Abstract
Large scale electrophysiological recordings from neuronal ensembles offer the opportunity to investigate how the brain orchestrates the wide variety of behaviors from the spiking activity of its neurons. One of the most effective methods to monitor spiking activity from a large number of neurons in multiple local neuronal circuits simultaneously is by using silicon electrode arrays1-3.
Action potentials produce large transmembrane voltage changes in the vicinity of cell somata. These output signals can be measured by placing a conductor in close proximity of a neuron. If there are many active (spiking) neurons in the vicinity of the tip, the electrode records combined signal from all of them, where contribution of a single neuron is weighted by its 'electrical distance'. Silicon probes are ideal recording electrodes to monitor multiple neurons because of a large number of recording sites (+64) and a small volume. Furthermore, multiple sites can be arranged over a distance of millimeters, thus allowing for the simultaneous recordings of neuronal activity in the various cortical layers or in multiple cortical columns (Fig. 1). Importantly, the geometrically precise distribution of the recording sites also allows for the determination of the spatial relationship of the isolated single neurons4. Here, we describe an acute, large-scale neuronal recording from the left and right forelimb somatosensory cortex simultaneously in an anesthetized rat with silicon probes (Fig. 2).
Keywords: Neuroscience, Issue 56, neuronal ensembles, silicon probes, spiking, local field potentials, tetrode, acute recordings, rat
Protocol
1. Surgery Preparation
Anesthetize the rat with the appropriate type and dose of anesthetics. Here we use urethane (1.5 g/kg, Sigma-Aldrich Co., St. Luis, MO) - prepared as 20% solution by diluting 20 g of urethane in 100 ml of phosphate buffered saline (PBS) i.e. the animal will receive 7.5 ml/kg of urethane solution intraperitonealy (IP). Because of its dose-response curve, the total dose of urethane is divided in to four applications, each separated by approximately 30 minutes. Before the first two applications of urethane, animal is anesthetized with isoflurane administered at a concentration of 3-4% (oxygen at 2 liters per minute) and maintained at 2% (oxygen at up to 2 liters per minute) to prevent an animal from getting stressed by injection. During surgery, an additional administration of isofluorane (2% concentration for 2 minutes) can be used if needed.
Shave the fur along the incision site (dorsal side of the head) to ensure that the fur will not contaminate the wound and that a sufficient area can be disinfected around the incision site.
Prepare surgical site antiseptically using organic iodine or chlorhexidine soap and antiseptics (ethanol).
Prepare silicon probes (NeuroNexus Technologies, Ann Arbor, MI). Probes can be disinfected using 70% ethanol followed by rinsing with distilled water. If needed, probes can be painted with a fluorescent marker prepared antiseptically (e.g. Dye I, Invitrogen Co.), to later reveal the position of the probe in histological analysis. To do this we use a small and very soft paint brush and delicately touch the back of the silicon probe with the dye. This procedure should always be done over a microscope to make sure the probe is not being damaged or bent. Always check to make sure that the dye is not blocking any of the channels. Any residual dye left on the probe can be later removed with alcohol, followed by rinsing with water.
Silicon electrodes care: Because of their small size, silicon probes are delicate and extremely susceptible to breaking even when using it with considerable care. Thus, we recommend monitoring potential hazards at all times, such as: (i) Keep the probes in an appropriately designated box; (ii) Prevent crashing or dropping probes when handling them; (iii) Make sure that the stereotaxic manipulators are well fixed to prevent any accidents; (iv) When recording, surrounding tissue and/or blood can attach to the probes. Therefore, it is important to be also careful when removing the probes after recording. Using a constant flow of PBS or saline solution is strongly recommended; (v) Always clean the probes after recording. To clean the probes, it is strongly recommended to submerge them in a liquid enzymatic cleaner (Boston, Bausch & Lomb) for a few minutes. Always rinse the probes with distilled water and return them to their dedicated box. In our experience, adequate care of the silicon probes ensures good quality recordings for more than 10 experiments. If a channel 'gets noisy' during use, it should be excluded from subsequent analyses. We have found that cross-talk between channels is minimal; therefore a noisy channel usually has very little effect on a signal in other channels.
2. Surgery
Apply an eye-ointment on eye surface before any surgery manipulations.
Administer Dexamethasone 5 mg/ml subcutaneously to reduce brain inflammation.
Fix the animal in stereotaxic frame throughout surgery.
Administer Lidocaine HCl 2% with Epinephrine (2-4 mg/kg diluted to 0.5 % solution) subcutaneously at the incision site before the incision is made to reduce the potential risk of the wound bleeding and for local anesthesia.
Make a 2 cm incision through the scalp midline and displace the skin laterally to expose the skull surface.
Clear the dorsal scalp from fascia by blunt dissection and control the bleeding by microcautery or by using bone wax.
Make two drilling holes to attach small screws in the occipital bone of the skull for animal grounding and reference. The reference screw must be in contact with dura. In order to reduce electrical noise during the recordings, it is recommended to keep the screws wet at all times. To do this, a small acrylic barrier surrounding the screws can be built or the screws can be covered with agar.
