Abstract
Identification of novel indications for commonly prescribed drugs could accelerate translation of therapies. We investigated whether any clinically-used drugs might have utility for treating prostate cancer by coupling an efficient, high-throughput laboratory-based screen and a large, prospective cohort study. In stage 1, we conducted an in vitro prostate cancer cell cytotoxicity screen of 3,187 compounds. Digoxin emerged as the leading candidate given its potency in inhibiting proliferation in vitro (mean IC50=163 nM) and common use. In stage 2, we evaluated the association between the leading candidate drug from stage 1 and prostate cancer risk in 47,884 men followed 1986–2006. Regular digoxin users (versus nonusers: RR=0.76, 95% CI 0.61–0.95), especially users for ≥10 years (RR=0.54, 95% CI 0.37–0.79, P-trend<0.001), had a lower prostate cancer risk. Digoxin was highly potent in inhibiting prostate cancer cell growth in vitro and its use was associated with a 25% lower prostate cancer risk.
Keywords: Digoxin, prostate cancer, risk, cohort, transdisciplinary, cytotoxicity
Introduction
In the midst of public debates on how to decrease health care costs, the exorbitant costs of new drug development, now estimated to exceed $1 billion for a drug receiving regulatory approval (1), has come into central focus (2). One approach to reduce these costs is to find drugs with established toxicologic, pharmacokinetic, and pharmacodynamic profiles that may be effective for unanticipated indications (3, 4). Rapid laboratory screening of such drugs followed by harnessing the strengths of existing, well-characterized cohort studies could, relatively inexpensively, expedite the identification of drugs that merit testing for novel indications in clinical trials.
With this goal in mind, our transdisciplinary prostate cancer research team investigated whether any clinically used drugs might have utility for prostate cancer treatment by using a novel laboratory-epidemiology two-staged approach. In stage 1, we used an in vitro screening of a library of drugs for prostate cancer cell growth inhibition. In stage 2, we evaluated the association between the leading candidate non-chemotherapy drug and prostate cancer risk in a large, prospective cohort study with long-term follow-up. In this paper we illustrate our strategy toward drug repositioning and present findings that provide compelling justification for further mechanistic and possibly clinical testing of the leading non-chemotherapy candidate, digoxin, a cardiac glycoside, as a drug for prostate cancer treatment.
Results
STAGE 1
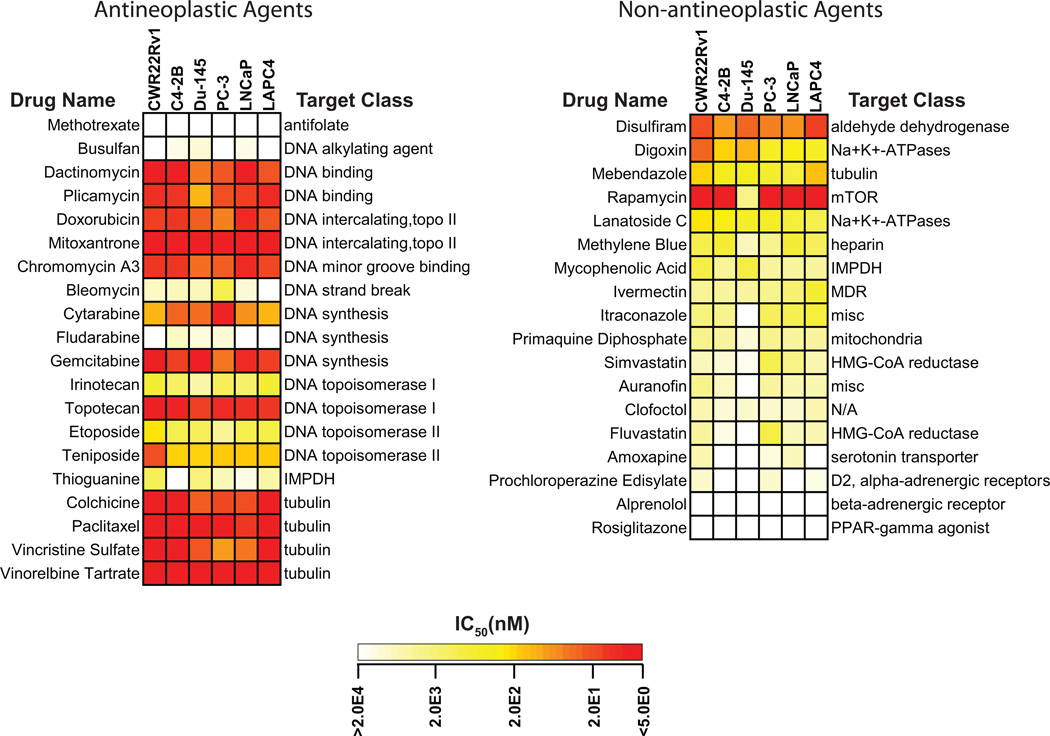
The primary screen yielded 70 compounds that demonstrated >50% inhibition of LNCaP or PC3 human prostate cancer cell proliferation at a concentration of 5 µM. Of these, 38 compounds are FDA-approved or have a history of clinical use internationally, including 20 that are known antineoplastic agents (“antineoplastic”) and 18 that are not typically used as antineoplastic agents (“non-neoplastic”). We focused on these 38 drugs in more detail in the secondary screen (Figure 1). Among the non-antineoplastic agents, the class of cardiac glycoside Na+/K+ ATPase inhibitors showed the most striking inhibition of prostate cancer cell growth, with two of these drugs, digoxin (mean across the six prostate cancer cell lines: IC50=163 nM, range 23–255 nM) and lanatoside C (mean IC50=408 nM, range 176–843 nM), ranking in the top 5 most potent of all non-antineoplastic agents. A third compound from the class of glycoside Na+/K+ ATPases, proscillaridin A, was the most potent of all non-antineoplastic compounds from the primary screen (mean IC50=13 nM, range <5–24 nM), but was not considered further because of the lack of clinical experience with this compound. The potency of this class of drug compounds was comparable to that of several known antineoplastic drugs (Figure 1). Given its potency in inhibiting prostate cancer cell proliferation in vitro and common use, digoxin was the leading candidate to carry forward into stage 2.
