Abstract
Maedi-visna virus (MVV) is a lentivirus of sheep, causing slowly progressive interstitial pneumonia and encephalitis1. The primary target cells of MVV in vivo are considered to be of the monocyte lineage2. Certain strains of MVV can replicate in other cell types, however3,4. The green fluorescent protein is a commonly used marker for studying lentiviruses in living cells. We have inserted the egfp gene into the gene for dUTPase of MVV. The dUTPase gene is well conserved in most lentivirus strains of sheep and goats and has been shown to be important in replication of CAEV5. However, dUTPase has been shown to be dispensable for replication of the molecular clone of MVV used in this study both in vitro and in vivo6. MVV replication is strictly confined to cells of sheep or goat origin. We use a primary cell line from the choroid plexus of sheep (SCP cells) for transfection and propagation of the virus7. The fluorescent MVV is fully infectious and EGFP expression is stable over at least 6 passages8. There is good correlation between measurements of TCID50 and EGFP. This virus should therefore be useful for rapid detection of infected cells in studies of cell tropism and pathogenicity in vitro and in vivo8.
Protocol
1. Transfection
The molecular clone is contained in two plasmids, p8XSp5-egfp and p67r, of 12 kb and 4.5 kb respectively.9,10
Cut equimolar quantities of the two plasmids, i.e. 4.4 μg of p8XSp5-egfp and 1.6 μg of p67r with XbaI and ligate before transfection.
For ligation, use 1 Weiss unit of ligase in a 50 μl reaction and incubate at 16°C overnight.
When ligation is completed, incubate the ligation mix at 65°C for 15 min for inactivation of the ligase. This step may be omitted.
Two days before transfection, seed 106 SCP cells in a T25 tissue culture flask, and grow cells for two days in 5 ml DMEM + 10% lamb serum and 2mM glutamine without antibiotics to a monolayer of 90% confluency. If the cells have not reached 90% confluency after two days, leave the cells to grow one additional day.
Note: The lamb serum can be substituted by fetal bovine serum (FBS). We routinely use lamb serum, since some strains of MVV are inhibited by FBS; this MVV strain is not inhibited by FBS, however.
When the cells are ready for transfection, change medium to DMEM with 1% serum (lamb serum or FBS) without antibiotics. We use Lipofectamin 2000 (Invitrogen) for transfection.
Dilute the DNA in 500μl Opti-MEM medium.
Dilute 20μl Lipofectamin 2000 in 500μl Opti-MEM medium and leave at room temperature (RT) for 5 min.
After the 5 min incubation, combine the diluted DNA with diluted Lipofectamine 2000 (total volume = 1000 μl). Mix gently and incubate for 20-30 min at RT.
Add the transfection mix (1 ml) to the T25 flask containing SCP cells and medium (5 ml). Mix by rocking gently.
Incubate at 37°C and 5% CO2 overnight (18 – 24 h).
Change medium the following day to DMEM with 1% serum, 2mM glutamine, 100IU penicillin and 100IU streptomycin.
Monitor the production of GFP in the SCP cells by inverted fluorescent microscopy. Low transfection efficiency can be expected with these primary cells, usually 5 – 15%.
Incubate further at 37°C, 5% CO2 for several days to allow spreading of the virus in the flask. It usually takes 7-14 days for full infection.
Harvest the virus by aliquoting the supernatant into Eppendorf tubes. Spin in a microfuge at 3000 rpm for 5 min to remove cell debris. Transfer the supernatant containing the virus to new tubes. Store at -80°C.
2. Titration of virus
Seed 3x104 SCP cells in 100 μl DMEM medium supplemented with 10% serum, 2mM glutamine, 100IU/ml penicillin and 100IU/ml streptomycin, per well in a 96 well plate. Incubate at 37°C, 5% CO2.
If the cells are confluent the following day, change medium to DMEM with 1% serum, 2mM glutamine, 100IU/ml penicillin and 100IU/ml streptomycin, 100 μl in each well.
- Dilute the virus in the following way:
- Add 180 μl DMEM with 1% serum, 2mM glutamine, 100IU/ml penicillin and 100IU/ml streptomycin per well to 4 x 10 wells in a 96 well round bottom or V-bottom plate.
- For serial tenfold dilutions in quadruplicate, add 20 μl of virus to each of 4wells in the first column of the plate, and using a four-channel pipette, pipette up and down to ensure that the samples are thoroughly mixed. Change pipette tips and transfer 20 μl to the next 4 wells and so on. Leave the last wells without virus for a negative control.
Add 100 μl of the virus dilutions to the SCP cells and medium.
Incubate at 37°C and 5% CO2. Observe for GFP fluorescence and cytopathic effects for two weeks. Cytopathic effects consist of cells becoming rounded and less transparent than normal cells with processes stretching out, ending with multinuclear cells appearing and cell death.
Calculate virus titer (TCID50) using the Reed Muench method11.
3. FACS analysis of infected cells
Note: For rapid monitoring of replication of the virus, infected cells can be examined by flow cytometry.
Trypsinize infected cells (ca 105), wash 1x with PBS and resuspend in 500μl PBS.
Add 500 μl 4% paraformaldehyde solution (2% final concentration), incubate at RT for 30 min.
Pellet cells in a microfuge at 3000 rpm for 5 min.