Make a 1-2 mm diameter craniotomy in the coordinates corresponding to the cortical and/or subcortical area(s) of interest 5. Use compressed air to remove bone dust as needed during craniotomy (drilling hole). Prevent skull warm up from drilling by applying drops of PBS. Make a second craniotomy if needed to insert the second probe. In this video, we are presenting a recording from two cortical areas simultaneously, therefore two craniotomies are made.
Build a small barrier of acrylic resin (e.g.: Lang Dental Manufacturing, Co., Inc., Wheeting, IL) surrounding the two craniotomies in order to keep PBS on top of exposed dura, preventing it from drying. Apply PBS constantly during the whole experiment.
Open the dura matter of both craniotomies. Two needles (30 ½ gauge) bended on the tips (forming a hook), may be used for this manipulation.
Attach the connector of the silicon probes to the stereotaxic manipulatiors. Connectors are attached to a pole in order to have the shanks of the probes perfectly parallel to the stereotaxic manipulator. This is crucial to maintain stable recordings and avoid probe damage.
Connect the reference screw to the silicon probe reference pin. To reduce electrical noise the ground screws have to be connected to: i) the ground of the probe plug; ii) the chassis ground from the recording device; and iii) the stereotaxic frame.
Place the tip of the silicon probes just in contact with the pia.
Slowly introduce the silicon probes and make sure that the shanks of the silicon probes (Fig. 1 & 2) can be inserted into the brain without resistance or bending.
Carefully lower the probes until reaching desired recording area. If needed, add drops of PBS on the top of craniotomies to avoid drying of the tissue.
Because urethane anesthesia should only be used for terminal procedures, the animal is euthanized by an i.p. injection of at least 200mg/kg of sodium pentobarbital diluted to a concentration of no more than 60 mg/ml. This is followed by decapitation of the animal.
3. Representative Results:
Representative recordings of local field potentials and spiking activity are illustrated in Figure 3A. After performing spike sorting (discriminating spikes originating from different neurons; Fig 3B,C; 6, 7, 8) silicon probe recordings provide the ability to investigate neuronal population activity involved in, among other, the following processes: memory and decision making 9, plasticity 10, stimulus coding 11, spontaneous activity 12 and effects of various drugs 13.
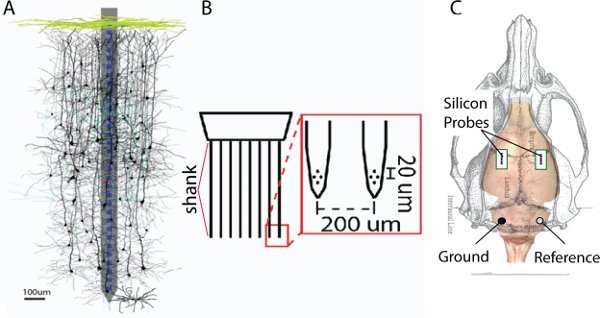 Figure 1. A. Example of a silicon probe with a single shank is superimposed on a montage of reconstructed neurons (courtesy of S. Sakata 14). B. Example of a probe with a tetrode configuration at each of the 8 shanks. C. Schematic of a rat skull. The green boxes represent the extent of the craniotomy over the right and left somatosensory cortex.
Figure 1. A. Example of a silicon probe with a single shank is superimposed on a montage of reconstructed neurons (courtesy of S. Sakata 14). B. Example of a probe with a tetrode configuration at each of the 8 shanks. C. Schematic of a rat skull. The green boxes represent the extent of the craniotomy over the right and left somatosensory cortex.
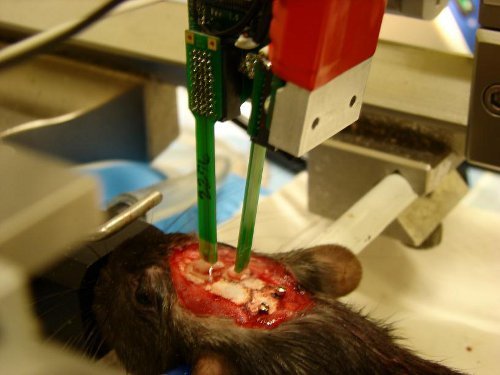 Figure 2. Example of experiment with silicon probes inserted in the prefrontal cortex and hippocampus. Drops of PBS cover the craniotomy to protect the exposed brain from drying. Two screws located over the cerebellum will be connected to ground and reference, respectively.
Figure 2. Example of experiment with silicon probes inserted in the prefrontal cortex and hippocampus. Drops of PBS cover the craniotomy to protect the exposed brain from drying. Two screws located over the cerebellum will be connected to ground and reference, respectively.