Figure 1.
Potency of prostate cancer cell growth inhibition of the 38 FDA-approved or clinically used compounds emerging from the primary screen. Shown are results from the secondary screen for these compounds. The 38 drugs are categorized as known anti-neoplastic or non-antineoplastic drugs based on their known clinical indications. The IC50 determined for each drug and each cell line in the secondary screen are color scaled as shown. Non-antineoplastic drugs are arranged by order of mean potency across the six prostate cancer cell lines.
STAGE 2
At baseline in 1986, 2.0% of the men reported regularly using digoxin. Digoxin users tended to be older, were more likely to be white, had a higher BMI, were less likely to have a family history of prostate cancer, had less vigorous physical activity, were more likely to be diabetic, and were more likely to regularly use cholesterol-lowering drugs, aspirin, and other cardiovascular drugs (Table 1). Users did not differ notably from non-users on food and nutrient intake (Table 1). Men who used digoxin to treat arrhythmia, congestive heart failure, or both conditions differed on their age-standardized characteristics (Table 1). The age-standardized prevalence of ever having had a screening PSA test by 1994 (the first year we asked the men to report on PSA screening), was 47.7% in nonusers and was 56.3%, 23.3%, and 46.3% in users whose indications were arrhythmia, congestive heart failure, or both, respectively.
Table 1.
Age-standardized* characteristics of digoxin users and nonusers, 47,884 men in the Health Professionals Follow-up Study, 1986
| Characteristic | Current use of digoxin at baseline | ||||
|---|---|---|---|---|---|
| Nonuser | Digoxin user |
Indication for use | |||
| Arrhythmia | Congestive Heart failure |
Both Indications |
|||
| Men (n) | 46,948 | 936 | 579 | 165 | 192 |
| Mean age (years) | 53.8 | 64.2 | 63.4 | 65.6 | 65.2 |
| White (%) | 90.8 | 94.3 | 95.9 | 89.0 | 88.2 |
| Mean current BMI (kg/m2) | 24.9 | 25.9 | 25.4 | 25.5 | 28.5 |
| Mean BMI at age 21 (kg/m2) | 23.0 | 23.6 | 23.2 | 23.9 | 25.9 |
| Mean height (inches) | 70.1 | 70.8 | 71.0 | 70.2 | 70.1 |
| Family history of prostate cancer (%) | 12.0 | 8.9 | 9.6 | 1.9 | 10.2 |
| Current smoking (%) | 9.7 | 9.9 | 9.5 | 4.6 | 14.7 |
| Mean vigorous physical activity (MET-hr/wk) | 12.8 | 7.4 | 8.8 | 4.0 | 2.0 |
| Diabetes mellitus (%) | 3.0 | 10.1 | 7.0 | 19.8 | 19.3 |
| Medications use (%) | |||||
| Cholesterol-lowering drugs | 0.7 | 4.8 | 4.0 | 3.0 | 10.6 |
| Aspirin | 29.2 | 46.6 | 44.9 | 60.2 | 47.9 |
| Ibuprofen | 5.5 | 7.9 | 8.0 | 2.9 | 9.7 |
| Furosemide diuretics | 0.9 | 14.8 | - | 51.3 | 53.2 |
| Other diuretics | 9.4 | 14.9 | - | 51.3 | 51.7 |
| Beta blockers | 9.4 | 26.3 | 26.4 | 34.4 | 18.8 |
| Calcium channel blockers | 2.0 | 12.1 | 10.8 | 9.1 | 19.5 |
| Antihypertensives | 3.1 | 10.7 | 6.8 | 23.0 | 18.2 |
| Antiarrythymics | 0.7 | 19.1 | 18.2 | 6.4 | 33.3 |
| Mean daily intake | |||||
| Energy (kcal) | 1985 | 1967 | 1972 | 1903 | 1980 |
| Energy-adjusted α-linolenic acid (g) | 1.07 | 1.07 | 1.06 | 1.09 | 1.14 |
| Energy-adjusted calcium (g) | 898 | 877 | 898 | 815 | 815 |
| Bacon (servings) | 0.82 | 0.66 | 0.63 | 0.63 | 0.88 |
| Fish (servings) | 0.39 | 0.43 | 0.41 | 0.49 | 0.50 |
| Tomato sauce (servings) | 0.96 | 0.98 | 0.94 | 1.24 | 0.99 |
| Vitamin E supplement use (%) | 40.5 | 37.9 | 37.2 | 51.5 | 39.3 |
All values (except age) were standardized to the age distribution of the analytic cohort at baseline in 1986.
Men who regularly used digoxin at baseline had about a 25% lower risk of prostate cancer compared with nonusers (Table 2). Current digoxin users and men who had ever used digoxin during follow-up had a lower risk of prostate cancer than men who were not currently using the drug or who had never used the drug (Table 2); the association was similar to overall when restricting to follow-up time during which digoxin information was not missing (RR=0.73, 95% CI 0.57 to 0.94). Risk decreased with increasing duration of digoxin use during the study period compared with never use (P-trend<0.001); the RR for ≥10 years of use was 0.54 (95% CI 0.37 to 0.79, Table 2).
