Abstract
mTOR kinase inhibitors block mTORC1 and mTORC2 and thus do not cause the mTORC2 activation of AKT observed with rapamycin. We now show, however, that these drugs have a biphasic effect on AKT. Inhibition of mTORC2 leads to AKT S473 dephosphorylation and a rapid but transient inhibition of AKT T308 phosphorylation and AKT signaling. However, inhibition of mTOR kinase also relieves feedback inhibition of RTKs leading to subsequent PI3K activation and rephosphorylation of AKT T308 sufficient to reactivate AKT activity and signaling. Thus, catalytic inhibition of mTOR kinase leads to a new steady state characterized by profound inhibition of mTORC1 and accumulation of activated AKT phosphorylated on T308 but not S473. Combined inhibition of mTOR kinase and the induced RTKs fully abolishes AKT signaling and results in profound cell death and tumor regression in vivo. These findings reveal the adaptive capabilities of oncogenic signaling networks and the limitations of monotherapy for inhibiting feedback-regulated pathways.
Keywords: mTOR kinase, feedback, AKT regulation
INTRODUCTION
Dysregulation of PI3K signaling is a common event in human cancer and mutations in multiple components of the pathway have been identified (1–3). Activation of PI3K signaling in tumors is responsible for key features of the transformed phenotype, suggesting that inhibition of the pathway could be a useful therapeutic strategy (1, 4). The mTOR protein kinase is an important component of the PI3K/AKT pathway that controls cell proliferation, size and metabolism by integrating the effects of growth factors and the availability of nutrients and of energy. mTOR exists in two complexes: mTORC1 and mTORC2. mTORC1 contains the mTOR, Raptor, mLST8/GβL and PRAS40 proteins and controls cell size and protein translation via its two major substrates p70S6K and 4E-BP1 (5, 6). Activated S6 kinase causes feedback inhibition of IGF-1/Insulin signaling by phosphorylating insulin receptor substrate 1 (IRS-1) and causing its degradation (7). The mTORC2 complex contains mTOR, Rictor, mSin1, Protor and mLST8/GβL and is also activated in response to growth factor stimulation (8). mTORC2 has been shown to phosphorylate AKT and SGK1 in a conserved hydrophobic domain (9, 10). Phosphorylation of AKT at the Serine 473 site (S473) by mTORC2 enhances the catalytic activity of AKT already phosphorylated on Threonine 308 (T308) (11). Thus, mTOR complexes function both upstream and downstream of AKT (12).
Inhibitors of PI3K, AKT and mTOR are currently being developed as potential therapeutics for tumors in which the pathway is dysregulated (13, 14). Initial studies have focused on inhibition of mTORC1 with the natural product rapamycin. Rapamycin binds to FKBP-12 and the complex binds to and causes the allosteric inhibition of mTORC1, suppressing CAP-dependent protein translation and, in model systems, inhibition of cell proliferation and tumorigenesis (15–17). In patients, rapamycin has been shown to have therapeutic activity in renal cell carcinoma, neuro-endocrine tumors and other cancers (18). However, significant therapeutic responses rarely occur in tumors in which mutations that activate PI3K/AKT signaling are prevalent such as in prostate and breast cancer and glioblastoma (19, 20).
We and others have observed that while rapamycin effectively inhibits S6K phosphorylation, it also induces AKT S473 phosphorylation and AKT activity in tumors in model systems and in patients as well (16, 21, 22). Physiologic activation of PI3K/AKT signaling is regulated by mTOR-dependent feedback inhibition of IRS expression and, consequently, IGF-1R/Insulin receptor signaling (7). Rapamycin relieves this feedback and induces AKT S473 phosphorylation in an mTORC2-dependent manner leading to AKT activation, which may attenuate its therapeutic effects (16, 23). In response to this problem, ATP-competitive inhibitors of mTOR kinase that potently inhibit both mTORC1 and mTORC2 complexes have now been developed. It has been hypothesized that such inhibitors will have greater antitumor activity than rapamycin because they inhibit mTORC2 and will therefore prevent feedback induction of AKT which may also directly affect its activity against certain substrates (14). Furthermore, this class of compounds has also been shown to inhibit mTORC1 more potently than does rapamycin (24).
We have now tested these assertions with the selective ATP-competitive mTOR kinase inhibitor AZD8055 (25). This drug inhibits 4E-BP1 phosphorylation more effectively than rapamycin. It also effectively inhibits mTORC2 and AKT S473 phosphorylation, which leads to AKT T308 dephosphorylation and inhibition of AKT activity and downstream signaling. However, these latter effects are transient. mTOR kinase inhibition also causes marked activation of receptor tyrosine kinase signaling (RTK), which induces PI3K signaling, reinduction of T308 phosphorylation and, despite persistent inhibition of mTORC2 activity and AKT S473 phosphorylation, reactivates AKT activity and signaling.
RESULTS
AZD8055 is a potent inhibitor of mTORC1 and mTORC2 complexes
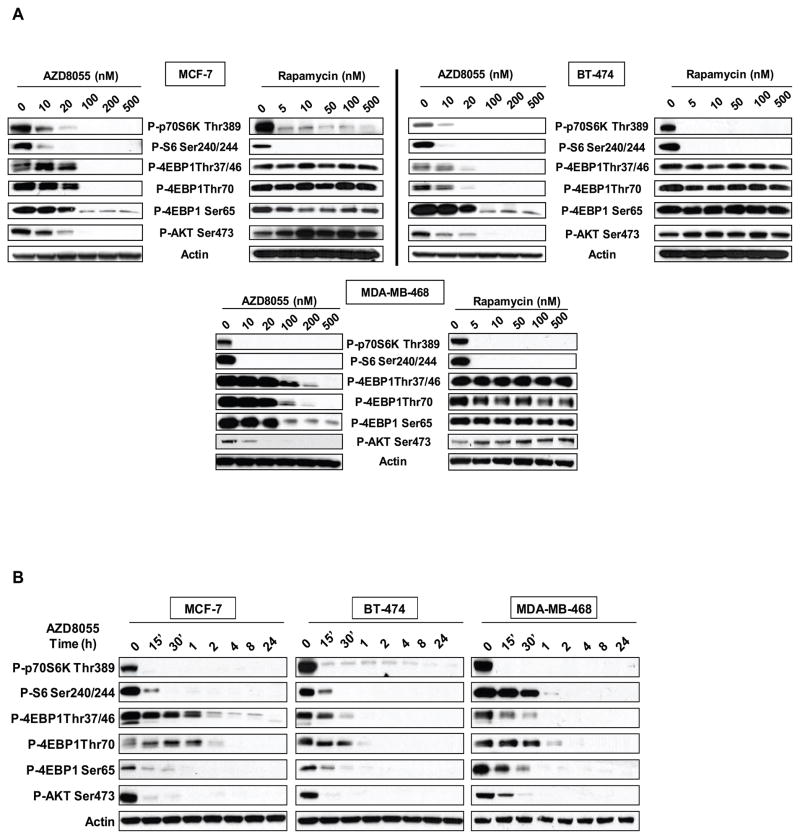
mTOR kinase inhibitors have now been developed and shown to effectively inhibit mTORC1 and mTORC2 (12, 24–27). AZD8055 is an ATP-competitive inhibitor of mTOR kinase that inhibits the enzyme with a Ki of 1.3 nM in vitro (25) and inhibits S6K and 4E-BP1 phosphorylation in cells with IC50’s of 10 nM and 100 nM respectively (data not shown). AZD8055 is selective, in that it displayed a potency of more than a thousandfold against all related kinases (25). In Figure 1A, the effects of AZD8055 on mTORC1 and mTORC2 signaling were compared with those elicited by rapamycin in three breast cancer cell lines with different mechanisms of activation of the PI3K pathway— BT-474 (HER2 amplified, PI3K mutant), MCF-7 (PI3K mutant), and MDA-MB-468 (EGFR amplified, PTEN deficient). Inhibition of mTORC1 with rapamycin potently inhibits the phosphorylation of p70S6 kinase and its substrate S6, but only poorly inhibits 4E-BP1 phosphorylation as has been previously described (28, 29). In contrast, AZD8055 potently inhibits both S6K and 4E-BP1 phosphorylations, although more drug (Figure 1A) and time (Figure 1B) are required to inhibit the latter. As reported previously, rapamycin does not inhibit mTORC2 and instead induces AKT S473 phosphorylation due to relief of feedback of IGF1-R signaling (16, 22). In contrast, AZD8055 potently and rapidly inhibits S473 phosphorylation and, therefore, despite inhibiting S6K phosphorylation, prevents the induction of S473 phosphorylation that results from relief of mTORC1-dependent negative feedback. The inhibition of the phosphorylation of these mTORC1 and mTORC2 substrates with AZD8055 was sustained for at least twenty-four hours (Figure 1B). We conclude that AZD8055 is a potent inhibitor of both mTORC1 and mTORC2.
