Abstract
The genomic RNA of poliovirus and closely related picornaviruses perform templated and non-templated functions during viral RNA replication. The non-templated functions are mediated by cis-active RNA sequences that bind viral and cellular proteins to form RNP complexes. The RNP complexes mediate temporally dynamic, long-range interactions in the viral genome and ensure the specificity of replication. The 5’ cloverleaf (5’ CL)-RNP complex serves as a key cis-active element in all of the non-templated functions of viral RNA. The 5’CL-RNP complex is proposed to interact with the cre-RNP complex during VPgpUpU synthesis, the 3’NTR-poly(A) RNP complex during negative-strand initiation and the 3’ end negative-strand-RNP complex during positive-strand initiation. Coordinating these long-range interactions is important in regulating each step in the replication cycle.
Introduction
A large number of viruses that cause human, animal and plant diseases contain RNA as their genetic material. The genomic RNA performs both templated and non-templated functions during the viral replication cycle. In positive-strand RNA viruses, the genomic RNA serves as a mRNA and as template for viral RNA replication. Besides storing genetic information and functioning as the template for RNA replication, the viral genome contains RNA determinants which perform non-templated functions that are mediated by distinct RNA sequences and structures present in the noncoding and coding regions of the viral genome. These RNA sequences and structures function as cis-active elements that participate in both RNA-RNA and protein-RNA interactions and are essential for viral replication. These cis-active RNA elements recruit and assemble trans-acting viral and cellular proteins to form membrane bound viral replication complexes that are sites of viral RNA replication.
In this review, we have discussed some of the non-templated functions of viral RNA during RNA replication. We have limited the discussion primarily to the replication of human enteroviruses belonging to the Picornaviridae family of positive-strand RNA viruses. Poliovirus has served as the prototype virus for studying the assembly of viral replication complexes on membrane vesicles using cell-culture systems permissive to viral replication. An important advance in the study of the molecular mechanisms regulating viral RNA replication has been the development of experimental systems to investigate the individual steps in the replication cycle using cell-free reactions [1–6]. Membrane-bound replication complexes isolated from cell-free reactions have been used to follow the synchronous initiation and sequential synthesis of authentic negative-and positive-strand RNAs [7]. This is in contrast to the situation in infected cells where a significant overlap develops between translation and RNA replication because of their mutual interdependence [8]. In addition, trans-replication assays have been developed to separate viral protein synthesis and RNA replication in the cell-free system [9–11]. This assay makes it possible to identify and characterize cis-active elements that are required for the replication of viral RNA templates. [9–11].
The 5’ end of the picornavirus genome is covalently linked to a viral protein, VPg, and is polyadenylated at the 3’ end. The RNA genome contains a large open reading frame flanked by 5’ and 3’ nontranslated regions (NTRs) (Fig. 1A). Conserved RNA sequences and structures present in the 5’ and 3’ NTRs and in the coding region of the genome participate in non-templated RNA functions that regulate the stability, translation and replication of the viral genome [12;13]. The 5’ cloverleaf (5’CL), the cre hairpin, the 3’NTR-poly(A) tail in positive-strand RNA and the 3’ and 5’ terminal sequences in negative-strand RNA are cis-active elements that are functionally important for picornavirus RNA replication. In this review, the non-templated functions of these RNA elements during viral RNA replication will be discussed.
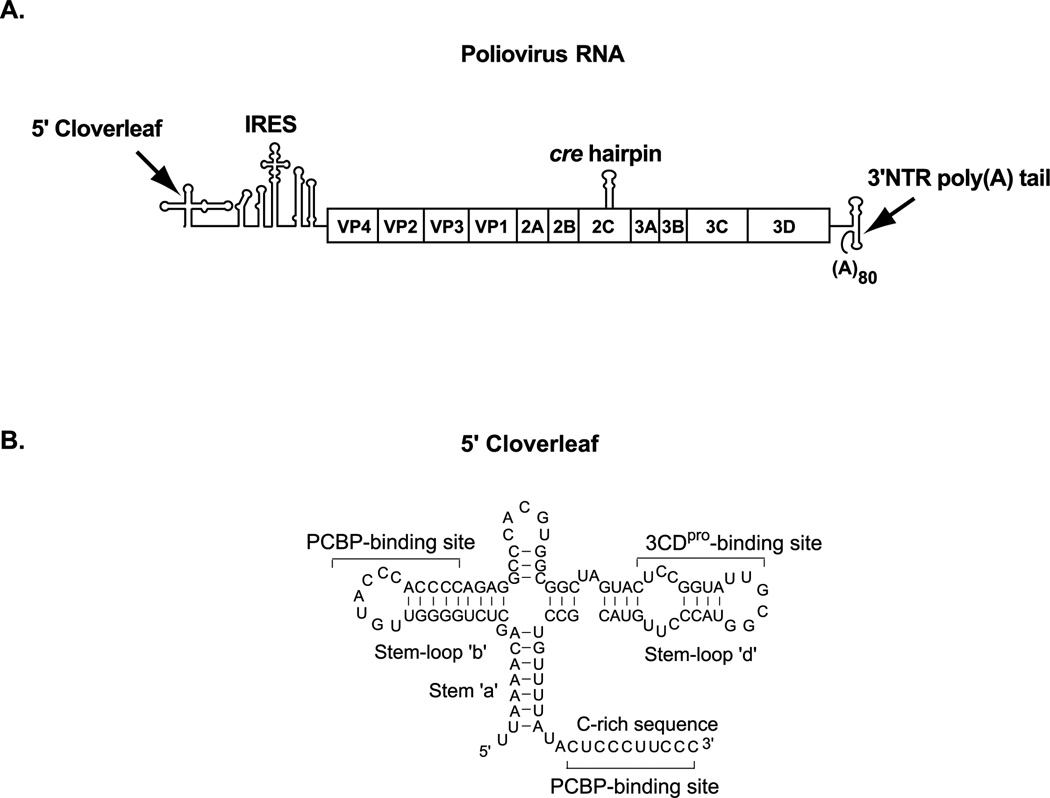
Figure 1.