Wash with PBS 2x5 min and analyze on a FACScan analyzer. Count 10,000 events.
4. Representative results:
This virus should be fully infectious and replicate to a titer of 106 – 107 TCID50/ml. Infected cells are fluorescent and can be detected by flow cytometry and fluorescence microscopy. Typically, GFP can be detected in more than 60% of the cells in the infected cell cultures using flow cytometry (Fig. 1). Likewise, when visualized by fluorescence microscopy, a majority of the cells are fluorescent (Fig. 2A and B).
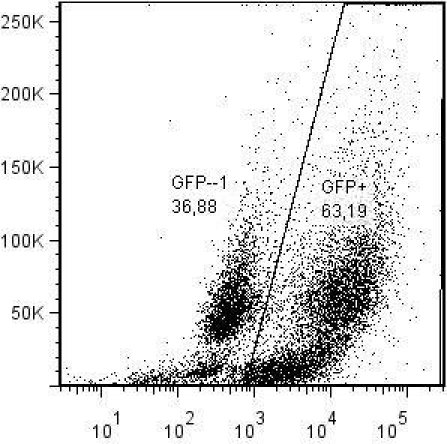 Figure 1. FACScan analysis of SCP cells infected with KV1772-egfp virus after 7 days of infection.
Figure 1. FACScan analysis of SCP cells infected with KV1772-egfp virus after 7 days of infection.
 Figure 2. Phase contrast microscopy of SCP cells infected with KV1772-egfp after 7 days of incubation (A). Same cells visualized by fluorescence microscopy (B).
Figure 2. Phase contrast microscopy of SCP cells infected with KV1772-egfp after 7 days of incubation (A). Same cells visualized by fluorescence microscopy (B).
Discussion
The GFP expressing molecular clone of MVV presented here should be useful for analyzing the host-cell interactions and pathogenicity of MVV both in vitro and in vivo. The virus obtained after 7-14 days of replication has undoubtedly acquired some mutations due to the inaccuracy of reverse transcriptase. However, there are limitations to the use of this clone as a single cycle vector. One is that use of the clone is restricted to cells of sheep and goat origin. The transfection efficiency of these cells using our construct is only 5-15%, and using a pEGFP-N3 vector the transfection efficiency is 15-20% compared to over 90% using 293-T cells. 293-T cells have been tested as packaging cells for MVV vectors, but the resulting virus particles proved non-infectious both in sheep cells and in 293-T cells12 probably due to unidentified restriction factors. The infectious clone is contained in two plasmids, which is another shortcoming. This is due to the fact that the full-length clone is unstable in E. coli. However, it should be possible to clone the full-length clone in a low copy plasmid.
Plasmids and cells can be obtained from the authors.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was funded by the Icelandic Research Fund and the University of Iceland Research Fund.
References
- Sigurdsson B, Palsson PA, Grimsson H. Visna, a demyelinating transmissable disease of sheep. J. Neuropathol. Exp. Neurol. 1957;14:389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- Gorrell MD, Brandon MR, Sheffer D, Adams RJ, Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J. Virol. 1992;66:2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresdottir V. Biological and genetic differences between lung- and brain-derived isolates of maedi-visna virus. Virus Genes. 1998;16:281–293. doi: 10.1023/a:1008030706308. [DOI] [PubMed] [Google Scholar]
- Georgsson G, Houwers DJ, Palsson PA, Petursson G. Expression of viral antigens in the central nervous system of visna-infected sheep: an immunohistochemical study on experimental visna induced by virus strains of increased neurovirulence. Acta Neuropathol. 1989;77:299–306. doi: 10.1007/BF00687582. [DOI] [PubMed] [Google Scholar]
- Turelli P, Guiguen F, Mornex JF, Vigne R, Querat G. dUTPase-minus caprine arthritis-encephalitis virus is attenuated for pathogenesis and accumulates G-to-A substitutions. J. Virol. 1997;71:4522–4530. doi: 10.1128/jvi.71.6.4522-4530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petursson G. Visna virus dUTPase is dispensable for neuropathogenicity. J. Virol. 1998;72:1657–1661. doi: 10.1128/jvi.72.2.1657-1661.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson B, Thormar H, Palsson PA. Cultivation of visna virus in tissue culture. Arch Gesamte Virusforsch. 1960;10:368–380. doi: 10.1007/BF01241886. [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir HS, Olafsdottir K, Franzdottir SR, Andresdottir V. Construction and characterization of an infectious molecular clone of maedi-visna virus that expresses green fluorescent protein. J. Virol. Methods. 2010;168:98–102. doi: 10.1016/j.jviromet.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Andresson OS. Nucleotide sequence and biological properties of a pathogenic proviral molecular clone of neurovirulent visna virus. Virology. 1993;193:89–105. doi: 10.1006/viro.1993.1106. [DOI] [PubMed] [Google Scholar]
- Skraban R. Naturally occurring mutations within 39 amino acids in the envelope glycoprotein of maedi-visna virus alter the neutralization phenotype. J. Virol. 1999;73:8064–8072. doi: 10.1128/jvi.73.10.8064-8072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- Berkowitz RD, Ilves H, Plavec I, Veres G. Gene transfer systems derived from Visna virus: analysis of virus production and infectivity. Virology. 2001;279:116–129. doi: 10.1006/viro.2000.0659. [DOI] [PubMed] [Google Scholar]