Figure 1. AZD8055 is a potent inhibitor of mTORC1 and mTORC2 signaling.
(A) Immunoblot analysis was performed on mTORC1 and mTORC2 effectors after treatment with increasing concentrations of AZD8055 or rapamycin for four hours in MCF-7, BT-474 and MDA-MB-468 breast cancer cells. (B) The same cell lines were treated with 500nM of AZD8055 and collected at indicated times and analyzed by immunoblotting.
mTOR kinase inhibition transiently inhibits AKT T308 phosphorylation and AKT function
PI3K activation causes the PIP3-dependent membrane localization of AKT and PDK1 where the latter is responsible for phosphorylation of AKT T308 (30). AKT T308 phosphorylation is required for AKT kinase activity, which is further enhanced by phosphorylation of S473 by mTORC2 (11). It has been proposed that phosphorylation of S473 stabilizes T308 phosphorylation and thereby enhances AKT catalytic activity (9, 31). In BT-474, MDA-MB-468 and MCF-7 cells, AZD8055 inhibits AKT T308 phosphorylation within one hour of treatment (Figure 2A and B, panel 1 and Supplemental Figure 1 for MCF-7 cells). Phosphorylation of T308 falls in parallel with that of the mTOR substrates AKT S473, S6K and 4E-BP1. These findings are consistent with data obtained with other mTOR kinase inhibitors (12, 24, 27). The phosphorylation of AKT substrates GSK3-β, FOXO1/3, and PRAS40 declines at one hour as well, suggesting that dephosphorylation of AKT in response to mTOR kinase inhibition results in the inhibition of AKT kinase activity. Phosphorylation of S6K, AKT S473, and 4E-BP1 at S65 and T70 remain inhibited for at least twenty-four hours after drug addition, showing that mTOR kinase inhibition persists over this period. However, phosphorylation of AKT at the T308 site and of the AKT substrates GSK3-β, FOXO1/3, and PRAS40 rebound four hours after drug addition and reach pre-treatment levels eight to sixteen hours later (Figures 2A and B, panel 1). The phosphorylation of FOXO is markedly enhanced compared to pretreatment levels. These data imply that inhibition of AKT in response to mTOR kinase inhibition is transient, despite continued inhibition of S473 phosphorylation. 4E-BP1 phosphorylation on T37/T46 also rises slightly compared to its nadir reaching a new steady state between eight and twenty-four hours after drug addition. Another mTOR kinase inhibitor, PP242, also caused transient inhibition of AKT T308 and AKT substrates phosphorylation suggesting that this is a general property of these drugs (Supplemental Figure 2) (32).
Figure 2. The mTOR kinase inhibitor leads to persistent inhibition of AKT S473 phosphorylation but transient inhibition of AKT effectors.
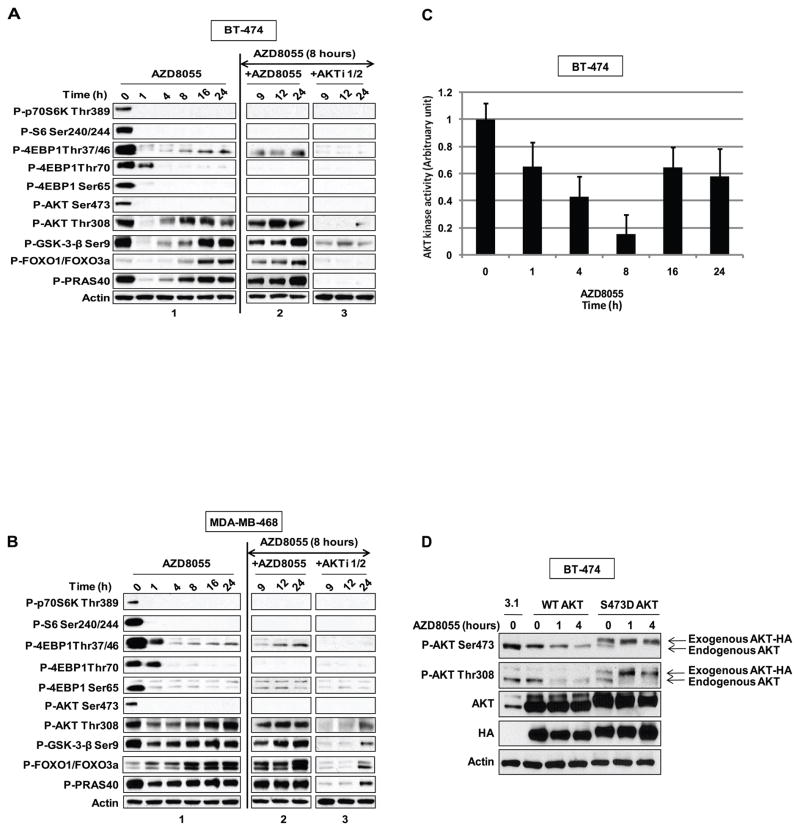
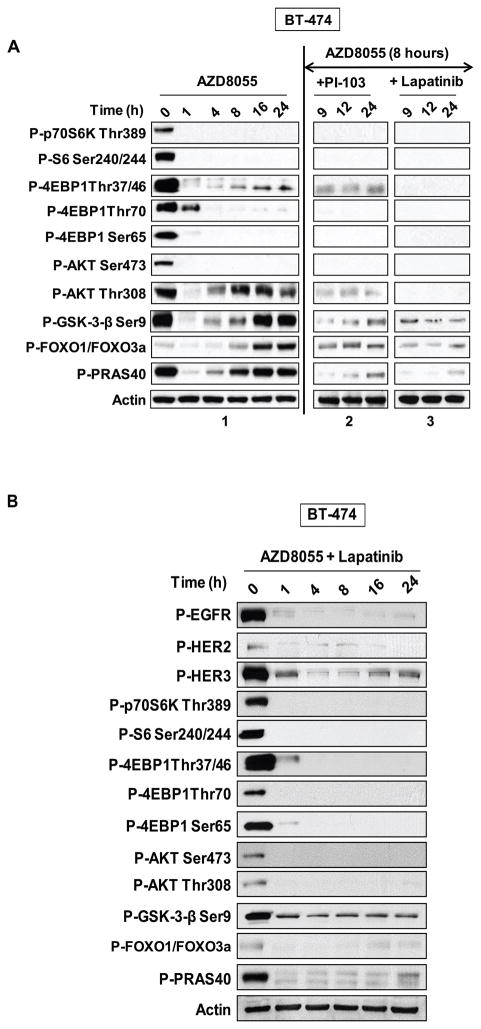
(A) BT-474 cells and (B) MDA-MB-468 cells were treated with 500nM of AZD8055 and collected at the indicated times and lysates were immunoblotted with indicated antibodies (panel 1) (see also Figure S1). After eight hours of AZD8055 treatment, the cells were treated with either 500nM of AZD8055 (panel 2), or 1μM of an AKT inhibitor (panel 3). Each inhibitor was added for one additional hour (indicated as time 9), four extra hours (indicated as time 12) or sixteen extra hours (indicated as time 24). Cell lysates were immunoblotted with the indicated antibodies (see also Figure S2). (C) BT-474 cells treated with 500nM of AZD8055 were collected at the indicated times and AKT kinase activity was performed; the results were quantified by densitometry. Error bars indicate standard error. (D) BT-474 cells were transfected with pcDNA3.1, HA-AKT Wild-Type (WT) or HA-S473D AKT plasmids and were treated for the indicated times with 2μM of AZD8055. Lysates were subjected to immunoblot analysis for P-AKT S473, T308, total AKT, HA and actin. The arrows refer to the exogenous and endogenous AKT species in the S473D transfectant.
