Abstract
Although the role of colour in mate choice is well known, few tests of colour vision have been based on mating behaviour. Females of the fiddler crab Uca mjoebergi have recently been shown to use claw coloration to recognize conspecific males. In this study I demonstrate that the females use colour vision for this task; preferentially approaching yellow claws over grey claws regardless of their intensity while failing to discriminate between yellow claws differing in intensity. This is one of only a handful of studies confirming the involvement of colour vision in mate choice and the first conclusive evidence in fiddler crabs.
Keywords: Crustacea, Ocypodidae, fiddler crab, colour vision, mate choice
1. Introduction
Colours can act as visual signals to attract the attention of conspecifics and to provide information about reproductive status, condition and individual or species identity (Roulin 2004; Price 2006). However, to be effective, such signals have to be detected by the intended receiver, and thus must be tuned to address their sensory and neural mechanisms (Johnstone 1997). While animals that lack colour vision may be able to differentiate between colours based on differences in intensity, they lose the more reliable information associated with spectral composition (Kelber et al. 2003).
A behavioural test is needed to prove that an animal is able to discriminate between stimuli that differ in spectral composition, irrespective of their relative intensity, and thus actually possesses colour vision (Menzel 1979; Burkhardt 1983; Goldsmith 1990; Kelber et al. 2003). If the animal's spectral sensitivity is known, it can be used to specifically choose test stimuli with the same probably perceived intensity, although this is not always reliable (see Kelber et al. 2003). Alternatively, an experiment can be designed to test the animal's ability to discriminate between a certain colour and various shades of grey, or between monochromatic colours varying in intensity (reviewed by Kelber et al. 2003).
Most tests of colour vision use food rewards to train test subjects to make these discriminations (reviewed by Kelber et al. 2003), e.g. deer (Birgersson et al. 2001), horses (Macuda & Timney 1999), birds (Goldsmith et al. 1981; Osorio et al. 1999), fishes (Neumeyer 1986), blowflies (Fukushi 1990), butterflies (Kelber & Pfaff 1999; Kinoshita et al. 1999), bees (Frisch 1971) and stomatopods (Marshall et al. 1996). However, as many animals use colour and achromatic vision for different tasks or in different situations (Giurfa et al. 1997; Osorio et al. 1999; Kelber et al. 2003; Osorio & Vorobyev 2005), it may be a mistake to assume that the use of colour vision in one task, such as feeding, necessarily translates to other behaviours, such as mate recognition.
There are numerous studies on the role of colour in mate choice in invertebrates, particularly butterflies (Rutowski 1977; Silberglied & Taylor 1978; Wiernasz & Kingsolver 1992; Jiggins et al. 2001) and damselflies (Gorb 1998; Córdoba-Aguilar 2002). However, although butterflies (Kelber & Pfaff 1999; Kinoshita et al. 1999), blowflies (Fukushi 1990), mantis shrimps (Marshall et al. 1996), bees (Frisch 1914), and jumping spiders (Nakamura & Yamashita 2000) and numerous other invertebrates have been shown to possess colour vision, these tests are all based on food training, phototaxis or avoidance behaviours (see Kelber et al. 2003). The one exception is glow-worms, which use colour vision to perceive the bioluminescent signals of potential mates (Booth et al. 2004). Even in vertebrates, proof of the involvement of colour vision in mate choice has only been shown conclusively in two species of toad (Gnyubkin et al. 1975; Kondrashev et al. 1976; Dimentman et al. 1978; Orlov & Maximov 1982). However, several birds, such as budgerigars (Pearn et al. 2001), bluethroats (Andersson & Amundsen 1997) and zebra finches (Bennett et al. 1996), and fishes, such as sticklebacks (Rick et al. 2006) and guppies (Smith et al. 2002) probably use colour vision to perceive ultraviolet signals involved in mate choice. Jumping spiders have also been shown to use ultraviolet signals in mate choice. However, this study cannot confirm the existence of colour vision as it failed to rule out the involvement of brightness cues (Lim et al. 2007).