Table 2.
Association between digoxin use and total prostate cancer, 47,884 men in the Health Professionals Follow-up Study, 1986–2006
| RR (95% CI) | |||||
|---|---|---|---|---|---|
| Prostate cancer cases (N) |
Person- years at risk |
Age-adjusted | Multivariable- adjusted* |
Additionally adjusted for use of other medications** |
|
| Regular digoxin use at baseline | |||||
| No | 4,923 | 804,663 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Yes | 79 | 11,001 | 0.74 (0.59 to 0.93) |
0.76 (0.61 to 0.95) |
0.76 (0.60 to 0.95) |
| Current digoxin use during follow-up | |||||
| No | 4,827 | 793,305 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Yes | 175 | 22,359 | 0.76 (0.65 to 0.88) |
0.78 (0.67 to 0.90) |
0.77 (0.66 to 0.90) |
| Ever use of digoxin during follow-up | |||||
| Never | 4,759 | 786,326 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Ever | 243 | 29,338 | 0.81 (0.71 to 0.92) |
0.83 (0.72 to 0.94) |
0.82 (0.72 to 0.94) |
| Duration of digoxin use (years) | |||||
| Never | 4,759 | 786,326 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| <5 | 125 | 14,736 | 0.84 (0.71 to 1.01) |
0.87 (0.73 to 1.04) |
0.87 (0.72 to 1.04) |
| 5–9.9 | 90 | 9,521 | 0.85 (0.69 to 1.05) |
0.87 (0.70 to 1.07) |
0.86 (0.70 to 1.07) |
| ≥10 | 28 | 5,082 | 0.53 (0.36 to 0.77) |
0.54 (0.37 to 0.79) |
0.54 (0.37 to 0.79) |
| P-trend | <0.001 | <0.001 | <0.001 | ||
Adjusted for calendar year, age, race, current body mass index (BMI; kg/m2), body mass index at age 21, height (inches), first degree family history of prostate cancer, pack-years smoked in the past 10 years, vigorous physical activity (metabolic equivalent [MET]-hours/week), diabetes mellitus, daily intake of energy (kcal/day), energy-adjusted α-linolenic acid (g/day), energy-adjusted calcium (mg/day), bacon (servings/day), fish (servings/day), tomato sauce (servings/day), and use of a vitamin E supplement.
Adjusted for use of cholesterol-lowering drugs, aspirin, ibuprofen, furosemide diuretics, other diuretics, beta blockers, calcium channel blockers, other antihypertensives, and antiarrhythmics.
Additionally adjusting for use of other medications did not appreciably change the results (Table 2). The associations for current use of other cardiovascular drugs and prostate cancer were null, including beta-blockers (RR=0.94, 95% CI 0.86 to 1.02), angiotensin-converting enzyme inhibitors (RR=1.04, 95% CI 0.95 to 1.14), calcium channel blockers (RR=1.03, 95% CI 0.94 to 1.14), furosemide diuretics (RR=0.99, 95% CI 0.84 to 1.17), and other diuretics (RR=1.07, 95% CI 0.97 to 1.19).
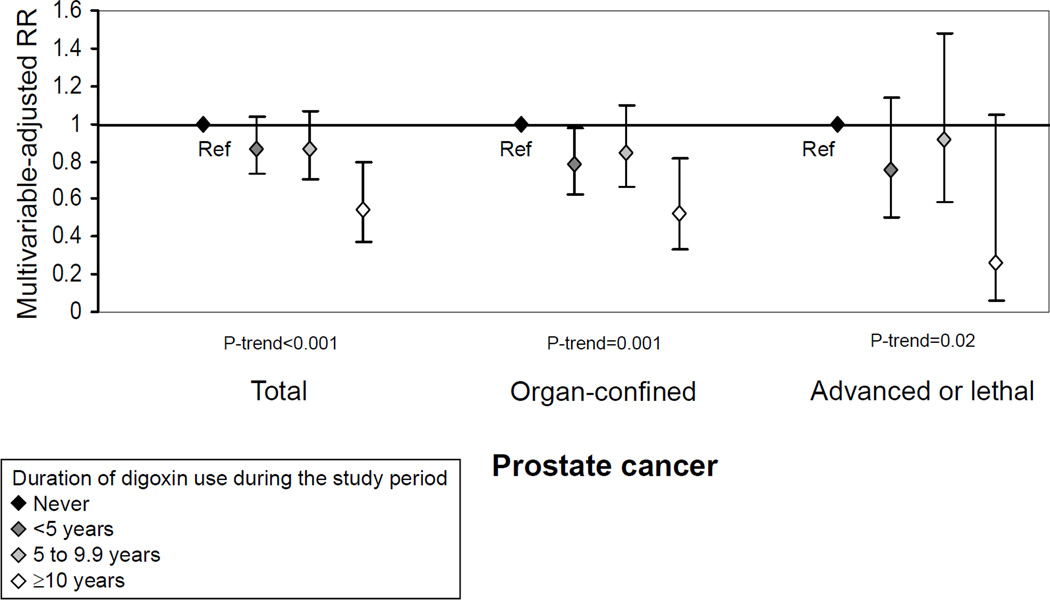
The inverse associations for regular use of digoxin at baseline and current use during follow-up were present for organ-confined, advanced stage or lethal disease, and lower and higher Gleason sum disease (Table 3). Long duration of use during the study period was inversely associated with organ-confined and advanced or lethal disease (Figure 2) as well as with lower (10+ years of use: RR=0.54, 95% CI 0.30 to 0.98, P-trend=0.03) and higher Gleason sum (RR=0.54, 95% CI 0.30 to 0.97, P-trend=0.009). The associations appeared inverse, but were not statistically significant for lethal, fatal, and very high-grade prostate cancer, endpoints for which sample sizes were smaller (Table 3 and data not shown). For both regular use at baseline and current use, inverse associations were stronger for cases that were organ-confined, but higher grade (N=1,285; RR=0.48, 95% CI 0.25 to 0.89 and RR=0.64, 95% CI 0.45 to 0.89, respectively) than for cases that were organ-confined but lower grade (N=1,962; RR=0.73, 95% CI 0.48 to 1.09 and RR=0.72, 95% CI 0.55 to 0.94, respectively).
