Abstract
The application of hung transplantation to the pediatric population was a natural extemion of the success realized in our adult transplantation program, which began in 1982. Twenty pediatric patients (age range 3 to 18 years) have had heart-lung (n = 11), double lung (n = 8), and single lung (n = 1) transplantation procedures. The causes of end-stage lung disease were primary pulmonary hypertension (n =7), congenital heart disease (n =5), cystic fibrosis (n =4), pulmonary arteriovenous malformation (n =2), graft-versus-host disease (n =1), and desquamative interstitial pneumonitis (n =1). Four (20%) patients bad thoracic surgicaJ procedures before the transplantation operation. The survival was 80% at a mean foDow-up of 2 years. Immunosuppressive drugs included cyclosporine (n =9) or FK 506 (n =11) based therapy with azathioprine and steroids. Children were followed up by meaDS of spirometry, t:rambroncbial biopsy, and primed lymphocyte testing of bronchoalveolar lavage fluid. The mean number of treated episodes of rejeclion was 1.4 at 30 days, 0.5 at 30 to 90 days, and 1.4 at more than 90 days, and the first treated rejection episode occurred on average 28 days after the operation. Obliterative bronchiolitis developed in four (25%) of 16 patients surviving more tban 100 days. Results of poImonary function tests have remained good in almost all recipients. The greatest infectious risk was that of cytomegalovirlti: one death and one case of pneumonia. Posttransplantation Iymphoproliferative disease was diagnosed in two (12.5 %) patients; both recovered. The most common complicatiom were hypertension (25 %) and postoperative bleeding (15 %). Early results indicate that lung transplantation is a most promising therapy for cbildren with severe vascular and parenchymal lung disease.
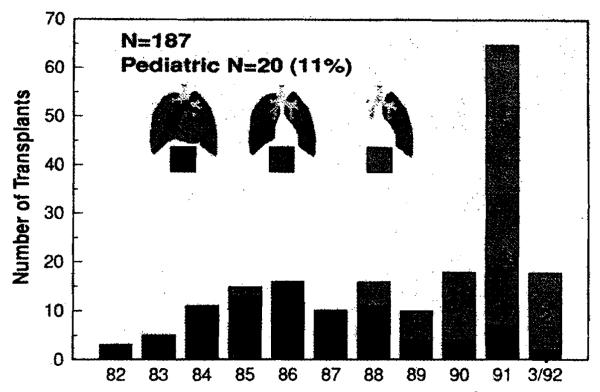
More than 90% of the 1500 lung transplantation procedures reported to the International Society for Heart and Lung Transplantation Registry as of 1991 had been performed since 1985,in over 90 centers worldwide.1 As in cardiac transplantation, the application of pulmonary transplantation to the pediatric age group has lagged behind the successes realized in the adult population. Less than 15% (n =220) of all lung transplantation procedures have been performed in patients under the age of 20 years and only 4% (n = 60) in patients less than 10 years of age.1 At the University of Pittsburgh, the first heartlung transplantation was performed in 1982, and it was not until 1985 that our program began accepting children as candidates. From 1985 through March 1992, 20 children <18 years) have undergone heart-lung, double lung, or single lung transplantation at Children’s Hospital of Pittsburgh (CHP). The growth of our pediatriclung transplantation program has mirrored the national experience and that of our adult program, both in terms of increased organ availability and in the trend toward single and double lung procedures and away from heart-lung transplantation (Fig. 1). In this report we include our pediatric experience with emphasis on our present management strategies and the preliminary results of the clinical trial of FK 506 immunosuppression.
Fig. 1.
A decade of lung transplantation, 1982 to 1992, University of Pittsburgh Medical Center.
Patients and methods
Patient group
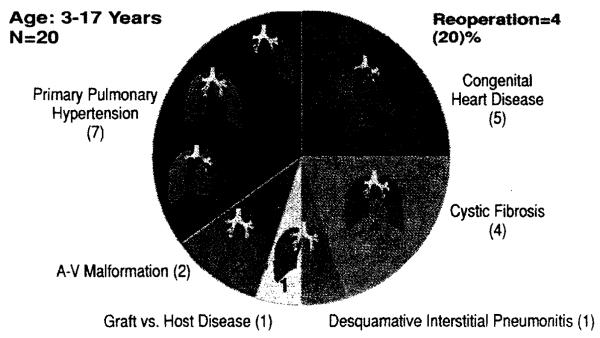
Twenty children, aged 3 to 18 years, have undergone heart-lung (n = 11), double lung (n = 8), or single lung (n = I) transplantation from 1985 through March 1992. Seventeen (85%) of these children were female and three (15%) were male. The indications for transplantation have been primary pulmonary hypertension (n = 7), congenital heart disease (n = 5), cystic fibrosis (n = 4), pulmonary arteriovenous malformation (n = 2), graft-versus-host disease (n = I), and desquamative interstitial pneumonitis (n = I) (Fig. 2). The congenital heart anomalies in our five patients were transposition of the great arteries (n = 3) and ventricular septal defect (n = 2).
Fig. 2.
Pediatric lung transplantation, 1985 to 1992. A-V, Arteriovenous.
Four (20%) of the twenty recipients had thoracic operations before transplantation. Three patients had one chest procedure (repair of ventricular septal defect, Mustard procedure, and right lower lobectomy in one patient each), and one patient had two prior operations (pulmonary artery banding at the age of 5 months and Mustard procedure at the age of 3 years).