The aim of this paper is to test the colour vision capabilities of fiddler crabs in a biologically significant context. The walking legs, carapaces and chelae of ocypodid crabs, particularly the well-studied fiddler crabs (genus Uca), are often brilliantly coloured (Crane 1975). However, despite this array of colours and their likely role in social displays (Detto et al. 2004, 2006; Hemmi et al. 2006), there is still some debate over whether ocypodid crabs are actually capable of colour vision and what role it might play. At least, two spectrally distinct classes of photoreceptor are required for colour vision (Goldsmith 1990) but the number possessed by fiddler crabs is still in question. Scott & Mote (1974) found evidence of only one receptor type with a peak sensitivity approximately 510 nm, Horch et al. (2002), on the other hand, found two receptor types with peak sensitivities approximately 430 nm and between 510 and 540 nm. The most recent work based on microspectrophotometry found a single visual pigment type with peak absorption between 508 and 530 nm in the main retinular cells (R1-7; Jordão et al. 2007). However, Jordão et al. (2007) suggest there may be an additional short wavelength-sensitive pigment in the eighth retinular cell (R8) of the rhabdom that would render fiddler crabs capable of dichromatic colour vision. Behavioural experiments based on spontaneous phototaxis further suggest that Uca pugilator females can distinguish between red and blue lights (Hyatt 1975). However an innate intensity-based preference overshadow any potential preference for colour in another ocypodid, Heloecius cordiformis (Detto 2007, unpublished data).
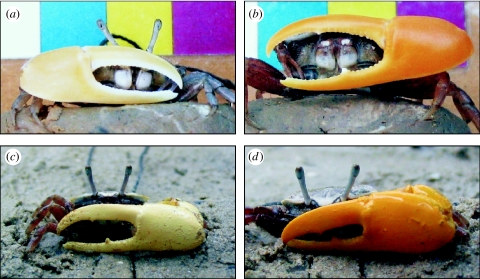
Uca mjoebergi is an endemic Australian fiddler crab characterized by a mottled brown carapace and the male's yellow major claw (figure 1a,b). Females leave their burrows and wander through the colony in search of a male before mating within his burrow. Males wave their enlarged yellow claw at any moving, crab-sized object, and females have been shown to use the coloration of the male's claw to recognize conspecifics (Detto et al. 2006). Females have a strong preference for uniformly yellow claws over those painted red and white, and are even able to discriminate between natural yellow claws and those painted with a yellow that appears very similar to the human visual system (Detto et al. 2006). The social importance of colour in these crabs and the demonstration of an apparently colour-based preference in an experimental situation make them ideal candidates for a behavioural test of colour vision in mate choice.
Figure 1.
Claw colours of Uca mjoebergi and paint colours used in experiments. (a,b) Extremes in natural variation of claw colour in Uca mjoebergi. (c,d) Uca mjoebergi males painted with ‘light yellow’ and ‘yellow’ paint.
2. Material and methods
To determine whether U. mjoebergi use differences in spectral composition to discriminate between colours, and thus possess colour vision, I tested their ability to consistently discriminate yellow from various shades of grey. Experiments were conducted in October 2005 on an intertidal mudflat in the East Point Reserve, Darwin, Australia (12°24′35″ S, 130°50′00″ E).