Table 3.
Association between digoxin use and prostate cancer by disease aggressiveness, 47,884 men in the Health Professionals Follow-up Study, 1986–2006.
| Regular digoxin use at baseline* | Current digoxin use during follow-up* | |||||
|---|---|---|---|---|---|---|
| Prostate cancer cases (N)** |
RR (95% CI) | Prostate cancer cases (N)** |
RR (95% CI) | |||
| Multivariable- adjusted*** |
Additionally adjusted for use of other medications¶ |
Multivariable- adjusted*** |
Additionally adjusted for use of other medications¶ |
|||
| Organ-confined | ||||||
| No | 3,465 | 1.00 (ref) | 1.00 (ref) | 3,402 | 1.00 (ref) | 1.00 (ref) |
| Yes | 44 | 0.70 (0.52 to 0.94) |
0.70 (0.52 to 0.95) |
107 | 0.72 (0.59 to 0.87) |
0.73 (0.59 to 0.88) |
| Advanced or lethal | ||||||
| No | 838 | 1.00 (ref) | 1.00 (ref) | 819 | 1.00 (ref) | 1.00 (ref) |
| Yes | 16 | 0.59 (0.36 to 0.97) |
0.60 (0.36 to 0.99) |
35 | 0.75 (0.54 to 1.06) |
0.76 (0.53 to 1.07) |
| Lethal | ||||||
| No | 617 | 1.00 (ref) | 1.00 (ref) | 601 | 1.00 (ref) | 1.00 (ref) |
| Yes | 15 | 0.67 (0.40 to 1.12) |
0.68 (0.40 to 1.14) |
31 | 0.83 (0.58 to 1.20) |
0.82 (0.56 to 1.19) |
| Fatal | ||||||
| No | 513 | 1.00 (ref) | 1.00 (ref) | 499 | 1.00 (ref) | 1.00 (ref) |
| Yes | 15 | 0.78 (0.46 to 1.30) |
0.79 (0.47 to 1.34) |
29 | 0.92 (0.63 to 1.35) |
0.93 (0.63 to 1.38) |
| Lower grade (Gleason sum <7) | ||||||
| No | 2,178 | 1.00 (ref) | 1.00 (ref) | 2,142 | 1.00 (ref) | 1.00 (ref) |
| Yes | 27 | 0.69 (0.47 to 1.01) |
0.68 (0.46 to 1.01) |
63 | 0.69 (0.54 to 0.89) |
0.68 (0.53 to 0.88) |
| Higher grade (Gleason sum ≥7) | ||||||
| No | 1,871 | 1.00 (ref) | 1.00 (ref) | 1,834 | 1.00 (ref) | 1.00 (ref) |
| Yes | 22 | 0.65 (0.42 to 0.99) |
0.66 (0.43 to 1.02) |
59 | 0.73 (0.56 to 0.94) |
0.76 (0.59 to 1.00) |
| Very high grade (Gleason sum ≥8) | ||||||
| No | 493 | 1.00 (ref) | 1.00 (ref) | 481 | 1.00 (ref) | 1.00 (ref) |
| Yes | 8 | 0.71 (0.35 to 1.44) |
0.74 (0.36 to 1.51) |
20 | 0.79 (0.50 to 1.24) |
0.81 (0.51 to 1.29) |
Reference: no use at baseline for regular use at baseline; not current use for current use during follow-up.
See Table 2 for numbers of person-years.
Adjusted for calendar year, age, race, current body mass index (BMI; kg/m2), body mass index at age 21, height (inches), first degree family history of prostate cancer, pack-years smoked in the past 10 years, vigorous physical activity (metabolic equivalent [MET]-hours/week), diabetes mellitus, daily intake of energy (kcal/day), energy-adjusted α-linolenic acid (g/day), energy-adjusted calcium (mg/day), bacon (servings/day), fish (servings/day), tomato sauce (servings/day), and use of a vitamin E supplement.
Adjusted for use of cholesterol-lowering drugs, aspirin, ibuprofen, furosemide diuretics, other diuretics, beta blockers, calcium channel blockers, other antihypertensives, and antiarrhythmics.
Figure 2.
Association between duration of digoxin use during the study period and prostate cancer risk, 47,884 men in the Health Professionals Follow-up Study, 1986–2006.
The inverse association between current digoxin use and prostate cancer risk was suggested for each current indication for use, arrhythmia (RR=0.75, 95% CI 0.62 to 0.91), congestive heart failure (RR=0.70, 95% CI 0.38 to 1.26), or both (RR=0.86, 95% CI 0.65 to 1.15), including after adjustment for use of other medications (data not shown). Because patients with atrial fibrillation, the most common type of arrhythmia, are sometimes prescribed anticoagulants to reduce stroke risk and men taking an anticoagulant might be less likely to be biopsied after an elevated PSA, we ran subanalyses beginning follow-up in 1996, the first year we asked about warfarin (e.g., Coumadin) use: RR of total prostate cancer for current digoxin was 0.77 (95% CI 0.61 to 0.97), and was 0.70 (95% CI 0.51 to 0.96) after excluding warfarin users.
