt FM. Functional down-regulation of alpha 5 beta 1 integrin in keratinocytes is reversible but commitment to terminal differentiation is not. J Cell Sci. 1993;106:1131–1138. doi: 10.1242/jcs.106.4.1131. [DOI] [PubMed] [Google Scholar]
- Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, Nakano K, Takaishi K, Takai Y. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells—regulation by Rho, Rac and Rab small G proteins. Oncogene. 1999;18:6776–6784. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development. 1994;120:901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Michaely PA, Mineo C, Ying YS, Anderson RG. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J Biol Chem. 1999;274:21430–21436. doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- Murphy C, Saffrich R, Grummt M, Gournier H, Rybin V, Rubino M, Auvinen P, Lutcke A, Parton RG, Zerial M. Endosome dynamics regulated by a Rho protein. Nature. 1996;384:427–432. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte cell lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Robertson D, Paterson HF, Adamson P, Hall A, Monaghan P. Ultrastructural localization of ras-related proteins using epitope-tagged plasmids. J Histochem Cytochem. 1995;43:471–480. doi: 10.1177/43.5.7537292. [DOI] [PubMed] [Google Scholar]
- Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalzing G, Richter HP, Hansen A, Schwarz W, Just I, Aktories K. Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevis oocytes. J Cell Biol. 1995;130:1319–1332. doi: 10.1083/jcb.130.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL, McNiven MA, DeCamilli P. Dynamin and its properties: a progress report. Curr Opin Cell Biol. 1998;16:483–519. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996;10:659–671. doi: 10.1101/gad.10.6.659. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M, Pines J, Ryan K, Siemering KR, Haseloff J, Evans MJ, Gurdon JB. An indelible lineage marker for Xenopus using a mutated green fluorescent protein. Development. 1996;122:3719–3724. doi: 10.1242/dev.122.12.3719. [DOI] [PubMed] [Google Scholar]