Recipient selection
All children in this series had end-stage parenchymal or vascular lung disease that significantly affected their lifestyle. School-age children had either curtailed or discontinued school activity. All candidates with primary pulmonary hypertension had near-systemic or suprasystemic pulmonary artery pressures with associated signs of right-sided heart failure. Fifty percent (n = 10) of our patients were receiving supplementary oxygen and one patient was dependent on a ventilator before transplantation. Profound cyanosis, clubbing, and polycythemia were present in all candidates with congenital heart disease and pulmonary arteriovenous malformation. Five (20%) patients were receiving prednisone at the time of transplantation, and although attempts were made to wean steroids as tolerated, this was not considered a contraindication to lung transplantation. Likewise, we cautiously increased our experience in patients with previous thoracic and cardiac surgery. In candidates whose disease process affected their airway function, pulmonary function tests-first-second forced expiratory volume (FEYl), forced vital capacity (FYC), and forced expiratory flow (FEF25.27)-were less than 30% of predieted normal values for age, height, and weight. Whenever possible, an exercise study was performed with pulse oximetry, both as a measure of exercise-induced hypoxia and also as an objective test for follow-up of the candidate accepted for transplantation.
Implantation tecbniques
The implantation techniques for heart-lung (n = 11),2 double lung (n = 2),3 sequential bilateral single lung (n = 6),3 and single lung (n = 1)5 transplantation have been previously described and were used in this series of patients without significant changes. We no longer wrap the tracheal or bronchial anastomoses with omentum, and we have used a telescoping airway anastomosis with polypropylene suture. Only one of the six patients undergoing sequential bilateral single lung transplantation required cardiopulmonary bypass because of profound hypoxemia. This lo-year-old boy with pulmonary arteriovenous malformation and prior right upper lobectomyhad an arterial oxygen saturation of 46% under anesthesia before the skin incision.
Donor evaluation and lung preservation
Our criteria for acceptance of a lung donor has evolved over the past decade. In this series, the cause of brain death of the donors was equally distributed among gunshot wound to the head, blunt head trauma resulting from vehicular accidents, and intracerebral hemorrhage or tumor. The opportunity to observe the clinical course of a cadaveric donor over 24 to 72 hours of hospitalization was not always feasible but has been invaluable in discerning the quality of lungs for donation. Not only were a clear chest radiograph and an arterial oxygen tension of 400 torr on 100% oxygen in the organ donor desirable, the trend of these pulmonary variables, over time, was critical. Donor sputum Gram stain and culture rarely precluded lungs for consideration, with the exception of moderate growth of yeast. All donors underwent bronchoscopic and visual inspection of the lungs at the time of harvest. Pulmonary contusion, chest tubes, and tracheostomies were relative and not absolute contraindications to lung procurement.6
All lung donors received intravenous methylprednisolone, clindamycin, and ceftazidime, as well as aerosolized gentamicin.7 The first two donors in this series were transferred to CHP from other centers and underwent core-eooling for procurement in 1985. Although we first described long-distance lung procurement and transport with an ex vivo autoperfused heart-lung preparation,8 this technique was used in only one patient in this series. Four donors underwent core-eooling and 15 had rapid high-volume pulmonary flushing with either Euro-Collins (n = 4) or University of Wisconsin solution (n = II). Care was always taken to adequately vent the left side of the heart during lung flushing, which we accomplished by first administering cardioplegic solution with the left atrial appendage opened and then transecting the aorta to assure a pulmonary venous pressure of zero during the infusion. We have found it convenient to use a small roller-head pump and heat exchanger to deliver the 60 ml/kg flush solution at 4° C. Gentle hand ventilation was maintained during the pulmonary infusion. The flushed lungs were then transported, three-fourths inflated with oxygen, in the iced flush solution. The details of our explantation technique have been previously described.9
Immunosuppression
All patients in this series were treated with triple-drug immunosuppression. Nine patients (group I) had cyclosporine and II patients (group 2) had FK 506 as the basis of their immunosuppressive protocol. One patient, included in group 2, was begun on the cyclosporine regimen but was switched to FK 506 at 12 weeks after transplantation because of persistent rejection, severe hirsutism, and hypertension. Azathioprine, 4 mg/kg intravenously, was begun before transplantation and continued after transplantation, either intravenously or orally, at a dosage of 2 mg/kg per day. Azathioprine was discontinued or the dose was decreased with associated neutropenia. Methylprednisolone, 10 mg/kg, was given intraoperatively and 5 mg/kg, in three divided doses, on the first postoperative day. Thereafter, patients received prednisone, 0.1 mg/kg per day, by mouth. Four patients in group I received rabbit antithymocyte globulin, 1.5 mg/kg per day intramuscularly, for 5 days as immunoprophylaxis; no patients in group 2 received rabbit antithymocyte globulin. In group 2, steroid weaning was begun 6 months after transplantation.
Cyclosporine or FK 506 was begun in the postoperative period as an intravenous infusion and was continued for 48 to 72 hours. When gastrointestinal function returned, immunosuppressive drugs were administered orally. In group 1, the intravenous cyclosporine dosage was 1.5 mg/kg per day. The oral dosage of cyclosporine was 5 mg/kg per day, in two divided doses. The daily dose was adjusted to attain a trough level of 200 to 250 ng/ml by plasma high-performance liquid chromatography. In group 2 patients, the intravenous FK 506 induction dosage was 0.05 mg/kg per day and the oral maintenance dosage was 0.3 mg/kg per day in two divided doses. The oral dose of FK 506 was targeted for a serum trough level of I to 2 ng/ml (as measured by the enzyme-linked immunosorbent assay technique of Tamaura and associates10).