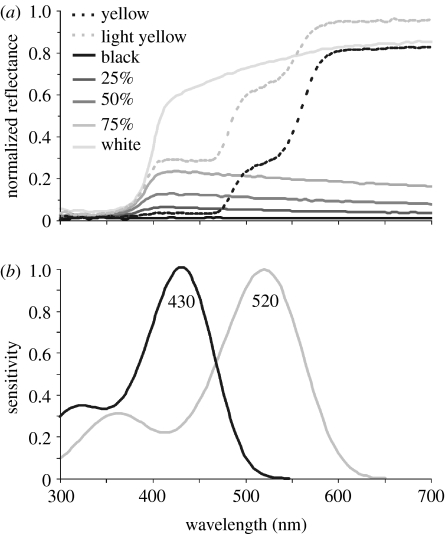
I quantified the colour of the paints, as perceived by the crabs, to ensure that they differed primarily in intensity or spectral composition. I measured the spectral reflectance of the yellow and grey paints relative to a white ‘Spectralon’ standard with a USB2000 UV–VIS portable spectrophotometer (Ocean Optics, Inc., Dunedin, FL, USA). I used the λmax values of 430 and 520 nm of the fiddler crab Uca thayeri (see Horch et al. 2002) to calculate the hypothetical spectral sensitivities of U. mjoebergi (Govardovskii et al. 2000; figure 2b). Then, following the methods reviewed by Kelber et al. (2003), I calculated the quantum catches of the two receptor types (Q430 and Q520) as the product of their spectral sensitivities, the reflectance spectra of the different colours, and the relative quantum units for standard daylight (D65, Wyszecki & Stiles (1982), kindly provided by Misha Vorobyev), integrated over wavelength (300–700 nm). I then calculated the receptor signals (Qs430 and Qs520) as a percentage of the quantum catch compared with a white surface reflecting 100%. Differences in spectral composition are characterized by the chromatic signal, calculated as follows:
| (2.1) |
The achromatic (intensity) signal is represented by the sum of the two receptor signals.
Figure 2.
Reflectance spectra of the different paints and the hypothetical spectral sensitivities. (a) The grey paints are relatively flat across the spectrum and vary in intensity, while the yellow paints plateau at approximately 500 nm and above 600 nm. None of the paints reflect in the ultraviolet. (b) The spectral sensitivities of U. thayeri used to model the chromatic and achromatic responses of U. mjoebergi to the different paints.
When the crabs were reproductively active, during neap tide, I caught wandering females and placed them under a container 15 cm from two identical plaster casts of a U. mjoebergi male's 21.8 mm claw. The claws were attached to servomotors programmed to move synchronously 40 times a minute over an angle of 15°, with a pause of 0.5 s at the beginning and apex of each cycle. This movement was not designed to mimic the wave of a real male, rather to attract the attention of the females and make the artificial claws more attractive. I started the servomotors before lifting the container remotely to release the female. I then recorded which claw she approached to within approximately 2 cm. Females that left the area without approaching either claw (approx. 75%) were considered not to have made a choice and new females were tested on the same combination until one made a choice.
Females were given a choice between a yellow claw (Dulux Tiny Tin yellow enamel) and a claw painted white, 75% grey, 50% grey, 25% grey or black (made by mixing appropriate amounts of white and black Dulux Tiny Tin enamel paint). The grey and yellow claws were randomly positioned and the combinations randomly tested throughout the low tide period to eliminate any directional or temporal effects. Each combination was tested until 15 females successfully made a choice.
I conducted the same experiment with two tethered, size-matched (within 1 mm claw length), and handedness-matched males in place of the plaster claws. The males were tethered with 1 cm of cotton super-glued to their carapace and tied to a nail stuck in the ground, allowing some restricted movement (as per Detto et al. 2006). I painted the males' claw either with the same yellow paint as above or with the light yellow paint, made by mixing one part yellow to 14 parts white paint (figure 1c,d). The experimental conditions were otherwise the same as when using plaster claws, and the females did not appear to treat the artificial and real males any differently. I repeated the experiment 25 times using different pairs of males and new females each time. If the crabs are basing their choice on intensity, they should be able to discriminate between the light and dark yellow claws. If they are unable to distinguish between the different yellows, they are most likely basing their choice on colour, independently of intensity.
3. Results
Spectral measurements confirmed that the grey paints were relatively flat from 400 to 700 nm. They varied primarily in their intensity, which decreased progressively from white through to black. Both the light and dark yellow paints reflected most strongly above 600 nm, but the light yellow reflected more strongly over the entire spectrum, and was less saturated than the dark yellow (figure 2a). Neither the yellow nor any of the grey paints reflected in the ultraviolet.