To reduce the potential for bias that might result from differences between digoxin users and nonusers in intensity of healthcare, we ran several subanalyses. When we restricted the cohort to men (N=9,576, 1,157 cases) who at baseline used at least one medication for cardiovascular indications (except aspirin), digoxin use (current use during follow-up: age-adjusted RR=0.67, 95% CI 0.54 to 0.83) was inversely associated with prostate cancer. The results were similar when restricting the cohort to men (N=6,700, 761 cases) using blood pressure medications at baseline (RR=0.70, 95% CI 0.53 to 0.92). Beginning follow-up in 1994, the first time we asked the men to report on PSA screening and which was concurrent with the routine use of PSA for prostate cancer screening in the United States, the RR of total prostate cancer for current digoxin use during follow-up was 0.78 (95% CI 0.63 to 0.96). The results were unchanged when we restricted the analysis to men only after a first screening PSA test without a prostate cancer diagnosis was reported on a biennial follow-up questionnaire (RR=0.76, 95% CI 0.61to 0.95).
The inverse association between current digoxin use during follow-up and prostate cancer did not differ between lean and overweight/obese men, smokers and nonsmokers, men with high and low vigorous physical activity, men with and without diabetes, and men who did and did not regularly use cholesterol-lowering drugs or aspirin (all P-interaction>0.25). The inverse association was present when restricting to white men (RR=0.76, 95% CI 0.64 to 0.89) and older men (RR=0.78, 95% CI 0.67 to 0.91). However, the magnitude of the inverse association differed by family history (P-interaction=0.02); among men without a history (156 cases in 19,870 person-years in current digoxin users and 3,837 cases in 698,425 person-years in nonusers) the RR was 0.84 (95% CI 0.71 to 0.99) and among those with a history (19 cases in 2,494 person-years in digoxin users and 990 cases in 95,052 person-years in nonusers) the RR was 0.48 (95% CI 0.30 to 0.76).
Discussion
In this transdisciplinary, two-stage approach, we first identified drugs that inhibited the proliferation of androgen-dependent and independent prostate cancer cell lines. Digoxin, a cardiac glycoside drug derived from foxglove, was the leading candidate for further study because of its strong anti-proliferative activity and long history of common use in treating congestive heart failure and arrhythmia. In the second stage, in a large prospective cohort study, we observed that men who used digoxin had a 25% lower risk of prostate cancer, including disease that was potentially lethal, than men who did not use digoxin. This inverse association was suggested for both major indications for digoxin prescription. These findings were not explained by differential uptake of PSA screening between men who used and did not use digoxin or by the use of other medications.
In general, the inverse association was comparable in magnitude between strata of prostate cancer risk and protective factors, with the exception of family history. The inverse association between digoxin use and prostate cancer was stronger in men with a family history than without. We stratified by family because these men may be enriched with a genetic predisposition or may have a different screening pattern than other men. Explanations should be sought for this observation.
The potential for cardiac glycosides as cancer therapeutic agents has been discussed previously (9, 10). The mechanisms by which digoxin may influence prostate cancer cell growth are not established, but some leads exist. Digoxin caused an influx of intracellular calcium into prostate cancer cells triggering apoptosis (11, 12), perhaps through effects on the Cdk5/p25 pathway (3). Digoxin and ouabain, which are structurally similar, activated Src/MAPK signaling resulting in inhibition of p53 synthesis, suggesting that cardiac glycosides may have utility in the treatment of cancers with gain of function P53 mutations (13). Digoxin inhibited HIF-1 (14), which is well recognized for its role in cancer development via its influence on VEGF and consequently angiogenesis. However, the antiproliferative effects of the drug also acted independently of HIF-1 (14). Gynecomastia is reported to be a side effect of digoxin possibly caused by shared structure with steroids including estrogen (15); estrogen has long been used to treat advanced prostate cancer. Interestingly, ouabain could selectively inhibit proliferation of oncogene-transformed cells compared to untransformed cells (16, 17), suggesting that a synthetic lethality paradigm may allow sparing of normal cells despite cytotoxicity of oncogene-transformed malignant cells. Whether the same differential effect would be observed for digoxin and prostate cancer cells with their array of oncogenic alterations remains to be assessed, but these findings suggest the intriguing possibility that a synthetic lethality paradigm (18) could be exploited in the development of digoxin and other cardiac glycosides as a prostate cancer drug. Finally, in vivo, digoxin reduced distant metastases in a mouse model of metastatic prostate cancer, an effect attributed to digoxin’s inhibition of Na+/K+ ATPase (19). For the treatment of congestive heart failure and arrhythmia, digoxin acts by inhibiting Na+/K+ ATPases in cardiac myocytes. That multiple cardiac glycosides showed potent inhibition of the growth of both androgen-dependent and independent prostate cancer cell lines in our screen provides further evidence that inhibition of these enzymes may at least in part underlie digoxin’s effects on prostate cancer and suggests that the Na+/K+ ATPases may be a relevant target for prostate cancer treatment. Nevertheless, studies are needed to determine whether any of these or other mechanisms explains digoxin’s anti-prostate cancer activity in both stages of our study.
The only two prior epidemiologic studies reporting on digitalis-derived drugs and prostate cancer, one a surveillance study of drugs prescribed in a California health maintenance organization (20) and the other a record linkage study in Norway (21), reported statistically significant positive associations (RR>1). The California study used pharmacy records from 1969 to 1973 to evaluate 215 drugs in association with 56 cancer sites with follow-up through 1984 (20). The Norway study was conducted among cardiac patients who had recently started taking digitoxin, a digitalis-derived drug, and their cancer rates were compared with age-specific population rates (21). Unlike in our prospective cohort study, these studies were limited by the inability to address potential observation bias and confounding resulting from differences in characteristics of users and nonusers of these drugs. Indeed, in the Norway study, the authors reported that the prostate cancer finding was likely to be due to bias because men who began using digitoxin had a higher risk of cancer before starting on the drug (21). No consistent pattern is apparent for cardiac glycosides and other cancers in the few epidemiologic studies that have been conducted (20–26). Additional large epidemiologic studies are needed that can evaluate duration of digoxin use and potentially confounding and modifying factors.