(A). A schematic diagram showing the RNA structures in the poliovirus RNA genome which perform non-templated RNA functions during viral replication. The 5’ cloverleaf (5’CL) and the internal ribosome entry site (IRES) are present in the 5’ non-translated region (NTR) of the genome. The open reading frame in the viral genome encodes a polyprotein which is cleaved to generate the structural and replication proteins. The cre hairpin is present in the 2C coding region and the 3’ NTR and poly(A) tail is at 3’ end of the genome. (B). The secondary structure and nucleotide sequence of the 5’CL showing the binding sites for the cellular poly(C) binding protein (PCBP) and viral protein, 3CDpro.
Cre-dependent VPgpUpU synthesis
Cre hairpin
VPg is covalently linked to the 5’ end of both negative- and positive-strand RNAs and the uridylylated form of VPg (VPgpUpU) functions as the primer to initiate RNA synthesis [14–18]. A highly conserved hairpin structure, originally identified as a cis-replication element (cre) in the RNA coding region of picornaviruses [19–22], is used as the template for VPgpUpU synthesis in reconstituted assays containing purified viral proteins, VPg, the polymerase (3Dpol) and 3CDpro [12;13;23;24]. The cre hairpin has been identified in the 2C region of poliovirus and Coxsackievirus [6;22;23], the VP1 region of human rhinovirus 14 (HRV14) [19], the 2A region of HRV2 [24], the VP2 region of cardioviruses [20] and in the 5’ NTR of foot and mouth disease virus (FMDV) [25]. Based on the above findings, it is not surprising that the cre hairpin has been shown to function in a position-independent manner [22;25–27].
The loop of the cre hairpin contains a conserved sequence (R1NNNA5A6R7NNNNNNR14) in enteroviruses and rhinoviruses [12;13]. It was shown that the A5 residue functions as the primary template for the addition of both U residues in VPgpUpU using a “slide-back” mechanism [28]. High resolution NMR structural studies together with mutational analysis of the cre hairpin provided evidence for the functional importance of the cre structure during VPgpUpU synthesis [21;27;29;30]. Biochemical and genetic studies suggest that the cre hairpin interacts with 3CDpro and 3Dpol to form a cre-RNA complex that is important for VPgpUpU synthesis [27;31–37]. In summary, the cre hairpin is a position-independent, cis-active element that forms an RNP complex with 3CDpro and 3Dpol and serves as the primary template for VPgpUpU synthesis by 3Dpol.
5’ Cloverleaf (5’CL)
In membrane-bound viral replication complexes isolated from cell-free reactions, it was shown that in addition to the cre hairpin, the 5’CL is also required for VPgpUpU synthesis [38]. In recent studies, using a trans-uridylylation assay in which the replication proteins were provided by a helper RNA, it was shown that the cre and the 5’ CL were the only cis-active elements required for VPgpUpU synthesis. In addition, positioning the cre hairpin adjacent to the 5’CL resulted in a large increase in VPgpUpU synthesis (Sharma and Flanegan, unpublished).
The 5′CL is organized into stem ‘a’ and stem-loops ‘b’, ‘c’ and ‘d,’ and an adjacent C-rich sequence (Fig. 1B). Stem-loop ‘b’ and the C-rich sequence are the binding sites for the cellular poly(C) binding protein (PCBP) and stem-loop ‘d’ is the binding site for 3CDpro[39–42]. Recent studies showed that 5’CL mutations which disrupt the binding of PCBP or 3CDpro to the 5’CL strongly inhibit VPgpUpU synthesis in viral replication complexes indicating that the 5’CL bound to PCBP and 3CDpro is required for VPgpUpU synthesis on the cre hairpin (Sharma and Flanegan, unpublished). This suggests a long-range interaction between the 5’CL-RNP complex and the cre-RNP complex is required for VPgpUpU synthesis as shown in Fig. 2. This idea appears to be consistent with the observation that VPgpUpU synthesis was significantly increased when the 5’CL and cre hairpin were positioned next to each other in the template RNA. It is possible that the 5’CL-RNP complex in concert with the cre-RNP complex provides the optimal conditions for VPgpUpU synthesis on the cre hairpin in the context of the membrane replication complex.
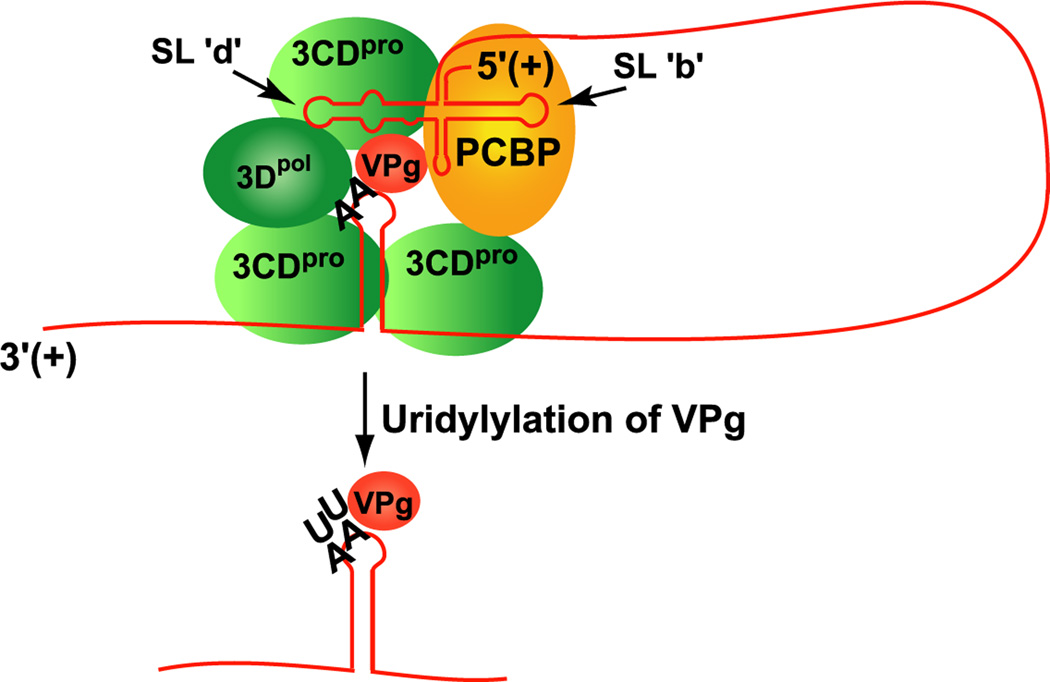
Figure 2.