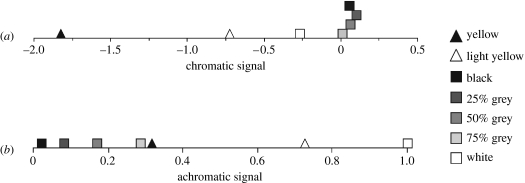
As perceived by theoretical spectral sensitivities (figure 2b), both the light and dark yellow paints are spectrally distinct from the different greys, and from each other (figure 3a). As the light yellow is less saturated, it is chromatically more similar to the greys than the dark yellow. Based on the achromatic model, light yellow is considerably brighter than dark yellow which is similar in intensity to 75% grey (figure 3b).
Figure 3.
Perceived spectral composition and intensity of the different claw colours. (a) The chromatic signal based on the difference between the natural logarithms of the receptor signals. The greys are spectrally similar, although white differs slightly. Both yellows are spectrally distinct from the greys and each other due to the low saturation of light yellow. (b) The achromatic signals based on the normalized sum of the two receptor signals. The greys differ significantly in intensity, as do dark and light yellow, which fall between 75% grey and white.
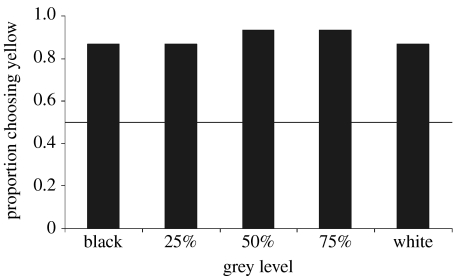
Uca mjoebergi females consistently (greater than 85%) approached claws painted yellow over those painted grey, regardless of their intensity (figure 4). Furthermore, the females did not discriminate between two shades of yellow differing significantly in intensity; 14 approached the males with dark yellow claws while 11 approached the males with light yellow claws (G-test, p=0.5, n=25).
Figure 4.
The proportion of U. mjoebergi females approaching yellow claws over claws when presented with various shades of grey. Females approached yellow significantly more than any of the grey levels (G-test, p<0.001, n=15).
4. Discussion
Colour is believed to play a role in the social interactions of ocypodid crabs (Crane 1975; Zeil & Hofmann 2001; Detto et al. 2004). However, until a recent study demonstrated that female U. mjoebergi use claw coloration to recognize conspecific males (Detto et al. 2006), there was no quantitative evidence that colour is important to fiddler crabs. Furthermore, although Hyatt (1975) suggested that the preference for red over blue light in U. pugilator was due to the social importance of their blue carapace colour, the role of colour vision in fiddler crab social interactions remained unproven.
I showed here that female U. mjoebergi are able to discriminate between stimuli based solely on chromatic differences; preferring claws painted yellow over those painted grey, regardless of their intensity. If the crabs were basing their choice on the relative intensity of the paints, they would have been able to discriminate between yellow and black or white. The ability to discriminate between stimuli based on chromatic differences, independently of intensity, is proof of the presence of colour vision (Menzel 1979; Kelber et al. 2003). This is one of only a handful of studies confirming the involvement of colour vision in mate choice (see Kelber et al. 2003; Booth et al. 2004) and the first conclusive evidence in fiddler crabs.
Although other studies have found that female birds prefer brighter males, as a signal of male quality (e.g. Hill 1991), U. mjoebergi females did not discriminate between male claws of two shades of yellow that differed in intensity and spectral composition. Furthermore, they were able to discriminate between yellow and several greys which were chromatically distinct but achromatically more similar than the two yellows. Thus, although there is considerable natural variation in the intensity of the yellow colour of U. mjoebergi males' claws (see figure 1a,b), females do not appear to approach males based on differences in claw brightness.
These results highlight the importance of examining an animal's capacity for colour vision within the context of interest. The vast majority of studies of colour vision are based on training regimes based on food rewards (reviewed by Kelber et al. (2003)), and as such are effective at examining the role of colour vision in foraging behaviour. While these studies demonstrate that an animal is capable of colour vision, this does not necessarily mean that the animal uses it in other situations.