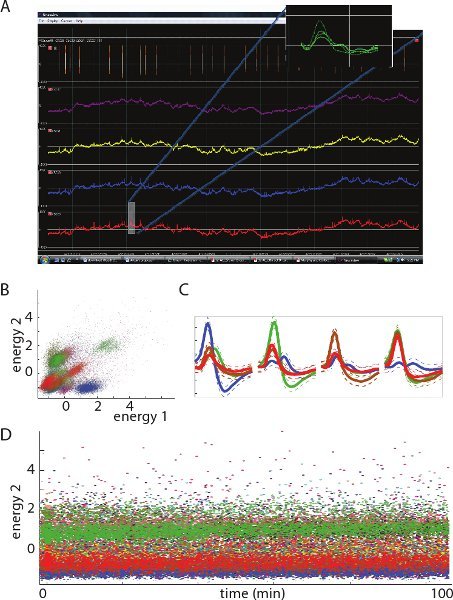 Figure 3. A. Image shows 500ms of a representative electrophysiological recording of Local Field Potential (LFP) from one tetrode (note: negative voltage is plotted up on the ordinate). Insert shows - a waveform of a spike. B. Two-dimensional views of unit clusters in feature space from 1 tetrode. Each cluster represents spikes from a single unit (neuron). C. Average spike waveforms from representative units (color coded) at each of the four recording sites from a single tetrode. D. Waveform energy (related to amplitude) of recorded spikes over time. Note the relatively constant energy values for each unit; this indicates a stable recording over the duration of the displayed period.
Figure 3. A. Image shows 500ms of a representative electrophysiological recording of Local Field Potential (LFP) from one tetrode (note: negative voltage is plotted up on the ordinate). Insert shows - a waveform of a spike. B. Two-dimensional views of unit clusters in feature space from 1 tetrode. Each cluster represents spikes from a single unit (neuron). C. Average spike waveforms from representative units (color coded) at each of the four recording sites from a single tetrode. D. Waveform energy (related to amplitude) of recorded spikes over time. Note the relatively constant energy values for each unit; this indicates a stable recording over the duration of the displayed period.
Discussion
This paper demonstrates how to use the silicon electrode arrays to record from large population of neurons (>100) in multiple cortical areas simultaneously. In order to be successful in surgery and recording, the following issues should be considered:
Introduction of the probes to a desired area: When inserting the probes in brain tissue, it is possible to cause considerable damage. This can result in a low quality of recorded units. To avoid this problem we recommend the following: (i) Introduce the probes at a certain degree angle (recommended to use a 10 degree angle). By doing this, dendritic damage of the recorded neurons can be reduced; (ii) After the probes are inserted in the brain tissue, they are initially lowered at a faster rate (approximately 50 to 100 microns per 10-30 sec) until they get closer to the desired area of recording. When the probes are about 200 microns from the target area, the position is adjusted more slowly (approximately 10 microns every 2 or 3 minutes).
Stabilization of recordings: When the probes are in the designated area and considerable activity is detected (distinctive spikes in the majority of the channels), it is recommended to wait approximately 30 minutes before starting a recording. This will allow brain tissue to mechanically stabilize after the insertion of probes and ensure a more stable recording.
Brain pulsation: Occasionally it is possible to see brain pulsation that can significantly reduce the quality of a recording. A small craniotomy (just enough space to fit the probe) can reduce brain pulsation in a recording area. If necessary, the cisterna magna can be punctured. This reduces cerebrospinal fluid pressure and decreases swelling and pulsation.
Silicon probe configuration: Silicon probes can have a variety of shapes and recording site configurations. For example, they can vary in the number of shanks, the length and thickness of shanks, and in the arrangement of recording sites (e.g. tetrode vs. linear configuration; see: www.neuronexustech.com). The choice of probe used with a specific configuration depends on the scientific question needing to be answered. For example, if the objective is to record populations of neurons in multiple locations in one specific layer (as presented in these recordings), the best choice is to use a probe with eight shanks and a tetrode configuration. This allows for recordings of multiple single units at each tetrode and sampling of eight locations across a 1.4mm span. In another instance, if someone would like to study activity propagations across cortical layers, the best choice would be to use a probe with one shank with regularly distributed recording sites along that shank which allows recording in multiple cortical layers simultaneously 14.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by NSERC & AHFMR. The authors thank Mariam Alaverdashvili and Amanda Mauthe-Kaddoura for comments on the manuscript and Hiroe Yamazaki for help on the surgery preparation.
References
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Kipke DR. Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities. J. Neurosci. 2008;28:11830–11838. doi: 10.1523/JNEUROSCI.3879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J. Neurophysiol. 2003;90:1314–1323. doi: 10.1152/jn.00116.2003. [DOI] [PubMed] [Google Scholar]
- Bartho P. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4th Edition. San Diego: Academic Press; 1998. [Google Scholar]
- Lewicki MS. A review of methods for spike sorting: the detection and classification of neural action potentials. Network. 1998;9:R53–R78. [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Luczak A, Narayanan NS. Spectral representation--analyzing single-unit activity in extracellularly recorded neuronal data without spike sorting. J. Neurosci. Methods. 2005;144:53–61. doi: 10.1016/j.jneumeth.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD. How do neurons work together? Lessons from auditory cortex. Hear. Res. 2011;271:37–53. doi: 10.1016/j.heares.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc. Natl. Acad. Sci. U.S.A. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat. Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