Model showing the non-templated functions of the 5’CL and cre hairpin during cre-dependent VPgpUpU synthesis. The model shows long-distance interaction between the 5’CL-RNP complex, formed with 3CDpro and PCBP, and the cre-RNP complex, formed with 3CDpro, 3Dpol and VPg. The interaction between the 5’CL-RNP complex and the cre-RNP complex results in the efficient synthesis of VPgpUpU by 3Dpol. For clarity, cellular membranes are not depicted and additional viral and cellular proteins that may be required are not shown in this model and in the other models in this review.
Negative-strand RNA synthesis
3’ Nontranslated Region (NTR) and poly(A) tail
During RNA replication, negative-strand synthesis initiates at the 3’ end of the RNA genome. A highly structured 3’NTR is present at the 3’ end of the genome of picornaviruses [43–46]. Stem-loops X and Y are predicted to form a pseudoknot structure through base-pairing in a “kissing interaction” in the 3’NTR [46]. Mutations that disrupt the formation of the kissing interaction block viral RNA replication [47;48]. Alignment of the 3’NTR sequences of enteroviruses revealed an invariable region of five nucleotides (GUAAA) which forms a single-stranded region between stem Y and stem X [48;49]. Deleting this sequence from the 3’NTR results in a dramatic inhibition of negative-strand synthesis which suggests that it plays a critical role in viral replication [9;10;50]. Interestingly, a poliovirus mutant RNA in which the entire 3’NTR was deleted was still capable of replicating in infected cells suggesting that the 3’NTR is not absolutely required for negative-strand synthesis [51;52]. Finally, the binding of the cellular protein, nucleolin, to the 3’ NTR of poliovirus RNA has been shown to be important for early viral replication [53]. In addition to nucleolin, the viral proteins 3AB and 3CDpro together form an RNP complex with the 3’NTR [54]. These findings suggest that the 3’NTR serves as the platform for the assembly of an RNP complex containing viral and cellular proteins that facilitate the efficient initiation of negative-strand synthesis. Importantly, specific point mutations in the 3’NTR appear to have a more severe effect on viral RNA replication than deletions of the entire 3’NTR. It is possible that in contrast to the deletion mutant, the point mutations allow the mutant 3’NTR to form a nonfunctional RNP complex that interferes with negative-strand initiation.
The 3’ terminal poly(A) tail is also important for several aspects of viral replication. Shortening the poly(A) tail reduced the infectivity of the genomic RNA indicating that the length of the poly(A) tail directly correlates with the infectivity of the genomic RNA [55;56]. Consistent with these findings is the observation that a poly(A) tail 20 nucleotides long or longer is needed for efficient negative-strand initiation [57;58]. It has been suggested that the poly(A) tail plays a role in the formation of a 5’-3’ circular RNP complex [10;57;58]. In addition, VPgpUpU is reported to serve as the preferred primer for negative-strand initiation at internal sites on the poly(A) tail [59;60]. Taken together, this suggests that the poly(A) tail needs to be long enough to be both part of the circular RNP complex and to serve as an efficient template for VPgpUpU priming during negative-strand synthesis (Fig. 3).
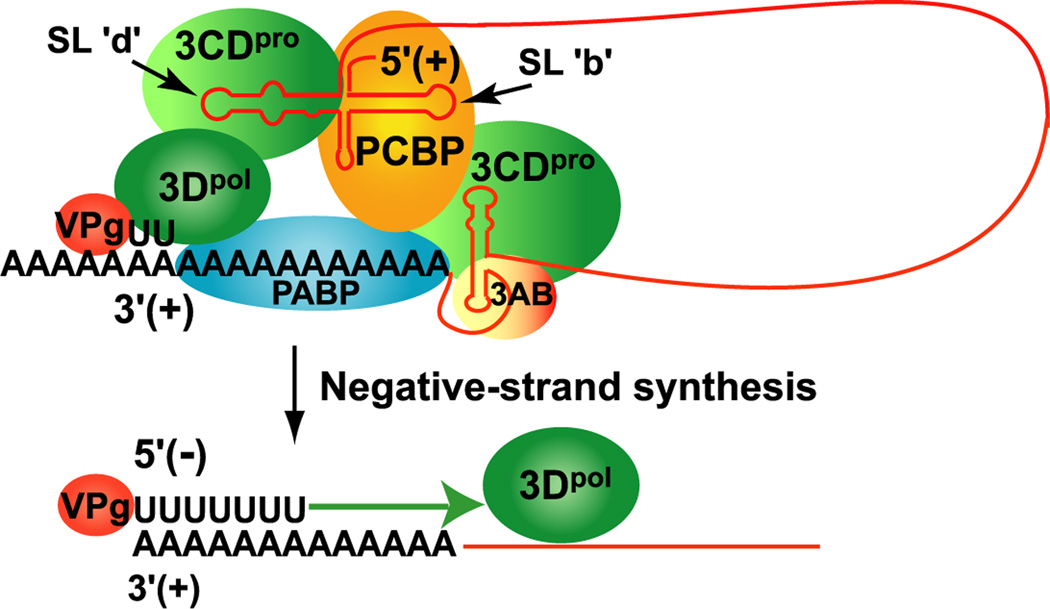
Figure 3.
Model showing non-templated functions of the 5’CL and 3’ NTR-poly(A) tail during initiation of negative-strand synthesis. The model shows the circular RNP complex used to initiate negative-strand synthesis. The long-range interaction between the 5’CL-RNP complex and the 3’ NTR-poly(A) RNP complex results in the formation of a 5’-3’ circular complex. This circular complex is thought to facilitate the initiation of negative-strand synthesis by using VPgpUpU as a primer on the 3’ poly(A) tail. This results in the synthesis of negative-strand RNA containing a 5’ terminal poly(U) sequence.