Reactivation of AKT signaling could be due to a fall in drug concentration in the cell or to establishment of a new steady state of the signaling network with higher levels of AKT activity. To distinguish between these possibilities, either AZD8055 or a selective allosteric inhibitor of AKT1 and 2 was added to BT-474 (Figure 2A, panels 2 and 3) and MDA-MB-468 cells (Figure 2B, panels 2 and 3) eight hours after exposure of the cells to AZD8055. Re-addition of AZD8055 had essentially no effect; phosphorylation of AKT T308, AKT substrates and 4E-BP1 T37/46 remained elevated. In contrast, phosphorylation of AKT T308, GSK3-β, FOXO1/3, and PRAS40 were all-sensitive to the AKT inhibitor. This suggests that the increased phosphorylation of AKT substrates is due to reactivation of AKT. The residual phosphorylation of 4E-BP1 T37/46 was also sensitive to AKT, but not to mTOR kinase inhibition, suggesting that there may be AKT-dependent, but mTOR-independent signals that regulate phosphorylation of this site. These data and the persistent suppression of AKT S473 and S6K phosphorylation suggest that the reinduction of phosphorylation of AKT substrates is not due to decreased levels of drug in the cells. Furthermore, these data suggest that reinduction is due to reactivation of AKT and not another kinase.
To confirm that the rapid inhibition and subsequent reinduction of phosphorylation of AKT substrates is due to changes in AKT activity, we performed in vitro AKT kinase assays on immunoprecipates from cells treated with AZD8055 for up to twenty-four hours. AKT kinase activity declines within one hour of drug addition, reaches a nadir of fifteen percent of baseline at eight hours, and then rises to sixty percent of baseline by twenty-four hours after drug addition (Figure 2C).
The biphasic inhibition and subsequent mTOR-independent reactivation of AKT is likely due to parallel changes in T308 phosphorylation. In order to determine whether the initial rapid decline in T308 phosphorylation was due to the inhibition of mTORC2-dependent S473 phosphorylation, we utilized the AKT S473D mutant, which mimics constitutive phosphorylation of the site. BT-474 cells transfected with either AKT wild-type (WT) or AKT S473D were treated with AZD8055 for one or four hours. Phosphorylation of endogenous AKT S473 (lower band) falls within one hour of drug treatment in both transfectants (Figure 2D). As expected, the binding of the anti-phospho 473 antibody to the S473D mutant (upper band) is unaffected by the drug treatment, confirming that the aspartate substitution is phosphomimetic. Drug treatment also caused the rapid inhibition of T308 phosphorylation of endogenous WT AKT in both transfectants. However, T308 phosphorylation of the AKT S473D mutant (upper band) does not decline; in fact, it increases after drug treatment. These data support the work of others that suggests that inhibition of AKT S473 phosphorylation causes a decline in T308 phosphorylation (9, 27, 32). The rapid induction of T308 phosphorylation in mutant S473D confirms the conclusion that this induction is not due to declining intracellular drug concentrations. The rapid loss of T308 phosphorylation in WT AKT and rise in AKT S473D mutant suggest that, in these cells, two separate processes account for the decline and subsequent reinduction of T308 phosphorylation and AKT activity after mTOR kinase inhibition.