Monitoring and treatment of rejection
All patients in this series had bronchoscopic treatment with bronchoalveolar lavage approximately 2 and 4 weeks after transplantation. Since 1989, transbronchial biopsy has been perfonned at the time of the lavage. The lavage specimen was evaluated for total cell count and differential, microbiologic assays, and immunologically with a donor-specific primed lymphocyte test (PLT).11 Patients received empiric or “clinical” treatment of rejection only during the first 7 to 10 days after transplantation if clinical and radiographic findings were both consistent with lung rejection and there was no evidence of infection.
Treatment of lung rejection since 1989 has been based on standardized histologic criteria.12 Acute rejection, grade 2 to 4, and chronic airway rejection with active bronchiolitis obliterans were first treated with intravenous methylprednisolone, 10 mg/kg per day, for 3 consecutive days. Refractory or severe episodes or associated obliterative bronchiolitis were treated with courses of rabbit antithymocyte globulin or Minnesota antilymphocyte globulin.
Outpatient evaluation consisted of clinic visits, blood tests, chest radiographs, pulmonary function tests, bronchoalveolar lavage, and transbronchial biopsy. Although follow-up visits were individualized on the basis of clinical and rejection history, they were generally scheduled for 2, 6, and 12 weeks after discharge. Thereafter, regular follow-up was every 3 to 4 months for the first year after transplantation and every 4 months for subsequent years. All patients were instructed in the use of and discharged home with hand-held spirometers. When able, recipients used a portable spirometer to record daily measurements of airflow. A persistent 10% fall in FYC or FEY, initiated a return visit to CHP for bronchoalveolar lavage and transbronchial biopsy. A recent cytomegalovirus infection or persistent elevation in primed lymphocyte test results increased our surveillance and concern for lung rejection.
Patients with heart-lung transplants had one endomyocardial biopsy before discharge. Thereafter, endomyocardial biopsy was done at year I and every 2 years thereafter, with complete left and right heart catheterization. All heart biopsy specimens were graded according to standardized histologic criteria.13
Monitoring for infection and prophylaxis
All patients were observed by a specialist in infectious disease. Before transplantation, titers for hepatitis A, B, C, and D, herpesvirus, EpsteinBarr virus, human immunodeficiency virus, Toxoplasma, and cytomegalovirus were perfonned on all donors and recipients. Skin testing for tuberculosis and anergy was routine. Sputum cultures were obtained every 3 months from candidates with cystic fibrosis to guide perioperative antimicrobial prophylaxis.
Unless surveillance cultures suggested otherwise, perioperative antibiotics consisted of clindamycin, ceftazidime, and amphotericin B. They were maintained for 48 hours after the operation and were adjusted to cover bacterial sensitivities obtained from the donor culture. Patients with cystic fibrosis always received an aminoglycoside for 10 to 14 days. Since 1989, our prophylactic strategy for cytomegalovirus has included intravenous ganciclovir for 4 weeks, followed by acyclovir (650 mg/m2 per day) for 6 months. Only cytomegalovirus-negative blood products were administered. Oral mycostatin and trimethoprim sulfamethoxazole were given to all patients after their transplant operation. Surveillance cultures from the trachea or bronchoalveolar lavage fluid were obtained on patients who remained dependent on a ventilator for more than 72 hours and at the time of routine transbronchial biopsy. Serologic follow-up was performed on all seronegative patients and on seropositive patients to monitor for evidence of reactivation.14
Results
Survival and mortality
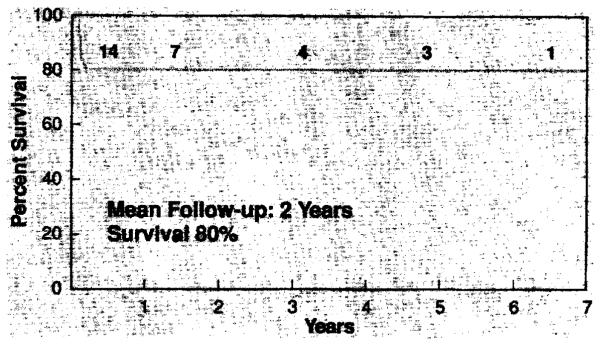
Sixteen of the 20 (80%) patients in this series survived. The perioperative mortality (45 days after transplantation) was 20%. There have been no late deaths. An overview of the survival and follow-up is depicted in Fig. 3. The mean follow-up was 2 years and the longest follow-up, 6½ years. Eleven of the lung transplants in this series were done within the last year, and nine (82%) of the patients have survived. There were no deaths and no cases of postoperative bleeding in the four patients who had had prior thoracic operations. The four deaths in this series are detailed as follows:
Fig. 3.
Pediatric lung transplantation, survival—1985 to 1992.