Species-specific colours, like those common in fiddler crabs (Crane 1975), may influence the evolution of visual pigments (Goldsmith 1991; Endler 1992) and vice versa (Endler 1992; Ryan & Keddy-Hector 1992; Ryan 1998; Carleton et al. 2005). For instance, the females of two sympatric species of Lycaena butterflies appear to use wing colour to identify conspecific males and their visual pigments are well matched to facilitate such discriminations (Bernard & Remington 1991). The coloration of cichlid fish is believed to play a role in speciation (Seehausen & von Alphen 1998; Couldridge & Alexander 2001) and there is some evidence that their spectral sensitivities match their nuptial coloration (Carleton et al. 2005). Reproductive isolation in fiddler crabs may be aided by their species-specific coloration, but whether their colours evolved in response to a pre-existing sensory bias or drove the evolution of the visual pigments is unknown. To answer this question, the spectral sensitivities of fiddler crab photoreceptors will need to be accurately identified, and compared across a variety of differently coloured species.
Acknowledgments
This research conformed to the ethical guidelines of The Australian National University.
This work was supported by an ANU PhD Scholarship with an additional contribution by the Centre for Visual Sciences and with the assistance of the Australian Research Council under the ARC Centres of Excellence program. I thank Almut Kelber, Justin Marshall, Patricia Backwell, Jan Hemmi, Misha Vorobyev and Jochen Zeil for their advice, discussions and comments on the manuscript and Mark Snowball for construction of the servomotor-controlled claws.
References
- Andersson S, Amundsen T. Ultraviolet colour vision and ornamentation in bluethroats. Proc. R. Soc. B. 1997;264:1587–1591. doi:10.1098/rspb.1997.0221 [Google Scholar]
- Bennett A.T.D, Cuthill I.C, Partridge J.C, Maier E.J. Ultraviolet vision and mate choice in zebra finches. Nature. 1996;380:433–435. doi:10.1038/380433a0 [Google Scholar]
- Bernard G.D, Remington C.L. Color vision in Lycaena butterflies: spectral tuning of receptor arrays in relation to behavioral ecology. Proc. Natl Acad. Sci. USA. 1991;88:2783–2787. doi: 10.1073/pnas.88.7.2783. doi:10.1073/pnas.88.7.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgersson B, Alm U, Forkman B. Colour vision in fallow deer: a behavioural study. Anim. Behav. 2001;61:367–371. doi:10.1006/anbe.2000.1603 [Google Scholar]
- Booth D, Stewart A.J.A, Osorio D. Colour vision in the glow-worm Lampyris noctiluca (L.) (Coleoptera: Lampyridae): evidence for a green–blue chromatic mechanism. J. Exp. Biol. 2004;207:2373–2378. doi: 10.1242/jeb.01044. doi:10.1242/jeb.01044 [DOI] [PubMed] [Google Scholar]
- Burkhardt D. Wavelength perception and colour vision. In: Cosens D.J, Vince-Price D, editors. The biology of photoreception. Symposia of the society for experimental biology no. XXXVI. Cambridge University Press; Cambridge, UK: 1983. pp. 371–397. [PubMed] [Google Scholar]
- Carleton K.L, Parry J.W.L, Bowmaker J.K, Hunt D.M, Seehausen O. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Mol. Ecol. 2005;14:4341–4353. doi: 10.1111/j.1365-294X.2005.02735.x. [DOI] [PubMed] [Google Scholar]
- Córdoba-Aguilar A. Wing pigmentation in territorial male damselflies, Calopteryx haemorrhoidalis: a possible relation to sexual selection. Anim. Behav. 2002;63:759–766. doi:10.1006/anbe.2001.1974 [Google Scholar]