Although our findings from stage 2 suggest that digoxin might reduce the risk of developing prostate cancer or might treat yet undetected prostate cancer, we do not suggest that digoxin be tested for chemoprevention at this time. Digoxin’s therapeutic range of 0.5–2.5 nM for cardiac indications is narrow (27). While not strictly comparable because it is unknown to what extent digoxin accumulates in prostate tissue, digoxin’s IC50 in our screen was 10 times the therapeutic range in blood. However, we observed in stage 2 that digoxin as prescribed was inversely associated with prostate cancer risk, suggesting that the therapeutic range for cardiac indications may be sufficient. If future investigations confirm that digoxin may inhibit or delay prostate cancer, then the development of related molecules with lower IC50s or drug delivery systems targeting the prostate might be warranted.
Our study has several strengths that suggest that our findings are not due to bias or chance. We used a two-stage approach in which the top candidate from stage 1 was confirmed in stage 2. Stage 1 used both androgen-dependent and androgen-independent prostate cancer cell lines. Stage 2 used a prospective design, included 5,002 cases, and had rich information on risk factors. In stage 2, we conducted subanalyses that diminish the possibility that differential intensity of care, including PSA screening and diagnostic work-up, between digoxin users and nonusers could explain the inverse association. The specificity of the association for digoxin, and not other cardiovascular drugs, also helps rule out bias or confounding as an explanation for our findings.
We were unable to study the other non-antineoplastic top candidate drugs identified in stage 1 because those drugs are not commonly used or were not assessed in the stage 2 cohort. However, some members of our study group are pursuing some of the other top candidates from the stage 1 screen, such as disulfiram, in mechanistic, preclinical, and early clinical studies. The stage 1 assay was based on an in vitro system using six prostate cancer cell lines, which may not have yielded IC50s that reflect the circulating concentration that would need to be achieved to prevent or treat prostate cancer. Because prevalence of digoxin use is low and the number of men who have died of prostate cancer is small in the HPFS, in stage 2 we were unable to study men who already had a prostate cancer diagnosis to determine whether digoxin users have better survival than nonusers. This information is needed to support the testing of digoxin as a prostate cancer drug. The stage 2 analysis was based on self-reported digoxin use; however, any inaccuracy in the reports by these health professionals prior to their prostate cancer diagnosis is unlikely to explain the inverse association that we observed. We assumed continued use/nonuse when drug use information was missing in a subsequent follow-up time period; a sensitivity analysis supported that this assumption did not distort the results. We did not collect information on use of the drug prior to baseline or the dose taken.
In summary, with an eye toward translation, our transdisciplinary team identified that digoxin was highly potent in inhibiting prostate cancer cell growth in vitro and that its use was associated with a 25% lower risk of prostate cancer. The mechanism by which digoxin may influence the development or progression of prostate cancer is uncertain, but may be related to Na+/K+ ATPase inhibition. Our study illustrates the power of the transdisciplinary approach in translational cancer research. By coupling laboratory and epidemiologic methods and thinking, we reduced the probability of identifying false positive candidate drugs for the next steps in testing. Our work should motivate additional basic science, epidemiologic, and translational research on the potential of digoxin or related molecules in the treatment of prostate cancer.
Materials and Methods
STAGE 1: IN VITRO SCREEN FOR DRUGS WITH PROSTATE CANCER CELL CYTOTOXICITY
Cell Lines
LNCaP, PC-3, CWR22Rv1, and DU-145 cell lines were obtained from the American Type Culture Collection (Rockville, MD) more than 9 years ago. LAPC-4 and C4-2B cell lines were provided by Dr. John T. Isaacs (Johns Hopkins University, Baltimore, MD) in 2002. Authentication of these cell lines is routinely carried out by the Powerplex 2.1 STR genotyping assay (Promega Corporation, Madison, WI) and was last performed at the close of the these studies in late 2009.
Primary In Vitro Screen
A growth-inhibition screen of all compounds from the Johns Hopkins Drug Library (JHDL), which includes 1,811 (57%) US Food and Drug Administration approved drugs among 3,187 total compounds (28% of all known drugs worldwide), was conducted using two commonly studied human prostate cancer cell lines, androgen-dependent LNCaP and androgen-independent PC3, propagated using previously described methods (5) in 96-well plates. Cells were treated with JHDL compounds, one per well, at a final concentration of 5 µM for 24 h and then treated with 1 µCi [3H]-thymidine for an additional 6 h. Cells were harvested and the amount of incorporated [3H]-thymidine was counted using the MicroBeta plate reader, providing a measure of DNA synthesis/cell proliferation (4).
Secondary In Vitro Screen
Those compounds that inhibited LNCaP or PC3 cell proliferation by more than 50% were further characterized by determining the concentration resulting in a 50% reduction in cell proliferation (IC50) in LNCaP, PC3, and four additional human prostate cancer cell lines, androgen dependent LAPC4, and androgen independent C42B, CWR22Rv1, and DU145. Digoxin emerged as one of the most potent hits for a non-chemotherapy drug (see Results). We carried digoxin forward for testing in stage 2 of this study. Other non-antineoplastic drugs passing the primary screen were not prioritized for study in stage 2 because, i) participants in the cohort were not asked to report on their use, ii) the association between their use and prostate cancer risk had already been assessed in the cohort (e.g., statin drugs (6)), and/or iii) they failed to show high potency (mean IC50≥10 µM) in the secondary in vitro screen.