CASE I. Our first pediatric heart-lung transplant recipient (June 1985) was a 14-year-old girl from Oklahoma who had congenital heart disease (double-inlet left ventricle, hypoplastic right ventricle with discordant ventriculoarterial connection, and transposition of the great arteries). She returned to the hospital on the day of discharge (postoperative day 20) with a mild cough, shortness of breath, and a diffuse interstitial pulmonary infiltrate. Bronchoalveolar lavage failed to reveal a pathogenic organism or cell differential suggestive of infection. She was treated with intravenous methylprednisolone for 3 days. She died on postoperative day 43, despite extracorporeal membrane oxygenation support, of cytomegalovirus pneumonia. Lung procurement and transportation were done with the autoperfused heartlung block. Immunosuppressive drugs included cyclosporine, azathioprine, and steroids. This patient’s course and treatment antedated our routine use of intravenous ganciclovir and transbronchial biopsy.
CASE 2. Our second pediatric recipient was a 12-year-old girl with primary pulmonary hypertension who underwent heart-lung transplantation in July 1985. She died on the second postoperative day of primary organ nonfunction, despite initial adequate arterial oxygenation. The donor had been injured in a motor vehicle accident and had had long bone and pelvic fractures. Postmortem examination of the lungs revealed bone and cartilage emboli in the pulmonary vasculature. Lung preservation in this case was core-cooling of a donor transported to our center. Immunosuppressive therapy comprised cyclosporine, azathioprine, and steroids.
CASE 3. A 13-year-old girl with cystic fibrosis had waited more than I year for lung transplantation when she had a severe pulmonary exacerbation with a temperature of 104° F in July 1991, necessitating intubation and mechanical ventilation. She underwent double lung transplantation on the third day of mechanical ventilation. Cultures of blood drawn before transplantation subsequently grew two Pseudomonas organisms. Although. initial lung function was excellent, she was never extubated and died on postoperative day 30 of disseminated aspergillosis despite continued prophylaxis with intravenous amphotericin B. Lung preservation was by flushing with University of Wisconsin solution and ischemic times were 390 minutes and 420 minutes. Immunosuppressive agents included FK 506, azathioprine, and steroids.
CASE 4. A 1O-year-old girl with cystic fibrosis had double lung transplantation in February 1992. She was extubated on postoperative day 2 with excellent oxygenation and a clear chest radiograph. She required reintubation on postoperative day 10, and results of both transbronchial biopsy and open lung biopsy were consistent with diffuse alveolar damage and no acute cellular rejection. The patient had a tracheostomy and her condition began to improve slowly. However, an adenoviral pneumonia developed and she died on postoperative day 45. Lung preservation was by flushing with University of Wisconsin solution and ischemic times were 213 minutes and 333 minutes. Immunosuppressive therapy comprised FK 506, azathioprine, and steroids.
Rejection
Despite the advent of standardized nomenclature for the histologic features of lung rejection and retrospective reclassification of all lung biopsy specimens in this series, we have chosen to define rejection as a “treated” episode. There have been no deaths as a result of rejection. No patient had a panel reactive antibody greater than 10%. All retrospective crossmatches were negative. The mean number of treated episodes of rejection in the 14 patients observed for more than 100 days was 1.4 at less than 30 days, 0.5 at 30 to 90 days, and 1.4 at more than 90 days. The mean number of days to the first treated rejection episode was 28 days and to the second episode, 100 days. There was no statistically significant difference in these parameters of rejection between patients treated with cyclosporine and those treated with FK 506. No patient in the FK 506 group has required treatment with any antilymphocyte agent. Six of seven patients in the cyclosporine group have required an average of two treatments per patient, with mouse antilymphocyte globulin, rabbit antithymocyte globulin, or OKT3. However, only three of these patients received the antilymphocyte therapy within the first year after transplantation.
Four (25%) patients in this series have obliterative bronchiolitis. Two of these patients have mild obliterative bronchiolitis with only moderate diminution in pulmonary function at 4 and 5 years after transplantation. The other two patients have obliterative bronchiolitis and chronic rejection associated with substantial reduction in small airway function, although both patients remain quite active. One patient with mild obliterative bronchiolitis and one with obliterative bronchiolitis and chronic rejection have associated Pseudomonas bronchitis.
Cardiac function has been excellent in all heart-lung recipients. There have been no significant episodes of cardiac rejection with the exception of one patient who had grade 3B rejection after reduction in immunotherapy for the treatment of posttransplantation lymphoproliferative disease. Coronary angiograms have shown no abnormalities.
Spirometry
Six months after the operation, the average FEV1 was 82% of predicted (range 66% to III %), FVC was 89% of predicted (range 72% to 108%), and FEF25.75 was 72% of predicted (range 54% to 98%). Patients at I-year follow-up showed continued improvement in these measures of pulmonary function. Only two patients with obliterative bronchiolitis had FEV1, FVC, or FEF25.75 of less than 80% of predicted normal values. The patient with a single lung transplant was excluded from these figures: she has had three courses of intravenous steroids and one course of rabbit antithymocyte globulin in 9 months. Her FEV1 is 64%, FVC 57%. and FEF2575 77% of predicted normal values.
Infection
Infection caused two deaths, one as a result of cytomegalovirus pneumonia and the other as a result of aspergillosis. There were four other major infections among the 16 survivors. (1) Cytomegalovirus pneumonia occurred in a cytomegalovirus-negative recipient receiving a cy10mcgalovirus-positivc donor organ, despite 4 weeks of intravenous ganeiclovir after transplantation. (2) A pneumocoecal pneumonia developed 2 days after a bronchoalveolar lavage and transbronchial biopsy but quickly resolved with intravenous antibiotics. (3) A respiratory syncytial viral pneumonia occurred in one young girl who required prolonged intubation; this resolved with ribavirin therapy. (4) Hepatitis caused hy Epstein-Barr virus developed 2 years after transplantation in an Epstein-Barr–positive recipient and resolved with reduction in immunosuppression. There were three minor infections: One patient had coxsackie virus infection and two patients had mild bacterial bronchitis. Six cytomegalovirus-negative recipients received cytomegaloviruspositive donor organs, and as yet only one patient has cytomegalovirus disease. Two patients had cytomegalovirus-positive bronchoalveolar lavage studies, but the infection resolved with intravenous ganciclovir treatment.