- Couldridge V.C.K, Alexander G.J. Color patterns and species recognition in four closely related species of Lake Malawi cichlid. Behav. Ecol. 2001;13:59–64. doi:10.1093/beheco/13.1.59 [Google Scholar]
- Crane J. Princeton University Press; Princeton, NJ: 1975. Fiddler crabs of the world. Ocypodidae: genus Uca. [Google Scholar]
- Detto T, Zeil J, Magrath R.D, Hunt S. Sex, size and colour in a semi-terrestrial crab, Heloecius cordiformis (H. Milne Edwards, 1837) J. Exp. Mar. Biol. Ecol. 2004;302:1–15. doi:10.1016/j.jembe.2003.09.023 [Google Scholar]
- Detto T, Backwell P.R.Y, Hemmi J.M, Zeil J. Visually mediated species and neighbour recognition in fiddler crabs (Uca mjoebergi and Uca capricornis) Proc. R. Soc. B. 2006;273:1661–1666. doi: 10.1098/rspb.2006.3503. doi:10.1098/rspb.2006.3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimentman, A. M., Kondrashev, S. L. & Orlov, O. Y. 1978 A study of the mechanism of colour constancy in grey toad (Bufo bufo L.). In Mechanisms of vision in animals, pp. 85–95. Moscow, Russia: Nauka. [In Russian.]
- Endler J.A. Signals, signal conditions, and the direction of evolution. Am. Nat. 1992;139:S125–S153. doi:10.1086/285308 [Google Scholar]
- Frisch K. von. Der Farbensinn und Formensinn der Biene. Zoologische Jahrbücher. Abteilung für allgemeine Zoologie und Physiologie der Tiere. 1914;35:1–188. [Google Scholar]
- Frisch K. von. Cornell University Press; Ithaca, NY: 1971. Bees; their vision, chemical senses, and language. [Google Scholar]
- Fukushi T. Colour discrimination from various shades of grey in the trained blowfly, Lucilia cuprina. J. Insect Physiol. 1990;36:69–75. doi:10.1016/0022-1910(90)90152-6 [Google Scholar]
- Giurfa M, Vorobyev M, Brandt R, Posner B, Menzel R. Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J. Comp. Physiol. A. 1997;180:235–243. doi:10.1007/s003590050044 [Google Scholar]
- Gnyubkin V.D, Kondrashev S.L, Orlov O.Y. Constancy of colour perception in the grey toad. Biofizika. 1975;20:725–730. [PubMed] [Google Scholar]
- Goldsmith T.H. Optimisation, constraint and history in the evolution of the eyes. Q. Rev. Biol. 1990;65:281–322. doi: 10.1086/416840. doi:10.1086/416840 [DOI] [PubMed] [Google Scholar]
- Goldsmith T.H. The evolution of visual pigments and colour vision. In: Gouras P, editor. The perception of colour. Macmillan Press Ltd; London, UK: 1991. pp. 62–89. [Google Scholar]
- Goldsmith T.H, Collins J.S, Perlman D.L. A wavelength discrimination function for the hummingbird Archilochus alexandri. J. Comp. Physiol. A. 1981;143:103–110. doi:10.1007/BF00606073 [Google Scholar]
- Gorb S.N. Visual cues in mate recognition by males of the damselfly, Coenagrion puella (L.) (Odonata: Coenagrionidae) J. Insect Behav. 1998;11:73–92. doi:10.1023/A:1020818617066 [Google Scholar]
- Govardovskii V.I, Fyhrquist N, Reuter T, Kuzmin D.G, Donner K. In search of the visual pigment template. Vis. Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. doi:10.1017/S0952523800174036 [DOI] [PubMed] [Google Scholar]
- Hemmi J.M, Marshall J, Pix W, Vorobyev M, Zeil J. The variable colours of the fiddler crab Uca vomeris and their relation to background and predation. J. Exp. Biol. 2006;209:4140–4153. doi: 10.1242/jeb.02483. doi:10.1242/jeb.02483 [DOI] [PubMed] [Google Scholar]