5’ Cloverleaf (5’CL)
Mutational analysis of the 5’CL revealed its importance in the initiation of negative-strand synthesis [5;10;11;40;42;61;62]. The finding that the 5’CL is required for the initiation of negative-strand synthesis at its 3’ end suggests an interaction between the 5’ and 3’ ends to form a circular complex [10;58]. Mutations that disrupted the 5’CL-PCBP interaction exhibited dramatically reduced negative-strand synthesis [40–42;61;63]. Using the (MS2)2 protein-RNA tethering system, PCBP could be artificially tethered to the 5’CL to partially restore negative-strand synthesis. This established that the 5’CL-PCBP interaction was required for efficient negative-strand synthesis and PCBP does not have to directly bind to the 5’CL to function in RNA replication [61]. Genetic evidence showed that the binding of PCBP to the C-rich sequence adjacent to the 5’CL was also important for RNA replication [5;39;64]. This suggests that PCBP binding to both stem-loop ‘b’ and the C-rich sequence is required for efficient negative-strand synthesis. Mutational analysis of stem-loop ‘d’ showed that the 5’CL-3CDpro interaction also plays a critical role in viral RNA replication and in negative-strand synthesis [5;10;40;41]. These findings clearly established the importance of the 5’CL-RNP complex during initiation of negative-strand synthesis. Interestingly, efficient negative-strand RNA synthesis and cre-dependent VPgpUpU synthesis requires the same 5’CL-RNP complex. Besides stem-loop ‘b’ and ‘d’, the duplex structure of stem ‘a’ is also required for efficient negative-strand synthesis [11]. This indicates that the duplex structure of stem ’a’ may play an important role in maintaining the overall structure of the 5’CL that is required to form the 5’CL-RNP complex. Therefore, the 5CL together with the 3’NTR-poly(A) tail may provide a scaffold to assemble a functional membrane-bound replication complex that is used to initiate negative-strand synthesis (Fig.3).
Cre hairpin
As discussed earlier, the cre hairpin serves as the template for the synthesis of VPgpUpU [19;22–24]. It was shown that disrupting the secondary structure of the cre hairpin by inserting multiple silent mutations resulted in the inhibition of VPgpUpU synthesis. These mutations totally inhibited positive-strand synthesis but had no significant effect on negative-strand synthesis [6;9;65]. In addition, in trans-replication assays, the complete deletion of the cre hairpin from the template RNA had no significant effect on negative-strand synthesis in reactions with helper RNAs containing either a wildtype cre or mutant cre. As expected, positive-strand synthesis was only observed with wildtype helper RNA [9]. These findings indicate that VPgpUpU synthesized on the cre hairpin is required for positive-strand synthesis but is dispensable for negative-strand synthesis. Therefore, VPgpUpU or VPg can serve as a primer to initiate negative-strand synthesis on the 3’ poly(A) tail. However, recent findings suggest that under conditions when the initiating nucleotide (UTP) is limiting, VPgpUpU synthesized on the cre hairpin is the preferred primer for negative-strand initiation [59]. In contrast to the mutations that completely disrupt the structure of the cre hairpin, point mutations in A5 and A6 nucleotides in the loop of the Coxsackievirus cre hairpin were found to completely inhibit VPgpUpU synthesis and to strongly inhibit negative-strand synthesis [6]. The question is why the effect on negative-strand synthesis was so different with the disrupted cre and the mutant cre containing the point mutations. This difference may be related to the observation that point mutations in the cre hairpin that inhibit VPgpUpU synthesis but do not completely disrupt its structure, have a trans-dominant negative effect on the replication of wildtype poliovirus [6;66]. This result was not observed with mutants that contained a disrupted cre. In contrast to the disrupted cre, point mutations in the cre, which disrupt its function, may allow the mutant cre to participate in protein-RNA interactions and form inhibitory RNP complexes by sequestering replication proteins that may be limiting and are required for negative-strand synthesis [66].
Positive-strand RNA synthesis
3’ and 5’ terminal sequences in negative-strand RNA
Once negative-strand synthesis is complete, positive-strand synthesis initiates at the 3’ end of the negative strand RNA. The synthesis of negative-strand RNA may result in the formation of a double-stranded RNA (dsRNA) intermediate known as replicative form RNA (RF). Partial denaturation of the duplex RNA would be necessary to allow VPgpUpU to anneal to the AA sequence at the 3’ end of the negative-strand and function as a primer for positive-strand synthesis. However, the exact mechanism by which the two strands in the RF RNA separate to initiate positive-strand synthesis is not known.
The primary sequence and structure of stem ‘a’ in the 5’CL are highly conserved and the duplex structure of stem ‘a’, as part of the 5’CL is required for negative-strand synthesis [11]. Interestingly, the conserved 5’ terminal sequence 5’UUAAAACAG3’ in stem ‘a’, or more appropriately, the 3’ terminal sequence (3’AAUUUUGUC5) in the negative-strand is a cis-active sequence required for positive-strand synthesis [11]. In addition, it was shown that deleting either one or both of the 3’ terminal A nucleotides dramatically reduced positive-strand synthesis [11]. This finding is consistent with a model in which preformed VPgpUpU base-pairs with the 3’ terminal AA sequence to initiate positive-strand synthesis. Supporting this model is the observation that wildtype virus was recovered from cells transfected with RNAs in which either one or both 3’ terminal A nucleotides were deleted [11;67;68]. These findings demonstrate that even in the absence of the terminal A nucleotides, preformed VPgpUpU can prime and initiate positive-strand synthesis thereby restoring the 3’ terminal A nucleotides.
The cellular protein, hnRNPC, is a cofactor that is required for efficient synthesis of positive-strand RNA and has been shown to bind near the 3’ end of the negative-strand RNA [69;70]. Interestingly, the 3’ terminal sequence 3’AAUUUUGUC5 in the negative-strand RNA appears to be similar to the RNA sequence recognized by the hnRNPC proteins and may explain why this sequence is important for positive-strand initiation (Fig. 4). A second hnRNPC binding site has been recently identified at the 5’ end of the negative strand [71]. The authors of this study propose that binding of hnRNPC to both ends of the negative-strand RNA may stabilize the interaction between the 5’ and 3’ ends. This interaction may in turn assist in the formation of the replication complex required for efficient initiation of positive-strand synthesis.