Posttransplantation lymphoproliferative disease
Two (12.5%) Epstein-Barr–negative recipients have posttransplantation lymphoproliferative disease. In both patients the disease became apparent in the lung during the third month after transplantation, as a diffuse infiltrate in one and as multiple pulmonary nodules associated with tonsillopharyngitis and hepatosplenomegaly in the other. Each patient had an associated infection. respiratory syncy1ial virus and cytomegalovirus from a bronchoalveolar lavage, respectively. These patients had reduction in immunotherapy and recovered with no recurrent disease. The first patient had received rahbit antithymocyte glohulin immunoprophylaxis and cyclosporinc-based triple-drug therapy and the other received FK 506-hased triple-drug therapy without an antilymphocyte prcparation.
Other complications
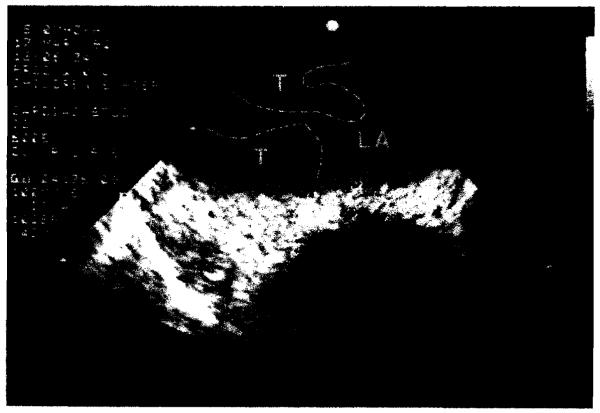
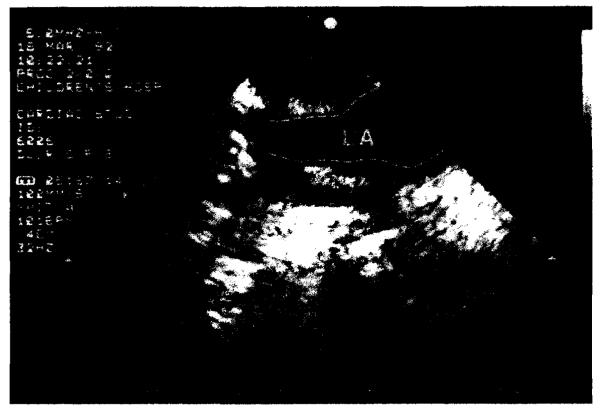
A summary of the complications obscrved in this group is outlined in Table 1. Postoperativc problcms ranged from minor drug-related toxicities to those necessitating operutive intervention. One patient who had right-sided pulmonary venous thrombosis was reintubated on postoperative day 4 and the diagnosis of pulmonary venous thrombosis was made by transesophageaI echocardiography (Figs. 4 and 5). At reaperation the anastomosis appeared usual except for obstructing thrombus. After revision, the patient was treated with intravenous heparin for 4 weeks and, todate, the problem has not recurred. The bronchial stenosis, which was mild, was due to a telescoped left main bronchial cartilage and has not required treatment. The uterine and nasal bleeding were systemic manifestations of capillary hemangiomatosis in a patient with pulmonary arteriovenous malformation. The gastric atony was due to vagal nerve paresis. which occurred during sequential bilateral lung transplantation, After the patient’s discharge from the hospital, gastric outlet obstruction developed, which necessitated readmission and pyloroplasty.
Table 1.
Complications
| No. | % | |
|---|---|---|
| Postoperativc bleeding | 3/20 | 15% |
| Phrenic paresis | 2/20 | 10% |
| Bronchial stenosi, | 1/16 | 6% |
| Pulmonary vein thrombosis | 1/16 | 6% |
| Hypertension | 4/16 | 25% |
| Group 1 (cyclosporine) | 4/8 | 50% |
| Group 2 (FK506) | 0/8 | 0% |
| Hirsutism (cyclosporine) | 1/16 | 6% |
| Gingival hyperplasia (cyclosporine) | 1/16 | 6% |
| Uterine/nasal bleeding | 1/16 | 6% |
| Gastrointestinal | 2/16 | 12% |
| Pathology | Treatment | Postoperativc day |
| Appendicitis | Appendectomy | 35 |
| Gastric atony | Pyloropldsty | 40 |
Fig. 4.
Transesophageal echocardiogram, posttransplantation day 4, demonstrating right pulmonary venous thrombus with extension into the left atrium. T, Thrombus; LA. left atrium.
Fig. 5.
Transesophageal echocardiogram after repair of right pulmonary venous anastomosis under cardiopulmonary bypass, posttransplantation day 5, LA, Left atrium.