- Hill G.E. Plumage colouration is a sexually selected indicator of male quality. Nature. 1991;350:337–339. doi:10.1038/350337a0 [Google Scholar]
- Horch K, Salmon M, Forward R. Evidence for a two pigment visual system in the fiddler crab, Uca thayeri. J. Comp. Physiol. A. 2002;188:493–499. doi: 10.1007/s00359-002-0325-7. doi:10.1007/s00359-002-0325-7 [DOI] [PubMed] [Google Scholar]
- Hyatt G. Physiological and behavioural evidence for colour discrimination by fiddler crabs (Brachyura, Ocypodidae, Genus Uca) In: Vernberg V, editor. Physiological ecology of estuarine organisms. University of South Carolina Press; Columbia, SC: 1975. pp. 333–365. [Google Scholar]
- Jiggins C.D, Naisbit R.E, Coe R.L, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. doi:10.1038/35077075 [DOI] [PubMed] [Google Scholar]
- Johnstone R.A. Recognition and the evolution of distinctive signatures: when does it pay to reveal identity? Proc. R. Soc. B. 1997;264:1547–1553. doi:10.1098/rspb.1997.0215 [Google Scholar]
- Jordão J.M, Cronin T.W, Oliveira R.F. Spectral sensitivity of four species of fiddler crabs (Uca pugnax, Uca pugilator, Uca vomeris and Uca tangeri) measured by in situ microspectrophotometry. J. Exp. Biol. 2007;210:447–453. doi: 10.1242/jeb.02658. doi:10.1242/jeb.02658 [DOI] [PubMed] [Google Scholar]
- Kelber A, Pfaff M. True colour vision in the orchard butterfly Papilio aegeus. Naturwissenschaften. 1999;86:221–224. doi:10.1007/s001140050601 [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. 2003;78:81–118. doi: 10.1017/s1464793102005985. doi:10.1017/S1464793102005985 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Shimada N, Arikawa K. Colour vision of the foraging swallowtail butterfly Papilio xuthus. J. Exp. Biol. 1999;202:95–102. doi: 10.1242/jeb.202.2.95. [DOI] [PubMed] [Google Scholar]
- Kondrashev S.L, Gnyubkin V.F, Dimentman A.M, Orlov O.Y. Role of visual stimuli in mating behavior of males in grass frog (Rana temporaria), grey toad (Bufo bufo) and green toad (Bufo viridis) Zool. J. USSR. 1976;55:1027–1037. [In Russian.] [Google Scholar]
- Lim M.L.M, Land M.F, Li D. Sex specific UV and fluorescence signals in jumping spiders. Science. 2007;315:481. doi: 10.1126/science.1134254. doi:10.1126/science.1134254 [DOI] [PubMed] [Google Scholar]
- Macuda T, Timney B. Luminance and chromatic discrimination in the horse (Equus caballus) Behav. Process. 1999;44:301–307. doi: 10.1016/s0376-6357(98)00039-4. doi:10.1016/S0376-6357(98)00039-4 [DOI] [PubMed] [Google Scholar]
- Marshall N.J, Jones J.P, Cronin T.W. Behavioural evidence for colour vision in stomatopod crustaceans. J. Comp. Physiol. A. 1996;179:473–481. doi:10.1007/BF00192314 [Google Scholar]
- Menzel R. Spectral sensitivity and color vision in invertebrates. In: Autrum H, editor. Comparative physiology and evolution of vision in invertebrates A: invertebrate photoreceptors. Springer; Heidelberg, Germany; New York, NY: 1979. pp. 503–580. [Google Scholar]
- Nakamura T, Yamashita S. Learning and discrimination of colored papers in jumping spiders (Araneae Salticidae) J. Comp. Physiol. A. 2000;186:897–901. doi: 10.1007/s003590000143. doi:10.1007/s003590000143 [DOI] [PubMed] [Google Scholar]
- Neumeyer C. Wavelength discrimination in the goldfish. J. Comp. Physiol. A. 1986;158:203–213. doi:10.1007/BF01338563 [Google Scholar]
- Orlov, Y. M. & Maximov, V. V. 1982 Color vision and behaviour of amphibians. In Sensory systems. Vision, pp. 114–125. Leningrad, Russia: Nauka.