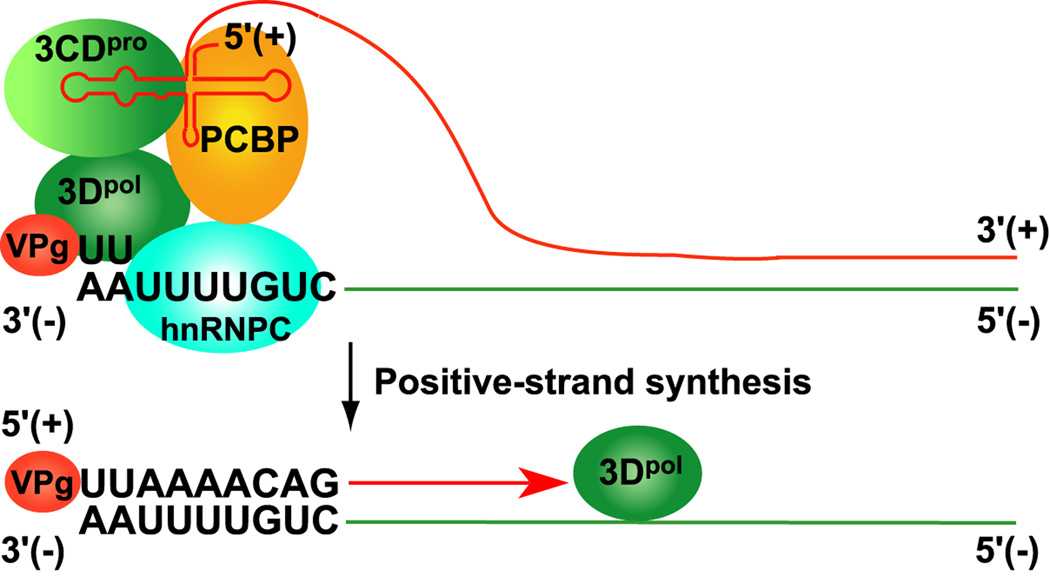
Figure 4.
Model showing non-templated functions of the 5’ CL and the 3’ terminal sequence of the negative-strand RNA during initiation of positive-strand synthesis. The model suggests that partial unwinding of the duplex RF RNA allows the cellular protein, hnRNPC, and VPgpUpU to bind at the 3’ end of the negative-strand RNA [69]. This would also facilitate the formation of the 5’CL-RNP complex in positive-strand RNA as shown in the model. It is proposed that the 5’CL-RNP complex functions in trans to promote the VPgpUpU primed initiation of positive-strand synthesis by 3Dpol [72]. Although not shown, it is possible that hnRNPC bound to the 5’ end of the negative-strand RNA may also be part of the positive-strand initiation complex [71].
5’ Cloverleaf (5’CL)
As discussed earlier, the 5’CL is an important cis-active RNA element that is required for cre-dependent VPgpUpU synthesis and negative-strand synthesis. Since positive-strand synthesis is dependent on both preformed VPgpUpU and negative-strand synthesis, it has been difficult to dissect the role of the 5’CL during positive-strand synthesis. To overcome this problem, a novel experimental approach was used in which a second CL was inserted near the 5’ end of the positive-strand RNA [72]. Using this approach, the authors confirmed the earlier finding that the sequence of stem ‘a’ is important for positive-strand initiation. In addition, they showed that the 5’ CL-RNP complex formed at the 5’ end of the positive-strand RNA, which includes PCBP and 3CDpro, is required for positive-strand synthesis. The authors suggest that after negative-strand synthesis is complete the RF RNA partially unwinds to allow the 5’CL in the positive-strand RNA to reform and assemble the 5’CL-RNP complex. According to this model, the 5’CL-RNP complex formed at the 5’ end of the positive-strand RNA functions in trans at the 3’ end of the negative-strand RNA allowing VPgpUpU to initiate positive-strand RNA synthesis [72] (Fig.4).
Conclusions
Cis-active RNA elements in the viral genome help regulate viral replication and ensure that this process is both efficient and specific for viral RNA templates. A major conclusion of this review is that the 5’CL serves as a key cis-active element in all of the non-templated functions of the viral RNA genome (Figs. 2–4). It is required for each step in the viral RNA replication cycle and serves as a scaffold for the assembly of the multi-protein viral RNA replication complex.
The cis-active elements in the viral genome contain conserved sequences and structures that provide specific binding sites for viral and cellular proteins. This results in the formation of RNP-complexes that serve as the functional form of the cis-active elements during the RNA replication cycle. Interestingly, point mutations in a cis-active RNA element sometime inhibit viral RNA replication at levels significantly higher than those observed with mutations that delete the element or disrupt its structure. With point mutations, it is possible that the formation of a non-functional RNP complex may further inhibit replication by sequestering replication proteins present in limiting concentrations. Examples of this point were discussed for the cre hairpin and the 3’ NTR in this review. In some cases, the formation of non-functional complexes may increase the fidelity of viral RNA replication by blocking the replication of defective viral genomes.
It is important to note that the cis-active RNA elements form multi-functional and temporally dynamic RNP complexes, which facilitate long-range interactions in the viral genome. The RNP complexes formed with the individual cis-active RNA elements interact with each other to form larger RNP complexes that are apparently stabilized by protein-protein interactions. These interactions change with each step in the replication cycle. For example, the models shown in Figs. 2–3 suggest that 5’CL-RNP complex interacts with 1) the cre-RNP complex during VPgpUpU synthesis, 2) the 3’NTR-poly(A) RNP complex during negative-strand synthesis and 3) the negative-strand 3’ end-RNP complex during positive-strand synthesis. Coordinating these long-range interactions is important in regulating the sequential steps in the viral replication cycle. Developing a better understanding of what promotes or inhibits these interactions is a challenge for the future. Clarifying the details of the protein-RNA and protein-protein interactions in the RNP complexes will be very important in further defining the molecular mechanisms that regulate viral RNA replication and in the development of new antiviral agents.
Highlights.