The average stay in the intensivecare unit was 12 days (range I to 90 days): nine of 16 patients stayed less than I week, The average period of postoperative intubation was 6 days (range I to 4S days); nine of 16 patients were intubated 3 days or less,
Discussion
Overall, we have been gratified with our results in these 20 patients: an 80% survival and no late deaths at a mean follow-up of 2 years. In many respects these children were the beneficiaries of the difTicult lessons learned in the arena of adult lung transplantation. An additional advantage for children is their youth–both their own youth and that of the donor. Compared with results in our adult series. primary nonfunction because of preservation injury was notably absent as a complication in this group, perhaps a testament to the higher threshold for ischemic injury of p(:diatric tissues.
We did not consider prior thoracotomy or sternotomy an absolute contraindication to lung transplantation, For example, one patient with pulmonary arteriovenous malformation underwent right lower lobectomy at 3 years of age and coil embolization of the right upper lobe at 4 and 5 years of age. This boy weighed 16 kg and was 117 cm tall. He had been wheelchair-bound and dependent on supplementary oxygen for 2 years and had never attended school despite his age of 10 years. Three other patients, as previously noted, had prior cardiac operations. There were no reoperations for bleeding and no deaths in this group. A prior thoracic operation is not an absolute contraindication to lung or heart-lung transplantation; it is advisable, however, to accrue experience in patients without prior thoracic operations before undertaking the additional challenges involved in reoperation. Another patient in our series was of particular note because of her pretransplantation status. A 15-year-old girl had received a bone marrow transplant from her sister (human leukocyte antigen-identical and mixed lymphocyte culturle-compatible) at the age of 7 years for aplastic anemia. Her course after bone marrow transplantation was complicated by severe graft-versus-host disease, pneumonia, respiratory insufficiency, dependence on mechanical ventilation, and tracheostomy. Eight years later the FEVI, FVC, and FEF25-75 were all 10% to 20% of predicted, and she was dependent on supplementary oxygen. Her bone marrow function was normal and she was accepted for lung transplantation.
Perhaps the most important lessons to be learned are those gleaned from our failures. Two of the four patients who died had cystic fibrosis. Although cystic fibrosis, as an indication for lung transplantation, represents an increasing population, in our opinion these patients are particularly challenging candidates and represent a highrisk group. The evaluation of patients with cystic fibrosis can be difficult, because the course of their pulmonary disease waxes and wanes; however, when a patient with cystic fibrosis becomes dependent on supplementary oxygen, it is reasonable to begin the evaluation for lung transplantation. Along with the problems associated with all other patients with end-stage lung disease, patients with cystic fibrosis have tracheal colonization with organisms that are difficult to control with antibiotics. These include Pseudomonas aerugiTUJsa. Pseudomonas cepacia, and frequently (60%) Aspergillus. Patients with cystic fibrosis also commonly are malnourished and have chronic sinusitis and osteoporosis. Indwelling intravenous or enteral catheters are frequently in place at the time of transplantation. Therapy directed toward each individual patient’s microbial colonization is mandatory. Compassionate but emotional decisions designed to salvage particularly those patients in extremis should be avoided. The third death in our series was due to occult pulmonary emboli in the donor; when present, this is a lethal complication for the recipient. In our experience with nearly 200 lung donors, four other such cases were observed, three with thrombotic emboli and one with a brain embolus.15 The fourth death in our series was due to cytomegalovirus pneumonia, in part the result of ill-advised empiric treatment of rejection without histologic confirmation. This occurred before our routine use of transbronchial biopsy and before ganciclovir was available.
We strongly concur with the national trend toward the use of single and double lung procedures whenever possible. Donor organs are thus used more efficiently and benefit a greater number of recipients. The first double lung transplantation performed in a child was done at CHP (January 1989) in a 15-year-old girl from Seattle with pulmonary arteriovenous malformation. The operation was done with cardiopulmonary bypass and tracheal, left atrial, and pulmonary arterial anastomoses. Our preferred technique for double lung transplantation is that of bilateral sequential single lung transplantation, for which we perform telescoped bronchial anastomoses through a transthoracic incision. Our second double lung transplantation was in a child with primary pulmonary hypertension. Dramatic resolution of right ventricular dilatation and hypertrophy was observed after transplantation. Eight of our adult patients have done well after repair of their congenital heart defects at the time of double lung transplantation. Clearly, this approach can be applied to the pediatric group as well. Only complex forms of congenital heart disease necessitate heart-lung transplantation, but when a heart-lung block is available and no heart candidate is identified, we have chosen to use heart-lung transplantation. The answers and long-term results are not yet available to questions regarding the differences in peak exercise capacity, right-sided heart function, pulmonary pressures, prevalence of obliterative bronchiolitis, and survival in patients with single versus double lung transplants; multicenter trials directed at specific patient groups are needed.
Infection is a constant threat to the lung transplant recipient, and in this pediatric group viral infections posed the greatest dangers. All recipients now receive intravenous ganciclovir for 4 weeks after transplantation and high-dose oral acyclovir for 6 months to protect them against cytomegalovirus. Conclusions regarding the effectiveness of this treatment strategy will require a larger patient group and longer follow-up. Cytomegalovirus is not just an infectious threat; rather, it places the recipient at significant risk of future rejection because of up-regulation of immune mechanisms systemicallyand in the graft, locally.16 In addition, Epstein-Barr virus is of particular concern to the pediatric lung recipient. We17 have shown, during a decade of heart and lung transplantation, that both the pediatric organ recipient and the lung recipient are at increased risk for the development of posttransplantation Iymphoproliferative disease. It is thus not surprising to observe a 12.5% prevalence of posttransplantation lymphoproliferative disease in our pediatric lung transplant group. We were fortunate to have recognized the disease and successfully treated both recipients in whom posttransplantation lymphoproliferative disease was diagnosed. It is helpful to remember that this disease is most likely to appear in the lung of a lung recipient and usually occurs within the first posttransplantation year. Other infections have been relatively few in this group.