mTOR kinase inhibition leads to activation of PI3K
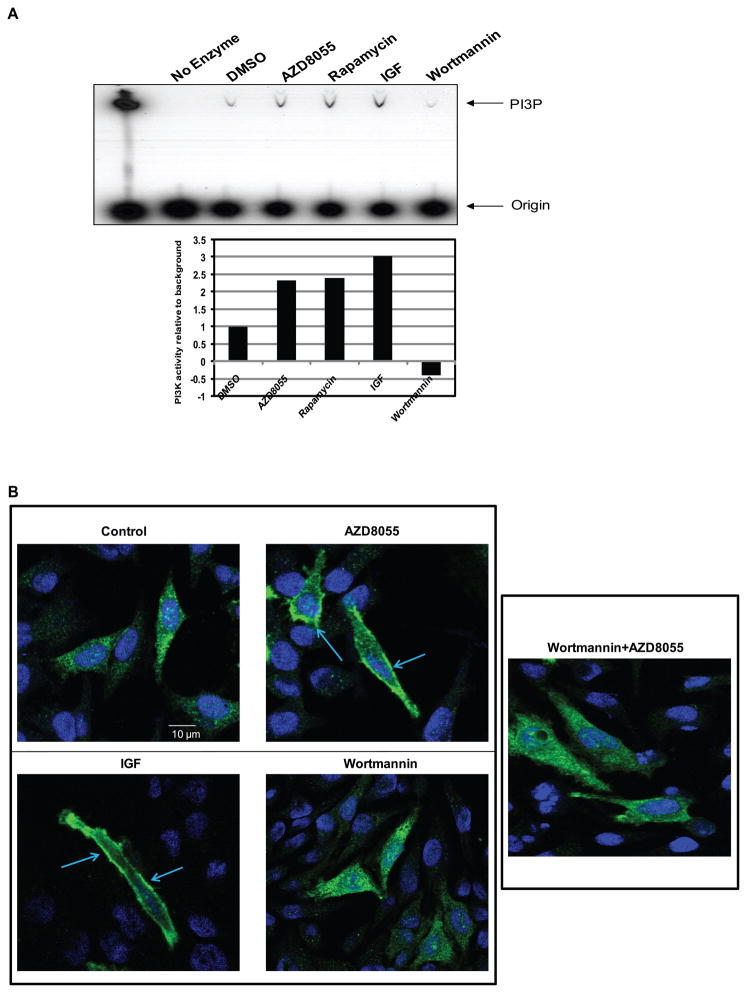
Phosphorylation of T308 is due to PI3K-dependent localization of PDK1, the T308 kinase, to the membrane. We asked whether the initial loss of T308 phosphorylation is counteracted by PI3K activation (Figure 3A). The p85 regulatory subunit of class 1 PI3K was immunoprecipitated from lysates of cells treated for four hours with drug and in vitro PI3K assays were performed on the precipitates in the presence of 32P-gamma-labeled ATP and phosphatidylinositol. Phosphatidylinositol 3-phosphate (PI3P, a product of PI3K) was significantly induced by IGF-1 and inhibited by the PI3K inhibitor wortmannin. Rapamycin and AZD8055 both significantly induced PI3K activity by more than two-fold (Figure 3A, lower panel). To investigate further whether the increase in the in vitro kinase activity is associated with increased intracellular levels of PIP3, we utilized an intracellular reporter assay in HeLa cells. The reporter is a fusion protein comprised of the AKT PH domain fused to the amino-terminus of GFP (33). PIP3 binding to the PH-domain causes the fusion protein to associate with the plasma membrane. In control cells, the PH-GFP fusion protein is largely cytoplasmic and translocates to the membrane after IGF-1 stimulation of PI3K signaling (Figure 3B). Treatment of cells with AZD8055 also causes a marked translocation of the reporter to the membrane within four hours of its addition that was prevented by pre-treatment with the PI3K inhibitor wortmannin. Thus, AZD8055 rapidly activates PI3K activity in cells and this causes induction of PIP3 levels sufficient to translocate PH-domain binding proteins to the membrane.
Figure 3. kinase inhibition leads to PI3K activation.
(A) MCF-7 cells were treated with 500nM of AZD8055, 50nM of rapamycin, or 100nM of wortmannin for four hours; or 60 ng/mL of IGF for ten minutes and a PI3K activity assay was performed. The product, PI3P, was resolved by thin layer chromatography and detected by autoradiography. A lane with a purified PI3K enzyme was used as a positive control and a lane without enzyme was used as a negative control for the assay. The results were quantified by densitometry (lower panel). (B) The pcDNA3-AKT-PH-GFP vector was transfected into HeLa cells. Twenty-four hours after the transfection, the cells were treated with either DMSO, 500nM of AZD8055, 100nM of wortmannin or combination of wortmannin and AZD8055 (wortmannin was added thirty minutes prior to AZD8055 for the combination treatment) for four hours or with 60 ng/mL of IGF for ten minutes. The GFP signal was detected using confocal microscopy. Shown are representative cells.
mTOR kinase inhibition activates RTKs
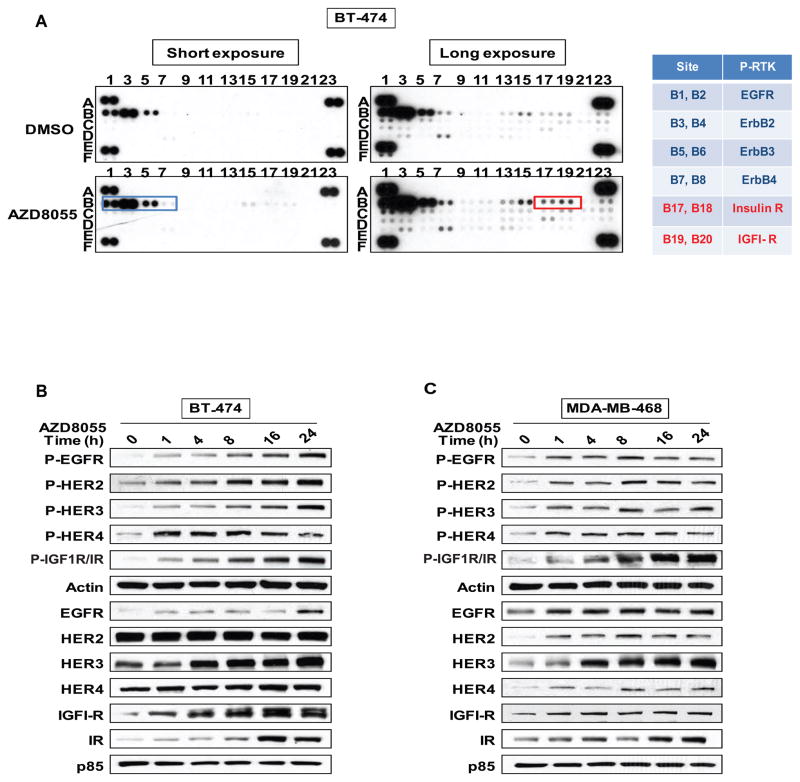
We have previously observed that mTORC1 inhibition leads to activation of upstream receptor tyrosine kinase signaling (16). Moreover, we and others have recently shown that PI3K and AKT inhibition induce expression and activation of multiple RTKs (34–36). We, therefore, hypothesized that induction of PI3K activation by AZD8055 is mediated in part by growth factor receptor activation. An array of forty-two anti-phosphotyrosine receptor antibodies was used to assess whether RTK phosphorylation levels were induced in breast cancer cell lines after their exposure to the drug (Figure 4A for BT-474 cells and Supplemental Figure 3 for MDA-MB-468 and MCF-7 cells). As shown in Figure 4A, phosphorylation of multiple RTKs was induced, including members of the HER kinase (EGFR, HER2, 3, 4), IGF-1R, Insulin receptor, and FGFR1-3 families. Induction occurred in all three models— BT-474, MCF-7 and MDA-MB-468. To confirm the increase in the levels of phosphorylated receptor, lysates of BT-474 and MDA-MB-468 cells treated with AZD8055 were analyzed by immunoblotting. The phosphorylation of EGFR family members and IGF-1R/Insulin receptor kinases was induced within one hour of exposure of cells to AZD8055 and persisted for twenty-four hours (Figures 4B and 4C). In BT-474 cells, in which HER2 is expressed at very high levels, we observed induction of both expression and phosphorylation of RTKs with greater induction of phosphorylation than expression (Figure 4B). A similar effect was observed in MDA-MB-468 cells, with levels of P-HER3 increasing five-fold by twenty-four hours after drug addition (P-EGFR— two-fold increase, P-HER4— two-fold and P-IGF1R/IR— six-fold) (Figure 4C).
Figure 4. mTOR kinase inhibition leads to RTK activation.