Cis-active elements regulate non-templated functions of viral RNA genome
Cis-active elements form RNP complexes that regulate viral RNA replication
RNP complexes mediate long-range interactions in viral RNA
5’CL-RNP complex is required in trans for VPgpUpU-primed (+) strand initiation
5’–3’ circular RNP complex regulates initiation of (−) strand synthesis
5’CL-RNP and cre-RNP interaction is required for VPgpUpU synthesis
Acknowledgements
We thank Joan Morasco for excellent technical assistance with the figures and for critically reading this review. This work was supported by Public Health Service grants (AI15539 and AI32123) from the National Institute of Allergy and Infectious Diseases and also by a grant from the American Heart Association (AHA-0555308B). Since this is a brief review, we apologize to colleagues for not discussing their findings related to this topic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Barton DJ, Black EP, Flanegan JB. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J.Virol. 1995;69:5516–5527. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton DJ, Flanegan JB. Coupled translation and replication of poliovirus RNA in vitro: Synthesis of functional 3D polymerase and infectious virus. J.Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molla A, Paul AV, Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 4.Nagashima S, Sasaki J, Taniguchi K. The 5'-terminal region of the Aichi virus genome encodes cis-acting replication elements required for positive- and negative-strand RNA synthesis. J.Virol. 2005;79:6918–6931. doi: 10.1128/JVI.79.11.6918-6931.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma N, Ogram SA, Morasco BJ, Spear A, Chapman NM, Flanegan JB. Functional role of the 5' terminal cloverleaf in Coxsackievirus RNA replication. Virology. 2009;393:238–249. doi: 10.1016/j.virol.2009.07.039. The authors show the function of the 5’ terminal cloverleaf in both negative- and positive-strand synthesis during Coxsackievirus RNA replication.
- 6.van Ooij MJ, Vogt DA, Paul A, Castro C, Kuijpers J, van Kuppeveld FJ, Cameron CE, Wimmer E, Andino R, Melchers WJ. Structural and functional characterization of the coxsackievirus B3 CRE(2C): role of CRE(2C) in negative- and positive-strand RNA synthesis. J Gen.Virol. 2006;87:103–113. doi: 10.1099/vir.0.81297-0. [DOI] [PubMed] [Google Scholar]
- 7.Barton DJ, Flanegan JB. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J.Virol. 1997;71:8482–8489. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak JE, Kirkegaard K. Coupling between genome translation and replication in an RNA virus. Genes Dev. 1994;8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 9.Morasco BJ, Sharma N, Parilla J, Flanegan JB. Poliovirus cre(2C)-dependent synthesis of VPgpUpU is required for positive- but not negative-strand RNA synthesis. J.Virol. 2003;77:5136–5144. doi: 10.1128/JVI.77.9.5136-5144.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton DJ, O'Donnell BJ, Flanegan JB. 5' cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J. 2001;20:1439–1448. doi: 10.1093/emboj/20.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma N, O'Donnell BJ, Flanegan JB. 3'-Terminal sequence in poliovirus negative-strand templates is the primary cis-acting element required for VPgpUpU-primed positive-strand initiation. J.Virol. 2005;79:3565–3577. doi: 10.1128/JVI.79.6.3565-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Wimmer E, Paul AV. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim.Biophys.Acta. 2009;1789:495–517. doi: 10.1016/j.bbagrm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steil BP, Barton DJ. Cis-active RNA elements (CREs) and picornavirus RNA replication. Virus Res. 2009;139:240–252. doi: 10.1016/j.virusres.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettersson RF, Flanegan JB, Rose JK, Baltimore D. 5'-Terminal nucleotide sequences of polio virus polyribosomal RNA and virion RNA are identical. Nature. 1977;268:270–272. doi: 10.1038/268270a0. [DOI] [PubMed] [Google Scholar]
- 15.Lee YF, Nomoto A, Detjen BM, Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc.Natl.Acad.Sci.U.S.A. 1977;74:59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wimmer E. Genome-linked proteins of viruses. Cell. 1982;28:199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]
- 17.Takeda N, Yang CF, Kuhn RJ, Wimmer E. Uridylylation of the genome-linked protein of poliovirus in vitro is dependent upon an endogenous RNA template. Virus.Res. 1987;8:193–204. doi: 10.1016/0168-1702(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 18.Toyoda H, Yang CF, Takeda N, Nomoto A, Wimmer E. Analysis of RNA synthesis of type 1 poliovirus by using an in vitro molecular genetic approach. J.Virol. 1987;61:2816–2822. doi: 10.1128/jvi.61.9.2816-2822.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKnight KL, Lemon SM. The rhinovirus type 14 genome contains an internally located RNA structure that is required for viral replication. RNA. 1998;4:1569–1584. doi: 10.1017/s1355838298981006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobert PE, Escriou N, Ruelle J, Michiels T. A coding RNA sequence acts as a replication signal in cardioviruses. Proc.Natl.Acad.Sci.U.S.A. 1999;96:11560–11565. doi: 10.1073/pnas.96.20.11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieder E, Paul AV, Kim DW, van Boom JH, Wimmer E. Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation. Journal of Virology. 2000;74:10371–10380. doi: 10.1128/jvi.74.22.10371-10380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodfellow I, Chaudhry Y, Richardson A, Meredith J, Almond JW, Barclay W, Evans DJ. Identification of a cis-acting replication element within the poliovirus coding region. Journal of Virology. 2000;74:4590–4600. doi: 10.1128/jvi.74.10.4590-4600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul AV, Rieder E, Kim DW, van Boom JH, Wimmer E. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. Journal of Virology. 2000;74:10359–10370. doi: 10.1128/jvi.74.22.10359-10370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber K, Wimmer E, Paul AV. Biochemical and Genetic Studies of the Initiation of Human Rhinovirus 2 RNA Replication: Identification of a cis-Replicating Element in the Coding Sequence of 2A(pro) J.Virol. 2001;75:10979–10990. doi: 10.1128/JVI.75.22.10979-10990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason PW, Bezborodova SV, Henry TM. Identification and characterization of a cis-acting replication element (cre) adjacent to the internal ribosome entry site of foot-and-mouth disease virus. J.Virol. 2002;76:9686–9694. doi: 10.1128/JVI.76.19.9686-9694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodfellow IG, Polacek C, Andino R, Evans DJ. The poliovirus 2C cis-acting replication element-mediated uridylylation of VPg is not required for synthesis of negative-sense genomes. J.Gen.Virol. 2003;84:2359–2363. doi: 10.1099/vir.0.19132-0. [DOI] [PubMed] [Google Scholar]