The trial of FK 506 in the pediatric group was begun because of the dramatic immune and therapeutic advantage this agent had in the pediatric cardiac transplant recipients. 18 We are hopeful that the improved freedom from rejection, steroid-sparing effects, and lack of hypertension, hirsutism, gingival hyperplasia, and facial bone growth abnormalities will be realized by the pediatric lung recipients as well. The FK 506 trial was also designed to determine if obliterative bronchiolitis may be less prevalent under the influence of this potent immunosuppressive macrolide. At this early juncture, we have not seen obliterative bronchiolitis in any patient receiving FK 506. Because of the relatively short follow-up in our FK 506-treated patients, direct comparisons between cyclosporineand FK 506 are of little value. It is safe to say that, under both protocols, most patients will have one or two episodes of acute lung rejection in the first 90 days. We have, however, successfully avoided the use of antilymphocyte agents thus far in the FK 506 group.
We stand at the beginning of an era when lung transplantation will bring new hope, life, and joy to young patients with end-stage lung disease. We hope this small series raises new questions and challenges to those involved in the field. Clearly, we can all broaden the scope of candidates who will benefit from lung transplantation and we should continue the endeavor to maximally use the available lung donor pool.
Acknowledgments
Special thanks for assistance to the Pediatric Lung Transplant Team of Children’s Hospital of Pittsburgh: Lynne A. Cipriani, RN; Blakeslee E. Noyes, MD; David Orenstein, MD; Arthur Atlas, MD; Melissa Chen-Woan, PhD; Adriana Zeevi, PhD; Susan Miller, MD; Marian Michaels, MD; Ellen Wald, MD;Steven Webber, MD; Kathy Lawrence, RN; Patty Weber, RN; Ko Bando, MD; and Mary Jane Hutchison.
APPENDIX
Discussion
Dr. R. Morton Holman III (Minneapolis. Minn.)
Dr. Annitage, I am amazed at your results. Pittsburgh continues to show tremendous leadership in the field of thoracic organ replacement. You mention a group of eight patients who had Eisenmenger’s physiology on whom you performed double lung transplantation in conjunction with repair of the cardiac defects. Could you extrapolate this to your pediatric population and tell us what congenital heart defects you consider repairable at the time of concomitant lung replacement?
I would also like some advice on redo operations. We are seeing, as you are, I am sure, many patients who have, until recently, been thought to be unable to have another operation because of multiple previous procedures for congenital heart disease. With the success that you and others have enjoyed in this area, can we reconsider these patients? Where do we draw the line? Is aprotinin going to have a role and how do you envision the future in this area? We have now performed II transplants in seven children. Six of seven have been long-term survivors, but we have observed the need for retransplantation in four of those patients. One patient has also had a recurrence of his original disease. Obliterative bronchiolitis has developed in two of the six, so we do see an approximate equivalent incidence to that observed in our adult population. Could you address these questions?
Dr. Hani Shennib (Montreal, Quebec, Canada)
You are achieving impressive results with pediatric transplantation. As a result of the initial poor results reported in pediatric transplantations, we reviewed our own data at the joint MarseilleMontreal lung transplant program and presented our data at the last Society of Thoracic Surgeons meeting. We showed that a 78% I-year survival can be obtained after bilateral lung transplantation in children, so I am very pleased to see you confirming our results.
I have a question with regard to your conclusion that patients with cystic fibrosis constitute a high-risk group. Perhaps the few patients with cystic fibrosis that you have treated in the pediatric population have been at particularly high risk because of their need for ventilatory support, because of resistant bacteria, or because of their need for high-dose steroids. Almost all of the patients in our series have had cystic fibrosis, except for one patient with complex congenital heart disease. I would like to know why you state that patients with cystic fibrosis are at higher risk and how you explain the discrepancy between our report and yours. I would also like to get your advice on what operation you would do, and for what indications, in patients having complex congenital heart disease involving transposition. Are there any technical precautions you would advise us to use?
Dr. Armitage
In the adult population we have done seven double-lung transplantations and one single lung transplantation at the time of a corrective procedure on the heart or great vessels. Most of these anomalies were atrial septal defect, ventricular septal defect, and patent ductus. The patient with patent ductus can be difficult to treat. The duct is usually quite large and the pulmonary pressures are usually quite high. Obtaining control of that ductus and clamping it before the start of cardiopulmonary bypass can be difficult.
Where does the limit lie as to what congenital defect can be repaired at the timeofdouble lung transplantation? I cannot say for sure. We have mainly addressed the more routine problems: atrial and ventricular septal defect and patent ductus. Clearly transpositions can be repaired. Our experience in children is that although heart-lung blocks are difficult to obtain for the child, they are not as difficult as in the adult, because there are not as many children needing a donor heart. Occasionally we are offered two lungs in a case in which there is no adequate recipient for the heart. If we have the opportunity, we prefer heartlung transplantation in a child ifthe organs are available and we are not depriving anyone else. Clearly, if there is a child who needs that heart, we use just the lungs.