(A) BT-474 cells were treated for four hours with either DMSO, 500nM of AZD8055 and phosphorylated levels of different RTKs were assessed by immunoblotting cell lysates applied directly to the AB-bound membranes (R&D systems). Each spot (in duplicate) corresponds to a specific P-RTK (see also Figure S3). (B) Immunoblots analysis were 28 performed on the EGF receptor family members and IGF1-R/IR in BT-474 cells and (C) in MDA-MB-468 cells after treatment with 500nM of AZD8055.
AKT reactivation is dependent on HER kinase activation of PI3K
Reinduction of AKT signaling after its initial inhibition in AZD8055-treated cells is accompanied by an increase in both PI3K and RTK activity. Addition of a class I PI3K inhibitor (PI-103) blocks reinduction of AKT T308 and AKT substrate phosphorylation in BT-474 (Figure 5A, panel 2) and MDA-MB-468 cells (Supplemental Figure 4, panel 2) that had been pretreated with AZD8055 for eight hours. BT-474 and MDA-MB-468 express high levels of HER2 and EGFR, respectively, due to gene amplification. The HER2-predominant HER kinase inhibitor lapatinib suppresses AKT signaling when added eight hours after exposure of BT-474 cells to mTOR kinase inhibition (Figure 5A, panel 3). Gefitinib, an EGR-predominant HER kinase inhibitor, has similar effects in MDA-MB-468 cells (Supplemental Figure 4, panel 3). Thus, in breast tumor cells in which mTOR kinase is inhibited, AKT signaling is dependent on the activation of upstream RTKs.
Figure 5. mTOR kinase inhibition-induced reactivation of AKT substrates is HER2 and PI3K dependent.
(A) BT-474 cells were treated with 500nM of AZD8055 and collected at the indicated times and lysates immunoblotted with indicated antibodies (panel 1 is the same as Figure 2A panel 1). After eight hours of AZD8055 treatment, the cells were treated with either 1μM of PI-103 (panel 2), or 200nM of lapatinib (panel 3 in A). Each inhibitor was added for one additional hour (indicated as time 9), four extra hours (indicated as time 12) or sixteen extra hours (indicated as time 24) and the lysates were immunoblotted with the indicated antibodies (see also Figure S4). (B) BT-474 cells were treated simultaneously with both 500nM AZD8055 and 1μM of lapatinib and were collected at the indicated times and lysates immunoblotted with indicated antibodies.
In the steady state more than eight hours after mTOR kinase inhibition, breast tumor cells are characterized by high levels of RTK phosphorylation and PI3K activity, phosphorylation of AKT T308, but not S473, phosphorylation of AKT substrates, and profound mTORC1 inhibition. To model the consequences of mTOR kinase inhibition in cells in which the relief of RTK feedback does not occur, we treated BT-474 cells with AZD8055 and lapatinib at the same time. We observed that the phosphorylation of EGFR, HER2 and HER3 was inhibited, and reinduction of AKT T308 and AKT substrates phosphorylation did not occur (Figure 5B). In these cells, chronic mTOR kinase inhibition is characterized by potent inhibition of both mTORC1 and AKT signaling. The data support the hypothesis that the effects of mTOR kinase inhibition will vary as a function of the degree of reactivation of upstream signaling.
Combined inhibition of the mTOR and AKT kinases induces tumor cell death
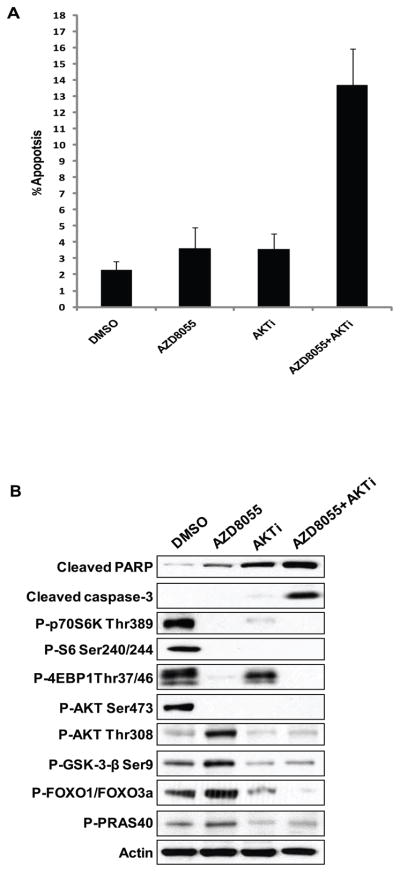
Reinduction of AKT activity in tumors treated with mTOR kinase inhibitors may attenuate the biologic and therapeutic effects of these drugs. To test this hypothesis, BT-474 cells were treated with AZD8055, an AKT inhibitor, or the combination for forty-eight hours. As seen in Figure 6A, the individual treatments had almost no effect on cell death (subG1 fraction) at 48 hours; however, the combination of both treatments greatly increased the level of apoptotic cells and the levels of cleaved PARP and cleaved caspase-3 (Figure 6B). Furthermore, the combination of both treatments inhibited the reinduction of AKT substrates due to mTOR kinase inhibition. These data support the hypothesis that restoration of AKT signaling helps to maintain cell survival under conditions in which mTOR kinase signaling is inhibited.
Figure 6. Addition of an AKT inhibitor to AZD8055 promotes apoptosis in BT-474 cells.
(A) BT-474 cells were treated with either 500nM of AZD8055 or 2μM of an AKT inhibitor or the combination for forty-eight hours. The fraction of apoptotic cells (sub-G1) was determined by flow cytometry. (B) BT-474 cells were collected after twenty-four hours of treatment and the lysates were analyzed by immunoblotting.