STAGE 2: EPIDEMIOLOGIC STUDY OF THE LEADING CANDIDATE DRUG AND PROSTATE CANCER RISK
Study Population
We included in the analysis participants in the Health Professionals Follow-up Study, an ongoing prospective cohort study on risk factors for cancer and other chronic diseases in men 40–75 years old in 1986. Details have been reported previously (7). Briefly, we asked the men to complete a mailed questionnaire on their medical history, including use of medications, and lifestyle factors at baseline and then again every two years. We also asked them to report on their diet at baseline and then again every four years. We excluded men who had a cancer diagnosis (except non-melanoma skin cancer) before baseline in 1986 (4.0%), returned an invalid food frequency questionnaire in 1986 (3.0%), or withdrew or were otherwise ineligible (0.2%), leaving 47,884 men. The Institutional Review Boards at the Harvard School of Public Health and the Johns Hopkins Bloomberg School of Public Health approved this study.
Assessment of Digoxin Use and Indication for Use
On the baseline questionnaire we asked the men to indicate their “Current Medications (mark if used regularly)”. On the follow-up questionnaires we asked them to “Please mark if you are currently using any of the following medications”. A list of medications was provided, including “Digoxin (e.g., Lanoxin)”. Duration of use during the study period was estimated by summing use across the two-year questionnaire periods. No information was available on duration of use prior to baseline; we assumed two years. Dose was not ascertained. We classified digoxin users by indication – arrhythmia, congestive heart failure, or both – which we inferred based on reported diagnoses and other medications used.
Ascertainment and Classification of Prostate Cancer Cases
Ascertainment of prostate cancer diagnoses and follow-up for recurrence and death were described previously (7, 8). Briefly, on each follow-up questionnaire, we asked the men to report a diagnosis of prostate cancer. We were able to obtain medical records and pathology reports pertaining to their diagnosis and treatment for 94.5% of the men who reported a prostate cancer diagnosis or for whom prostate cancer was mentioned on the death certificate. From baseline through January 31, 2006, we ascertained 5,002 cases of incident non-T1a prostate cancer in 815,664 person-years. Stage T1a cases (N=227) were excluded to reduce the possibility of detection bias due to differential rates of surgery for benign prostatic hyperplasia. We categorized cases as organ-confined (N=3,509; T1b to T2b and N0M0) or as advanced stage (≥T3b, N+, or M+ at diagnosis, progression to metastasis, or death during follow-up N=854; “advanced stage or lethal”). Of those with advanced stage or lethal disease, 528 died of prostate cancer. We abstracted Gleason sum from the pathology reports for the prostatectomy for men who were surgically treated and for the biopsy otherwise and classified the cases as lower (N=2,205; Gleason sum <7) and higher (N=1,893; Gleason sum ≥7) grade. Of the latter, 501 had a Gleason sum ≥8 (“very high grade”).
Statistical Analysis
We calculated age-standardized means and proportions for demographic and other factors by regular digoxin use and by indication for use at baseline. We calculated age-adjusted and multivariable-adjusted hazard ratios (RRs) and 95% confidence intervals (CIs) using Cox proportional hazards regression. The men contributed time at risk beginning at the month they returned the baseline questionnaire until the month of diagnosis of prostate cancer, month of death from other causes, or the end of follow-up on January 31, 2006. The proportional hazards assumption was met. We first evaluated whether regular digoxin use at baseline (reference: nonuse at baseline) was associated with prostate cancer risk. Next, we updated digoxin use to evaluate whether current use (reference: never or former use) or ever use (reference: never use) during follow-up was associated with risk. When digoxin use was missing, information from the prior questionnaire was carried forward and a term was included in the model for whether use was imputed. 3,186 PY of 22,359 PY of current use were imputed and 169,089 PY of 793,305 PY of nonuse were imputed. In a sensitivity analysis, we excluded follow-up time with missing information on digoxin use. Finally, we evaluated the association for updated duration of use during the study period by entering into the model three indicator variables: <5, 5–9.9, and ≥10 years of use (reference: never use). To test for trend, we entered into the model a continuous duration term and evaluated its coefficient using the Wald test. We stratified by age and calendar year and adjusted for factors that have been associated with prostate cancer risk previously in the cohort. We further adjusted for use of other medications that a priori were known or hypothesized to be associated with digoxin use – cholesterol-lowering drugs, aspirin, ibuprofen, furosemide diuretics, other diuretics, beta blockers, calcium channel blockers, other antihypertensives including angiotensin-converting enzyme inhibitors, and antiarrhythmics.
To assess whether the association differed by prostate cancer risk factors – family history of prostate cancer, current BMI (<25, ≥25 kg/m2), cigarette smoking in the past 10 years (yes, no), vigorous exercise (high, low), diabetes (yes, no), cholesterol-lowering drug use (yes, no), and aspirin use (yes, no) – we ran stratified models and tested interaction terms using the Wald test. We also conducted subanalyses restricting to whites and older men because prescription patterns and/or PSA screening behaviors may differ by race and age. All statistical tests were two-sided, with P<0.05 considered to be statistically significant. All analyses were performed using SAS release 9.1 (SAS Institute, Cary, NC).
Significance.