Our preferred technique in double lung transplantation is bilateral sequential single lung transplantation with telescoping bronchial anastomoses. Our one case of bronchial stenosis occurred in a lO-year-old patient with cystic fibrosis and a long, small left bronchus. I would concur with the previous comment to be careful with that technique in a child.
Which redos to do? Initially, we agonized over doing the redos at all. We became a little braver as we went along. The operations in both the children with Mustard procedures were a little bit difficult. One of them required end-to-end inferior and superior vena caval anastomoses. The baffle calcified, and it was difficult to identify the phrenic nerve. Those are the kind of problems encountered in patients having had a prior operation. They frequently require a little longer ventilation if phrenic nerve paresis develops.
I do not think that all patients with cystic fibrosis are at high risk, but some are. They have multiple antibiotic-resistant organisms; they are malnourished; they have osteoporosis; they have indwelling catheters. Those are all issues that we deal with and struggle with to get these patients through. I believe the overall world survival in cystic fibrosis patients with lung, heart-lung, or double lung transplantation is about 65%. That is in all centers. These patients will certainly represent an increasing candidate population, but I still believe that it is difficult to get them through the procedure.
Footnotes
Read at the Seventy-second Annual Meeting of The American Association for ThoracicSurgery, Los Angeles, Calif., April 26-29, 1992.
REFERENCES
- 1.Krien JM, Kaye MP. The Registry of the International Society for Heart and Lung Transplantation: eighth official report-1991. J Heart Lung Transplant. 1991;10(4):491–8. [PubMed] [Google Scholar]
- 2.Griffith BP. Heart and heart-lung transplantation. In: Welch KJ, Randolph JG, Ravitch MM, O’Neill JA, Rowe MI, editors. Pediatric surgery. 4th ed. Vol. 1. Chicago: 1986. pp. 383–92. Year Book. [Google Scholar]
- 3.Patterson GA, Cooper JD, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg. 1988;45:626–33. doi: 10.1016/s0003-4975(10)64763-7. [DOI] [PubMed] [Google Scholar]
- 4.Bisson A, Bonnette P. A new technique for double lung transplantation. J THORAC CARDIOVASC SURG. 1992;103:40–6. [PubMed] [Google Scholar]
- 5.Cooper JD, Pearson FG, Patterson GA, et al. Technique of successful lung transplantation in humans. J THORAC CARDIOVASC SURG. 1987;93:173–81. [PubMed] [Google Scholar]
- 6.Zenati M, Dowling RD, Armitage JM, et al. Organ procurement for pulmonary transplantation. Ann Thorac Surg. 1989;48:882–6. doi: 10.1016/0003-4975(89)90696-6. [DOI] [PubMed] [Google Scholar]
- 7.Zenati M, Dowling RD, Dummer JS, et al. Influence of the donor lung on development of early infections in lung transplant recipients. J Heart LungTransplant. 1990;9:502–9. [PubMed] [Google Scholar]
- 8.Hardesty RL, Griffith BP. Autoperfusion of the heart and lungs for preservation during distant procurement. J THORAC CARDIOVASC SURG. 1987;93:11–8. [PubMed] [Google Scholar]
- 9.Baldwin JC, Frist WH, Starkey TD, et al. Distant graft procurement for combined heart and lung transplantation using pulmonary artery flush and simple topical hypother- mia for graft preservation. Ann Thorac Surg. 1987;43:670–3. doi: 10.1016/s0003-4975(10)60249-4. [DOI] [PubMed] [Google Scholar]
- 10.Tamaura K, Kobayashi M, Hashimoto K, et al. A highly sensitive method to assay FK 506 levels in plasma. Transplant Proc. 1987;19(suppl 16):23–9. [PubMed] [Google Scholar]
- 11.Zeevi A, Rabinowich H, Yousem SA, et al. Presence of donor-specific alloreactivity in histologically normal lung allografts is predictive of subsequent bronchiolitis obliterans. Transplant Proc. 1991;23:1128–9. [PubMed] [Google Scholar]
- 12.Yousem SA, Berry GJ, Brunt EM, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1990;9:593–601. [PubMed] [Google Scholar]
- 13.Billingham ME, Cary NRB, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. J Heart Lung Transplant. 1990;9:587–92. [PubMed] [Google Scholar]
- 14.Kusne S, Dummer JS, Singh N, et al. Infections after liver transplantation: an analysis of 101 consecutive cases. Medicine. 1988;67:132. doi: 10.1097/00005792-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosendale BE, Keenan RJ, Duncan SR, et al. Donor cerebral emboli as a cause of acute graft dysfunction in lung transplantation. J Heart Lung Transplant. 1992;11:72–6. [PubMed] [Google Scholar]
- 16.Keenan RJ, Lega ME, Dummer JS, et al. Cytomegalovirus serologic status and postoperative infection correlated with risk of developing chronic rejection after pulmonary transplantation. Transplantation. 1991;51:433–8. doi: 10.1097/00007890-199102000-00032. [DOI] [PubMed] [Google Scholar]
- 17.Armitage JM, Kormos RL, Stuart RS, et al. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10:877–86. [PubMed] [Google Scholar]
- 18.Armitage JM, Fricker FJ, Del Nido P, Cipriani L, Starzl TE. The clinical trial of FK 506 as primary and rescue immunosuppression in pediatric cardiac transplantation. Transplant Proc. 1991;23:3058–60. [PMC free article] [PubMed] [Google Scholar]