- 27.Yin J, Paul AV, Wimmer E, Rieder E. Functional dissection of a poliovirus cis-acting replication element [PV-cre(2C)]: analysis of single- and dual-cre viral genomes and proteins that bind specifically to PV-cre RNA. J.Virol. 2003;77:5152–5166. doi: 10.1128/JVI.77.9.5152-5166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul AV, Yin J, Mugavero J, Rieder E, Liu Y, Wimmer E. A "slide-back" mechanism for the initiation of protein-primed RNA synthesis by the RNA polymerase of poliovirus. J.Biol.Chem. 2003;278:43951–43960. doi: 10.1074/jbc.M307441200. [DOI] [PubMed] [Google Scholar]
- 29.Thiviyanathan V, Yang Y, Kaluarachchi K, Rijnbrand R, Gorenstein DG, Lemon SM. High-resolution structure of a picornaviral internal cis-acting RNA replication element (cre) Proc.Natl.Acad.Sci.U.S.A. 2004;101:12688–12693. doi: 10.1073/pnas.0403079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Rijnbrand R, McKnight KL, Wimmer E, Paul A, Martin A, Lemon SM. Sequence requirements for viral RNA replication and VPg uridylylation directed by the internal cis-acting replication element (cre) of human rhinovirus type 14. J.Virol. 2002;76:7485–7494. doi: 10.1128/JVI.76.15.7485-7494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayak A, Goodfellow IG, Belsham GJ. Factors required for the Uridylylation of the foot-and-mouth disease virus 3B1, 3B2, and 3B3 peptides by the RNA-dependent RNA polymerase (3Dpol) in vitro. J.Virol. 2005;79:7698–7706. doi: 10.1128/JVI.79.12.7698-7706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nayak A, Goodfellow IG, Woolaway KE, Birtley J, Curry S, Belsham GJ. Role of RNA structure and RNA binding activity of foot-and-mouth disease virus 3C protein in VPg uridylylation and virus replication. J.Virol. 2006;80:9865–9875. doi: 10.1128/JVI.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pathak HB, Ghosh SK, Roberts AW, Sharma SD, Yoder JD, Arnold JJ, Gohara DW, Barton DJ, Paul AV, Cameron CE. Structure-function relationships of the RNA-dependent RNA polymerase from poliovirus (3Dpol). A surface of the primary oligomerization domain functions in capsid precursor processing and VPg uridylylation. J.Biol.Chem. 2002;277:31551–31562. doi: 10.1074/jbc.M204408200. [DOI] [PubMed] [Google Scholar]
- 34.Pathak HB, Arnold JJ, Wiegand PN, Hargittai MR, Cameron CE. Picornavirus genome replication: assembly and organization of the VPg uridylylation ribonucleoprotein (initiation) complex. J.Biol.Chem. 2007;282:16202–16213. doi: 10.1074/jbc.M610608200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen M, Wang Q, Yang Y, Pathak HB, Arnold JJ, Castro C, Lemon SM, Cameron CE. Human rhinovirus type 14 gain-of-function mutants for oriI utilization define residues of 3C(D) and 3Dpol that contribute to assembly and stability of the picornavirus VPg uridylylation complex. J.Virol. 2007;81:12485–12495. doi: 10.1128/JVI.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen M, Reitman ZJ, Zhao Y, Moustafa I, Wang Q, Arnold JJ, Pathak HB, Cameron CE. Picornavirus genome replication. Identification of the surface of the poliovirus (PV) 3C dimer that interacts with PV 3Dpol during VPg uridylylation and construction of a structural model for the PV 3C2-3Dpol complex. J.Biol.Chem. 2008;283:875–888. doi: 10.1074/jbc.M707907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Rijnbrand R, Watowich S, Lemon SM. Genetic evidence for an interaction between a picornaviral cis-acting RNA replication element and 3CD protein. J.Biol.Chem. 2004;279:12659–12667. doi: 10.1074/jbc.M312992200. [DOI] [PubMed] [Google Scholar]
- 38.Lyons T, Murray KE, Roberts AW, Barton DJ. Poliovirus 5'-Terminal Cloverleaf RNA is required in cis for VPg uridylylation and the initiation of negative-strand RNA synthesis. J.Virol. 2001;75:10696–10708. doi: 10.1128/JVI.75.22.10696-10708.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoda H, Franco D, Fujita K, Paul AV, Wimmer E. Replication of poliovirus requires binding of the poly(rC) binding protein to the cloverleaf as well as to the adjacent C-rich spacer sequence between the cloverleaf and the internal ribosomal entry site. J Virol. 2007;81:10017–10028. doi: 10.1128/JVI.00516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andino R, Rieckhof GE, Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990;63:369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- 41.Andino R, Rieckhof GE, Achacoso PL, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5'-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsley TB, Towner JS, Blyn LB, Ehrenfeld E, Semler BL. Poly (rC) binding protein 2 forms a ternary complex with the 5'-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA. 1997;3:1124–1134. [PMC free article] [PubMed] [Google Scholar]
- 43.Pilipenko EV, Maslova SV, Sinyakov AN, Agol VI. Towards identification of cis-acting elements involved in the replication of enterovirus and rhinovirus RNAs: a proposal for the existence of tRNA-like terminal structures. Nucleic.Acids.Res. 1992;20:1739–1745. doi: 10.1093/nar/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duque H, Palmenberg AC. Phenotypic characterization of three phylogenetically conserved stem-loop motifs in the mengovirus 3' untranslated region. J.Virol. 2001;75:3111–3120. doi: 10.1128/JVI.75.7.3111-3120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saiz M, Gomez S, Martinez-Salas E, Sobrino F. Deletion or substitution of the aphthovirus 3' NCR abrogates infectivity and virus replication. J.Gen.Virol. 2001;82:93–101. doi: 10.1099/0022-1317-82-1-93. [DOI] [PubMed] [Google Scholar]
- 46.Zoll J, Heus HA, van Kuppeveld FJ, Melchers WJ. The structure-function relationship of the enterovirus 3'-UTR. Virus Res. 2009;139:209–216. doi: 10.1016/j.virusres.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Melchers WJG, Hoenderop JGJ, Bruins Slot HJ, Pleij CWA, Pilipenko EV, Agol VI, Galama JMD. Kissing of the two predominant hairpin loops in the Coxsackie B virus 3' untranslated region is the essential structural feature of the origin of replication required for negative-strand RNA synthesis. J.Virol. 1997;71:686–696. doi: 10.1128/jvi.71.1.686-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilipenko EV, Poperechny K, Maslova SV, Melchers WJG, Bruins Slot HJ, Agol VI. Cis-element,oriR, involved in the initiation of (−) strand poliovirus RNA: a quasi-globular multi-domain RNA structure maintained by tertiary ('kissing') interactions. EMBO J. 1996;15:5428–5436. [PMC free article] [PubMed] [Google Scholar]