Our two-stage transdisciplinary approach for drug repositioning provides compelling justification for further mechanistic and possibly clinical testing of the leading non-chemotherapy candidate, digoxin, a cardiac glycoside, as a drug for prostate cancer treatment. Perhaps equally importantly, our study illustrates the power of the transdisciplinary approach in translational cancer research. By coupling laboratory and epidemiologic methods and thinking, we reduced the probability of identifying false positive candidate drugs for the next steps in testing.
Acknowledgments
We thank the research staff of the Health Professionals Follow-up Study for their continued help in the conduct of this study. We also thank Yan Liu for programming support.
Financial support: Stage 1 of this work was supported by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (P50 CA58236); and the Patrick C. Walsh Prostate Cancer Research Fund (Hopkins). Stage 2 of this work was additionally supported by the National Cancer Institute (P01 CA55075 and R01 CA133891) and the National Heart, Lung, and Blood Institute (R01 HL35464), National Institutes of Health, Department of Health and Human Services (Harvard). None of the sponsors played a role in the study design, collection, analysis, and interpretation of the data, in the writing of this report, or in the decision to submit the paper for publication. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations list
- BMI
body mass index
- CI
confidence interval
- IC50
the concentration of the drug at which proliferation was inhibited by 50%
- JHDL
Johns Hopkins Drug Library
- PSA
Prostate specific antigen
- RR
relative risk
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that they have no competing financial interests.
References
- 1.Scherer FM. The pharmaceutical industry--prices and progress. N Engl J Med. 2004;351:927–932. doi: 10.1056/NEJMhpr040117. [DOI] [PubMed] [Google Scholar]
- 2.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 3.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 4.Chong CR, Sullivan DJ., Jr New uses for old drugs. Nature. 2007;448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 5.Yegnasubramanian S, Haffner MC, Zhang Y, Gurel B, Cornish TC, Wu Z, et al. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 2008;68:8954–8967. doi: 10.1158/0008-5472.CAN-07-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the Health Professionals Follow-up Study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JM, Holick CN, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States) Cancer Causes Control. 2006;17:199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 9.Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8:36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 11.McConkey DJ, Lin Y, Nutt LK, Ozel HZ, Newman RA. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000;60:3807–3812. [PubMed] [Google Scholar]
- 12.Yeh JY, Huang WJ, Kan SF, Wang PS. Inhibitory effects of digitalis on the proliferation of androgen dependent and independent prostate cancer cells. J Urol. 2001;166:1937–1942. [PubMed] [Google Scholar]
- 13.Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X, et al. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res. 2009;69:6556–6564. doi: 10.1158/0008-5472.CAN-09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci U S A. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewinn EB. Gynecomastia during digitalis therapy; report of eight additional cases with liver-function studies. N Engl J Med. 1953;248:316–320. doi: 10.1056/NEJM195302192480802. [DOI] [PubMed] [Google Scholar]
- 16.Benade LE, Talbot N, Tagliaferri P, Hardy C, Card J, Noda M, et al. Ouabain sensitivity is linked to ras -transformation in human HOS cells. Biochem Biophys Res Commun. 1986;136:807–814. doi: 10.1016/0006-291x(86)90512-7. [DOI] [PubMed] [Google Scholar]
- 17.Tagliaferri P, Yanagihara K, Ciardiello F, Talbot N, Flatow U, Benade L, et al. Effects of ouabain on NIH/3T3 cells transformed with retroviral oncogenes and on human tumor cell lines. Int J Cancer. 1987;40:653–658. doi: 10.1002/ijc.2910400514. [DOI] [PubMed] [Google Scholar]
- 18.Iglehart JD, Silver DP. Synthetic lethality--a new direction in cancer-drug development. N Engl J Med. 2009;361:189–191. doi: 10.1056/NEJMe0903044. [DOI] [PubMed] [Google Scholar]
- 19.Zavareh RB, Lau KS, Hurren R, Datti A, Ashline DJ, Gronda M, et al. Inhibition of the sodium/potassium ATPase impairs N-glycan expression and function. Cancer Res. 2008;68:6688–6697. doi: 10.1158/0008-5472.CAN-07-6833. [DOI] [PubMed] [Google Scholar]
- 20.Selby JV, Friedman GD, Fireman BH. Screening prescription drugs for possible carcinogenicity: eleven to fifteen years of follow-up. Cancer Res. 1989;49:5736–5747. [PubMed] [Google Scholar]
- 21.Haux J, Klepp O, Spigset O, Tretli S. Digitoxin medication and cancer; case control and internal dose-response studies. BMC Cancer. 2001;1:11. doi: 10.1186/1471-2407-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein L, Ross RK. Prior medication use and health history as risk factors for non-Hodgkin's lymphoma: preliminary results from a case-control study in Los Angeles County. Cancer Res. 1992;52:5510s–5515s. [PubMed] [Google Scholar]
- 23.Van Den Eeden SK, Friedman GD. Prescription drug screening for subsequent carcinogenicity. Pharmacoepidemiol Drug Saf. 1995;4:275–287. [Google Scholar]
- 24.Ewertz M, Holmberg L, Tretli S, Pedersen BV, Kristensen A. Risk factors for male breast cancer--a case-control study from Scandinavia. Acta Oncol. 2001;40:467–471. doi: 10.1080/028418601750288181. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15:419–428. doi: 10.1023/B:CACO.0000027506.55846.5d. [DOI] [PubMed] [Google Scholar]
- 26.Ahern TP, Lash TL, Sorensen HT, Pedersen L. Digoxin treatment is associated with an increased incidence of breast cancer: a population-based case-control study. Breast Cancer Res. 2008;10:R102. doi: 10.1186/bcr2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Merck Manuals Online Medical Library. [cited 2009 September 9, 2009];2009 Available from: http://www.merck.com/mmpe/lexicomp/digoxin.html. [Google Scholar]