- Osorio D, Vorobyev M. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B. 2005;272:1745–1752. doi: 10.1098/rspb.2005.3156. doi:10.1098/rspb.2005.3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Miklósi A, Gonda Zs. Visual ecology and perception of coloration patterns by domestic chicks. Evol. Ecol. 1999;13:673–689. doi:10.1023/A:1011059715610 [Google Scholar]
- Pearn S.M, Bennett A.T.D, Cuthill I.C. Ultraviolet vision, fluorescence and mate choice in a parrot, the budgerigar Melopsittacus undulatus. Proc. R. Soc. B. 2001;268:2273–2279. doi: 10.1098/rspb.2001.1813. doi:10.1098/rspb.2001.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T.D. Phenotypic plasticity, sexual selection and the evolution of colour patterns. J. Exp. Biol. 2006;209:2368–2376. doi: 10.1242/jeb.02183. doi:10.1242/jeb.02183 [DOI] [PubMed] [Google Scholar]
- Rick I.P, Modarressie R, Bakker T.C.M. UV wavelengths affect female mate choice in three-spined sticklebacks. Anim. Behav. 2006;71:307–313. doi:10.1016/j.anbehav.2005.03.039 [Google Scholar]
- Roulin A. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol. Rev. 2004;79:815–848. doi: 10.1017/s1464793104006487. doi:10.1017/S1464793104006487 [DOI] [PubMed] [Google Scholar]
- Rutowski R.L. The use of visual cues in sexual and species discrimination by males of the small sulphur butterfly Eurema lisa. J. Comp. Physiol. A. 1977;115:61–74. doi:10.1007/BF00667785 [Google Scholar]
- Ryan M.J. Sexual selection, receiver biases, and the evolution of sex differences. Science. 1998;281:1999–2003. doi: 10.1126/science.281.5385.1999. doi:10.1126/science.281.5385.1999 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Keddy-Hector A. Directional patterns of female mate choice and the role of sensory biases. Am. Nat. 1992;139:S4. doi:10.1086/285303 [Google Scholar]
- Scott S, Mote M.I. Spectral sensitivity in some marine Crustacea. Vision Res. 1974;14:659–663. doi: 10.1016/0042-6989(74)90061-3. doi:10.1016/0042-6989(74)90061-3 [DOI] [PubMed] [Google Scholar]
- Seehausen O, von Alphen J.J.M. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex) Behav. Ecol. Sociobiol. 1998;42:1–8. doi:10.1007/s002650050405 [Google Scholar]
- Silberglied R.E, Taylor O.R., Jr Ultraviolet reflection and its behavioural role in the courtship of the sulphur butterflies Colias erythema and C. philodice. Behav. Ecol. Sociobiol. 1978;3:203–243. doi:10.1007/BF00296311 [Google Scholar]
- Smith E.J, Partridge J.C, Parsons K.N, White E.M, Cuthill I.C, Bennett A.T.D, Church S.C. Ultraviolet vision and mate choice in the guppy (Poecilia reticulata) Behav. Ecol. 2002;13:11–19. doi:10.1093/beheco/13.1.11 [Google Scholar]
- Wiernasz D.C, Kingsolver J.G. Wing melanin pattern mediates species recognition in Pieris occidentalis. Anim. Behav. 1992;43:89–94. doi:10.1016/S0003-3472(05)80074-0 [Google Scholar]
- Wyszecki G, Stiles W.S. Wiley; New York, NY: 1982. Color science. [Google Scholar]
- Zeil J, Hofmann M. Signals from ‘crabworld’: cuticular reflections in a fiddler crab colony. J. Exp. Biol. 2001;204:2561–2569. doi: 10.1242/jeb.204.14.2561. [DOI] [PubMed] [Google Scholar]