HER kinase inhibition enhances the antitumor activity of AZD8055 in vivo
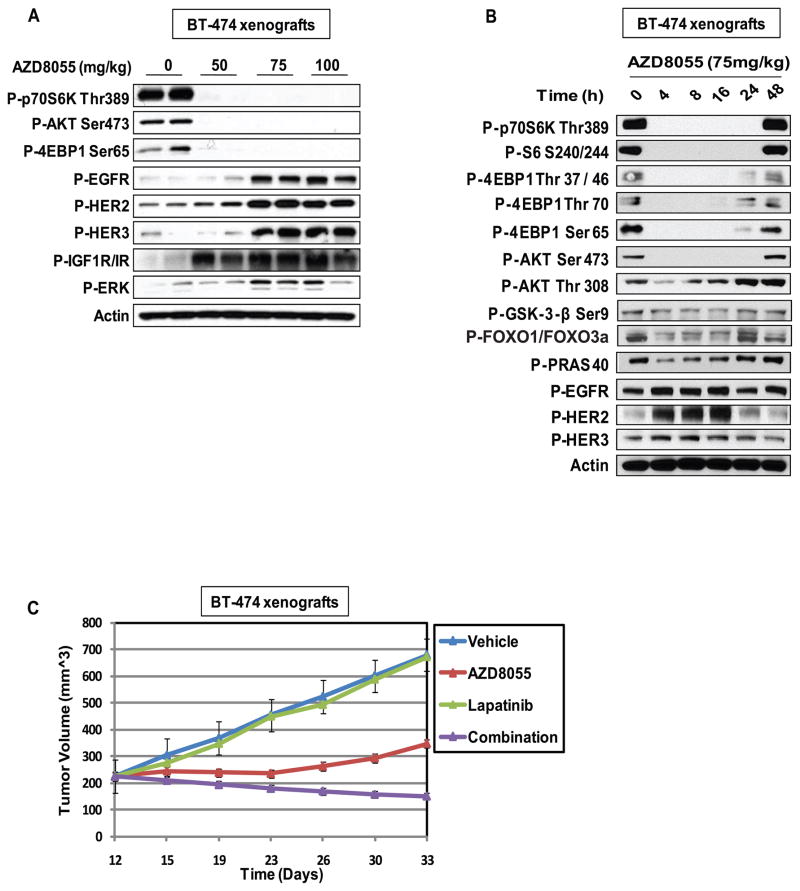
We previously showed that reactivation of AKT signaling might be in part responsible for the modest antitumor activity of mTORC1 inhibitors in patients (16). This may be the case for mTOR kinase inhibitors as well, even though they potently inhibit mTORC1 and mTORC2 (25, 26). We found that the maximal tolerated dose (MTD) of AZD8055 in mice is 150mg/kg, twice per week (data not shown). To determine if the induction of upstream RTKs in vitro could be observed in vivo; mice bearing BT-474 xenografts were treated for four hours with different concentrations of AZD8055. The mTOR kinase substrates S6K, 4E-BP1 and AKT S473 were maximally dephosphorylated in response to 75mg/kg of AZD8055 (Figure 7A). At this dose, there was a concomitant induction of the EGFR, HER2, HER3 and IGF-1/IR receptors and ERK phosphorylation. In mice, we have found that the regimen of AZD8055 that is most effective for antitumor therapy is 75mg/kg, three times per week (data not shown). In BT-474 xenografts treated with a single dose of 75mg/kg of AZD8055 (Figure 7B), we observed that AZD8055 effectively inhibited the phosphorylation of mTORC1 and mTORC2 substrates for at least twenty-four hours, but the effect was largely gone by forty-eight hours. As observed in tissue culture experiments (Figure 2, panel 1); phosphorylation of AKT T308 and the AKT substrates GSK3-β, FOXO1/3, and PRAS40 were initially inhibited (within four hours of treatment) and fall in parallel with that of the mTOR kinase substrates. However, we observed a subsequent increase in their phosphorylation eight hours after drug addition. Induction of phosphorylation of the EGFR, HER2 and HER3 also occurs in vivo at four hours. The phosphorylation of HER2 and EGFR but not HER3 decline after sixteen hours of drug exposure, after reactivation of AKT signaling. Of note, AKT T308 phosphorylation remains elevated at twenty-four hours despite loss of HER2 phosphorylation. This suggests that PI3K activity remains elevated, perhaps via activation of other HER3 or other receptors. In sum, the data suggest that chronic inhibition of mTOR kinase in vivo leads to a new steady state with persistent inhibition of mTORC1, activated AKT phosphorylated on T308 but not S473, and enough PI3K activation to support T308 phosphorylation.
Figure 7. mTOR kinase inhibition-induced reactivation of AKT substrates and RTKs in vivo and can be blocked by addition of a HER kinase inhibitor.
(A) Mice bearing BT-474 tumors were treated with increasing doses of AZD8055 for four hours; the samples were immunoblotted with the indicated antibodies. (B) Mice bearing BT-474 tumors were treated with 75mg/kg of AZD8055 for the indicated times; the samples were immunoblotted with the indicated antibodies. (C) Mice bearing BT-474 tumors were randomized to vehicle, AZD8055 (75mg/kg) three times per week, lapatinib (150mg/kg), three times per week or the combination for three weeks. Tumor size was measured twice per week. The results are presented as the mean tumor volume ± SEM (n = 5 mice/group).
To test whether inhibition of reactivated HER kinases sensitized the tumors to mTOR kinase inhibition; we evaluated the effects of combining AZD8055 with lapatinib on the growth of BT-474 xenografts (Figure 7C). We used a low dose of lapatinib administered three times weekly that had no antitumor activity when administered alone in order to distinguish sensitization of the tumor to mTOR kinase inhibition from additive activity of the two drugs. Chronic AZD8055 treatment causes complete arrest of tumor growth with little or no evidence for regression. After eleven days of treatment; the tumors began to re-grow, but more slowly than the controls. In contrast, combined treatment with AZD8055 and lapatinib caused persistent inhibition of growth over three weeks of treatment and was associated with thirty-five percent regression of the tumor.
DISCUSSION
AKT and mTOR are key enzymes controlling major cellular processes including cellular growth and metabolism; they each have been shown to regulate the activity of the other (12). We have now shown that the selective mTOR kinase inhibitor AZD8055 is an effective inhibitor of both mTORC1 and mTORC2 activity but has complex effects on AKT signaling. It potently inhibits both S6K and 4E-BP1 phosphorylation in cells, confirming that it is a better mTORC1 inhibitor than rapamycin; also, AZD8055 completely inhibits the phosphorylation of AKT S473, consistent with its efficient inhibition of mTORC2 as well. Loss of AKT S473 phosphorylation is accompanied by concomitant inhibition of AKT T308 phosphorylation and kinase activity and causes decreased phosphorylation of multiple AKT substrates. Some of these results were predicted from Rictor knockdown experiments, in which AKT T308 phosphorylation was shown to be inhibited along with that of S473 (9) and have been obtained with other mTOR kinase inhibitors as well (27, 32). They suggest that inhibition of mTORC2 will lead to the dephosphorylation of AKT at the T308 site and would lead to a more profound inhibition of AKT function than would be expected from dephosphorylation of AKT S473 alone. Thus, mTOR kinase inhibition should prevent the feedback activation of AKT signaling that has attenuated the response of patients with rapamycin therapy.
However, in tumor cells exposed to the drug, even though mTORC2 inhibition is potent and persistent, inhibition of phosphorylation of AKT T308 and of AKT substrates is only transient, occurring very quickly and then, four to eight hours after target inhibition, rising to baseline or higher than baseline levels. We show that this new steady state is due to reactivation of AKT after initial inhibition and not to a decrease in drug concentration in the cells. Reinduction of phosphorylation of AKT T308 and of AKT substrates is sensitive to AKT inhibition, but not to re-addition of the mTOR kinase inhibitor. Our data demonstrate that this reinduction is due to hyperactivation of PI3K. The induction of PI3K activation is due to the relief of feedback inhibition of RTK signaling. Although we have shown that AZD8055 activates RTK signaling more potently that rapamycin, the increase in PI3K activity observed with the two drugs is equivalent. It is not clear whether other factors play a role in limiting PI3K activation or that the in vitro kinase assays do not accurately reflect degree of induction of intracellular kinase activity. In tumors in which HER kinases are dysregulated, receptor blockade with tyrosine kinase inhibitors prevents reinduction of AKT T308 and AKT substrate phosphorylation. Taken together, our findings and those of others suggest the mechanisms that underlie the biphasic effects of mTOR kinase inhibitors (27, 32). Inhibition of mTORC2 leads to rapid inhibition of AKT S473 phosphorylation with attendant destabilization of phosphorylation at the T308 site. Release of feedback inhibition of receptor tyrosine kinase signaling function leads to activation of PI3K with the release of PIP3 which increases both PDK1 and AKT partition to the membrane and thus increases the rate of AKT T308 phosphorylation (37). The loss and then the reinduction of T308 phosphorylation and AKT activity are the result of these two opposing effects. This is supported by our data; in cells expressing the AKT S473D mutant, AZD8055 causes a rapid monophasic rise in T308 phosphorylation that is not preceded by a decline (Figure 2D). In contrast, in cells in which relief of RTK feedback is inhibited, AZD8055 causes stable inhibition of phosphorylation of T308 without rebound (Figure 5B).