- 49.Mirmomeni MH, Hughes PJ, Stanway G. An RNA tertiary structure in the 3' untranslated region of enteroviruses is necessary for efficient replication. J.Virol. 1997;71:2363–2370. doi: 10.1128/jvi.71.3.2363-2370.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steil BP, Barton DJ. Conversion of VPg into VPgpUpUOH before and during poliovirus negative-strand RNA synthesis. J.Virol. 2009;83:12660–12670. doi: 10.1128/JVI.01676-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown DM, Cornell CT, Tran GP, Nguyen JH, Semler BL. An authentic 3' noncoding region is necessary for efficient poliovirus replication. J Virol. 2005;79:11962–11973. doi: 10.1128/JVI.79.18.11962-11973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Todd S, Towner JS, Brown DM, Semler BL. Replication-competent picornaviruses with complete genomic RNA 3' noncoding region deletions. J.Virol. 1997;71:8868–8874. doi: 10.1128/jvi.71.11.8868-8874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waggoner S, Sarnow P. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J.Virol. 1998;72:6699–6709. doi: 10.1128/jvi.72.8.6699-6709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris KS, Xiang W, Alexander L, Lane WS, Paul AV, Wimmer E. Interaction of poliovirus polypeptide 3CDpro with the 5' and 3' termini of the poliovirus genome: Identification of viral and cellular cofactors needed for efficient binding. J.Biol.Chem. 1994;269:27004–27014. [PubMed] [Google Scholar]
- 55.Spector DH, Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc.Natl.Acad.Sci.U.S.A. 1974;71:2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarnow P. Role of 3'-end sequences in infectivity of poliovirus transcripts made in vitro. J.Virol. 1989;63:467–470. doi: 10.1128/jvi.63.1.467-470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silvestri LS, Parilla JM, Morasco BJ, Ogram SA, Flanegan JB. Relationship between poliovirus negative-strand RNA synthesis and the length of the 3' poly(A) tail. Virology. 2006;345:509–519. doi: 10.1016/j.virol.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 58.Herold J, Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Molecular Cell. 2001;7:581–591. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steil BP, Barton DJ. Poliovirus cis-acting replication element-dependent VPg Uridylylation lowers the Km of the initiating nucleoside triphosphate for viral RNA replication. J.Virol. 2008;82:9400–9408. doi: 10.1128/JVI.00427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Steil BP, Kempf BJ, Barton DJ. Poly(A) at the 3' end of positive-strand RNA and VPg-linked poly(U) at the 5' end of negative-strand RNA are reciprocal templates during replication of poliovirus RNA. J.Virol. 2010;84:2843–2858. doi: 10.1128/JVI.02620-08. The authors show that the poly(U) and poly(A) sequences in the poliovirus replication product RNAs are heterogeneous in length. In addition, they show that negative-strand synthesis initiates internally on the 3’ poly(A) tail and not at the very 3’ end.
- 61.Spear A, Sharma N, Flanegan JB. Protein-RNA tethering: the role of poly(C) binding protein 2 in poliovirus RNA replication. Virology. 2008;374:280–291. doi: 10.1016/j.virol.2007.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teterina NL, Egger D, Bienz K, Brown DM, Semler BL, Ehrenfeld E. Requirements for assembly of poliovirus replication complexes and negative-strand RNA synthesis. J Virol. 2001;75:3841–3841. doi: 10.1128/JVI.75.8.3841-3850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gamarnik AV, Andino R. Two functional complexes formed by KH domain containing proteins with the 5' noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 64.Zell R, Ihle Y, Seitz S, Gundel U, Wutzler P, Gorlach M. Poly(rC)-binding protein 2 interacts with the oligo(rC) tract of coxsackievirus B3. Biochem.Biophys.Res.Commun. 2008;366:917–921. doi: 10.1016/j.bbrc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 65.Murray KE, Barton DJ. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis. J.Virol. 2003;77:4739–4750. doi: 10.1128/JVI.77.8.4739-4750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crowder S, Kirkegaard K. Trans-dominant inhibition of RNA viral replication can slow growth of drug-resistant viruses. Nat.Genet. 2005;37:701–709. doi: 10.1038/ng1583. [DOI] [PubMed] [Google Scholar]
- 67.Harmon SA, Richards OC, Summers DF, Ehrenfeld E. The 5'-terminal nucleotides of hepatitis A virus RNA, but not poliovirus RNA, are required for infectivity. J.Virol. 1991;65:2757–2760. doi: 10.1128/jvi.65.5.2757-2760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klump WM, Bergmann I, M:uller BC, Ameis D, Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J.Virol. 1990;64:1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunner JE, Nguyen JH, Roehl HH, Ho TV, Swiderek KM, Semler BL. Functional interaction of heterogeneous nuclear ribonucleoprotein C with poliovirus RNA synthesis initiation complexes. J.Virol. 2005;79:3254–3266. doi: 10.1128/JVI.79.6.3254-3266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brunner JE, Ertel KJ, Rozovics JM, Semler BL. Delayed kinetics of poliovirus RNA synthesis in a human cell line with reduced levels of hnRNP C proteins. Virology. 2010;400:240–247. doi: 10.1016/j.virol.2010.01.031. The authors show that poliovirus replication was delayed in cells expressing low levels of hnRNPC proteins and suggest that hnRNPC functions as a cofactor for poliovirus RNA replication.
- 71. Ertel KJ, Brunner JE, Semler BL. Mechanistic consequences of hnRNP C binding to both RNA termini of poliovirus negative-strand RNA intermediates. J.Virol. 2010;84:4229–4242. doi: 10.1128/JVI.02198-09. The authors propose that the interaction of hnRNPC with the 5’ and 3’ ends of the negative-strand RNA may promote the initiation of positive-strand synthesis.
- 72. Vogt DA, Andino R. An RNA element at the 5'-end of the poliovirus genome functions as a general promoter for RNA synthesis. PLoS.Pathog. 2010;6:e1000936. doi: 10.1371/journal.ppat.1000936. The authors show that the 5’ cloverleaf on the positive-strand RNA regulates initiation of both negative- and positive-strand synthesis.