In cells in which mTOR kinase inhibitors relieve feedback inhibition of receptor tyrosine kinase leading to activation of PI3K, the result is a new steady state in which mTORC1 is potently inhibited and AKT is phosphorylated on T308 but not on the S473 site. This AKT species is activated and able to phosphorylate key substrates in the cell (Supplemental Figure 5). Whether the activity of AKT monophosphorylated on the T308 site differs from that of AKT phosphorylated on both residues in the range or intensity of substrate phosphorylation remains to be determined. Previously, selective deletion of mTORC2 activity in MEFs with Rictor and mLST8 knockouts has been used to show that phosphorylation of most AKT substrates is mTORC2 independent but that phosphorylation of FOXO proteins depends on intact mTORC2 activity (38). Of note, we show here that phosphorylation of multiple AKT substrates including FOXO declines and then rises with phosphorylation of AKT T308 showing that in this system, AKT T308 phosphorylation is enough to activate phosphorylation of AKT substrates, including FOXO.
The basis for the different effects of pharmacologic and genetic ablation of mTORC2 inhibition on FOXO-phosphorylation is unknown, but could have to do with the different cell types utilized in the studies. Our data show that mTOR kinase inhibition does initially inhibit AKT activity, but this inhibition is limited by relief of feedback inhibition of receptor tyrosine kinases, leading to induction of PI3K activity. The induction of PI3K activation is likely to be dependent on which receptor tyrosine kinases are activated and whether their ligands are present. It is conceivable that in certain lineages, feedback reactivation of receptor tyrosine kinases is weak or occurs in contexts in which ligands are not available. In such cases, mTOR kinase inhibition will lead to inhibition of AKT activity as well as inhibition of mTORC1 activity. In tumors in which mTORC1 inhibition leads to relief of RTK feedback, in the steady state, mTORC1 will be inhibited, but AKT, after initial inhibition, will be reactivated.
Emerging evidence suggests that dysregulated activation of onco-proteins leads to extensive feedback throughout the signaling network. We and others have partially characterized the relief of negative feedback induced by modest mTORC1 inhibition with rapamycin or the potent and selective inhibition of AKT (16, 35, 36). The results are consistent with a model in which activation of AKT by receptors causes the coordinate feedback inhibition of receptor tyrosine kinase signaling and expression by mTOR and FOXO-dependent mechanisms (35). mTOR activation causes the downregulation of IRS1 and other signaling intermediates and inhibition of the HER and IGF1-R/Insulin receptor tyrosine kinases as well (7). Inhibition of FOXO-transcription factors by AKT-dependent phosphorylation downregulates the expression of HER3, IGF1-R, and Insulin receptors (35).
AKT inhibition coordinately relieves this feedback, inhibits mTOR, activates FOXO function, and causes the induction of the expression and activity of HER3, IGF1-R/Insulin receptor and other receptors. Rapamycin relieves feedback differently; inhibition of mTORC1 also induces receptor activation and IRS1 expression and activates signaling. However, by further activating AKT, FOXO-remains inhibited and the receptor mRNAs are not induced (35). We show here that mTOR kinase inhibition leads to a third and more complex pattern of effects on these feedback pathways, with initial inhibition of AKT activity which then recovers. This is caused by re-induction of the phosphorylation of multiple HER kinases, IGF1-R, insulin receptor and other receptors that is much more marked than the one seen with rapamycin. This effect is likely due to a more complete inhibition of mTORC1 and to the transient potent inhibition of AKT activity by mTOR kinase inhibitors. This leads to an initial induction of both receptor expression and activity by these drugs but only the latter by rapamycin.
These findings have important implications for the biology of tumors with deregulated PI3K/AKT/mTOR signaling and for their treatment with inhibitors of components of the pathway. One prediction from the data is that certain receptor tyrosine kinases are likely to be downregulated in these tumors unless feedback inhibition by AKT or mTOR has been altered by other genetic lesions. These tumors are unlikely to be dependent on these receptors. This is especially true for IGF1-R, since IGF-1 signaling is powerfully downregulated by multiple AKT or mTOR dependent feedback mechanisms, including downregulation of the expression of IGF1-R, insulin receptor and their prime substrates, IRS1 and IRS2. In tumors treated with inhibitors of the pathway, the tumor cell reactivates IGF-1 signaling and may survive in an IGF1-R-dependent fashion (39).
This may be a general feature of these tumors; feedback reactivation of receptor tyrosine kinase signaling may significantly reduce their sensitivity to mTOR kinase inhibitors. This could occur via activation of PI3K/AKT alone or, more likely, together with activation of other downstream players of the signaling pathway. mTOR kinase inhibitors and rapamycin have been noted to be predominantly cytostatic and to prominently induce autophagy, with only modest induction of apoptosis (24, 25, 40). AKT activation has been shown to prevent apoptosis by multiple mechanisms, including phosphorylation of BAD (41) and activation of NFkB signaling (42). It is plausible that the reinduction of AKT signaling noted here plays an important role in suppressing apoptosis in tumors exposed to mTOR kinase inhibitors. Our finding that the AKT and mTOR kinase inhibitors induce synergistic apoptosis in the breast cancer cell line BT-474 is consistent with this hypothesis.
The idea that relief of feedback inhibition of receptor tyrosine kinases lessens the efficacy of the PI3K pathway inhibition in patients is extremely suggestive, but not yet proven. It does provide a framework for the rational design of therapeutic strategies that combine these drugs with inhibitors of reactivated pathways. The results of these trials will serve to test the hypothesis. It is not yet clear whether mTOR kinase, AKT or PI3K inhibitors will provide the greatest therapeutic index or whether to combine them with inhibitors of individual receptors (IGF1-R, HER kinase, etc.) or of common downstream targets of these pathways (eg AKT, MEK, etc.). The answer will probably vary as a function of tumor lineage and genotype as well as the therapeutic index of the combinations. Our studies do show that rapamycin, mTOR kinase inhibitors and AKT inhibitors relieve different aspects of PI3K pathway-dependent feedback and this may be important in differentiating their clinical effects (16, 35). We show here that combined inhibition of mTOR and HER kinase activity causes significant regression of a breast tumor xenograft model compared to the response elicited by the mTOR kinase inhibitor alone. These results and those of others with similar combinations chosen on an empirical basis suggest that this may be an effective therapeutic strategy (36).
MATERIALS AND METHODS
Cell culture and reagents
Human breast cancer cell lines were obtained from the American Type Culture Collection and maintained in a 1:1 mixture of DME: F12 medium supplemented with 4 mM glutamine, 100 units/mL each of penicillin and streptomycin, and 10% heat-inactivated fetal bovine serum and incubated at 37°C in 5% CO2. DNA fingerprinting was used for authentication of the MDA-MB-468 cell line; no further validation was performed. AZD8055 and Gefitinib were obtained from AstraZeneca Pharmaceuticals, rapamycin, PI-103, wortmannin, AKT1/2 inhibitor, IGF, EGF were purchased from EMD Bioscience, and the purified PI3K was purchased from Millipore. Lapatinib was provided by Tona Gilmer at GlaxoSmithKline and was dissolved in DMSO for in vitro studies and 0.5% hydroxypropylmethylcellulose/0.1% Tween-80 for in vivo studies.
Immunoblot analysis
Cells were washed with PBS once, disrupted on ice for thirty minutes in NP-40 (50 mM Tris [pH 7.4], 1% NP-40, 150 mmol/L NaCl, 40 mmol/L NaF) or RIPA lysis buffer supplemented with protease and phosphatase inhibitors (Pierce Chemical) and cleared by centrifugation. Protein concentration was determined with BCA reagent from Pierce. Equal amounts of protein (10 to 50 μg) in cell lysates were separated by SDS-PAGE, transferred to PVDF membranes (Millipore), immunoblotted with specific primary and secondary antibodies and detected by chemiluminescence with the ECL detection reagents from Amersham Biosciences. Antibodies used for P-AKT (S473), P-AKT (T308), P-GSK3α (S9), P-FOXO1 (T24)/FOXO3(T32), P-p70S6K (T389), P-S6 (S240/244 and S235/236), P-4EBP1 (T37/46), P-4EBP1 (S65), P-4EBP1 (T70), P-EGFR (Y1068), P-HER3 (Y1289), P-HER4 (Y1284), P-IGF1R/IR (Y1135/1136), c-PARP, caspase-3, P-ERK (T202/Y204) were purchased from Cell Signaling Technology. The agarose-conjugated PI3K p85, p85 and P-Her2 (Y1248) antibodies were obtained from Millipore. Antibodies against HER3, Insulin receptor, IGF-1R, Cyclin D1, Cyclin D2 and Cyclin D3 and HER2 were from Santa Cruz Biotechnology. The β-actin antibody was from Sigma. Antibody against P-PRAS40 (T246) was from Biosource International.
In vitro AKT kinase assay
Kinase activity was assayed using a Cell Signaling AKT kinase kit as described in (16). The full method is described in Supplemental Materials and Methods.
Mutagenesis
The HA-tagged AKT1 WT plasmid was used as template to create the HA-tagged S473D AKT plasmid using standard cloning methods. A point mutation in AKT1 WT (Ser473) converting Serine to Aspartate was introduced using the site-directed mutagenesis kit (Stratagene) by following the manufacturer’s instructions. See Supplemental Materials and Methods for transfection procedure.
PI3K activity assay
PI3K activity was determined as described previously (43). See Supplemental Material and Methods for additional information.
Immunofluorescence
Cells were plated on fibronectin-coated Lab-Tek chamber slides (VWR) and treated as described. After the treatments, cells were washed with phosphate-buffered saline and fixed in paraformaldehyde. Cells were incubated with primary antibody followed by fluorescein-conjugated secondary antibody. Nuclei were stained with DAPI. Slides were visualized using confocal microscopy. Confocal images were taken with Leica TCS AOBS SP2 using 63x/1.2NA objective. 405nm and 488nm laser lines were used to excite DAPI and Alexa488 dyes respectively. Same settings were used to take images of cells in different conditions.
RTK arrays
Phospho-RTK arrays (R&D systems) were utilized according to the manufacturer’s instructions. Cells were washed with cold PBS and lysed in NP-40 lysis buffer, and 400 μg of cell lysates were incubated with blocked membranes overnight. Membranes were subsequently washed and exposed to chemiluminescent reagent and developed by autoradiography. The RTK coordinates are listed in Supplemental Material and Methods.
Analysis of cell cycle and apoptosis
Cells were plated in ten-cm dishes and were treated with drug or vehicle (DMSO) the following day for forty-eight hours. Both adherent and floating cells were harvested, and the cell nuclei were prepared as described previously (44).
Animal studies
Six-week-old nu/nu athymic female mice (NCI-Frederick Cancer Center) were maintained in pressurized ventilated cages. Experiments were carried out under an IACUC approved protocol and institutional guidelines for the proper and humane use of animals in research were followed and as described previously (35). See Supplemental Materials and Methods for additional information.
Statistical analysis
Results are mean values ± standard error. Statistical analyses were performed by an unpaired, two-tailed Student t-test.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
The findings reveal the adaptive capabilities of oncogenic signaling networks as AKT signaling becomes re-activated through a feedback-induced AKT species phosphorylated on T308 but lacking S473. The addition of RTK inhibitors can prevent this reactivation of AKT signaling and cause profound cell death and tumor regressions in vivo highlighting the possible need for combinatorial approaches to block feedback regulated pathways.
Acknowledgments
Financial support: This work is supported by the National Institute of Health Program. Grants: 5P01CA094060-08, 5P50CA086438-10, 5P50CA092629-10 and the Breast Cancer Research Foundation.
We thank D. Domingo for assistance with FACS. Y. Qing, H. Zhao, W. Wong, J. Qiu and E. DeStanchina for assistance with nude mouse studies. S. Fujisawa and M. Manova for assistance with digital microscopy. We thank Dr. D. Foster, Dr. D. Solit, Dr. J. Fagin and Dr. C. Sawyers for reviewing the manuscript.
Footnotes
Conflict of interest: S.G. is an employee AstraZeneca and has declared that no competing interest exists.
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 7.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–61. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 11.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman ME, Shokat KM. New inhibitors of the PI3K-Akt-mTOR pathway: insights into mTOR signaling from a new generation of Tor Kinase Domain Inhibitors (TORKinibs) Curr Top Microbiol Immunol. 2010;347:241–62. doi: 10.1007/82_2010_64. [DOI] [PubMed] [Google Scholar]
- 13.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–83. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–44. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–94. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 16.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–42. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 18.Agarwala SS, Case S. Everolimus (RAD001) in the treatment of advanced renal cell carcinoma: a review. Oncologist. 2010;15:236–45. doi: 10.1634/theoncologist.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–9. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 23.Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–72. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–98. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 26.Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–40. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol. 2005;25:2558–72. doi: 10.1128/MCB.25.7.2558-2572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–64. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 31.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–16. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 32.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011 doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr Top Microbiol Immunol. 2010;346:31–56. doi: 10.1007/82_2010_58. [DOI] [PubMed] [Google Scholar]
- 38.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16 (Suppl 1):12–9. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 40.Sini P, James D, Chresta C, Guichard S. Simultaneous inhibition of mTORC1 and mTORC2 by mTOR kinase inhibitor AZD8055 induces autophagy and cell death in cancer cells. Autophagy. 2010:6. doi: 10.4161/auto.6.4.11671. [DOI] [PubMed] [Google Scholar]
- 41.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 42.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer cell. 2005;8:287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.