Abstract
The ability of an oocyte to successfully mature is highly dependent on intrafollicular conditions, including the size and structure of the follicle. Here we present a mathematical model of oxygen transport in the antral follicle. We relate mean oxygen concentration in follicular fluid of bovine follicles to the concentration in the immediate vicinity of the cumulus-oocyte complex (COC). The model predicts that the oxygen levels within the antral follicle are dependent on the size and structure of the follicle and that the mean level of dissolved oxygen in follicular fluid does not necessarily correspond to that reaching the COC.
1. Introduction
A mammalian oocyte develops within a follicle in the ovary. The presence and growth of the follicle is likely to affect nutrient levels reaching the oocyte, and intrafollicular conditions may have an influence on the developmental competence of oocytes (the ability to mature, be fertilized, and develop into an embryo [1]) that does not become evident until some time after fertilisation [2]. The success of assisted reproduction technologies relies on developmentally competent oocytes being selected for treatment. The level of follicular vascularity, which directly affects the supply of nutrient to the follicle, has been linked to in vitro fertilisation (IVF) outcomes [3], and the nutrient composition of intrafollicular fluid has been studied as a prognostic parameter for selecting oocytes for IVF [4].
While traditional IVF uses oocytes matured in vivo, it can be desirable, or even necessary, to use immature oocytes which must first be matured in the laboratory, a process known as in vitro maturation (IVM). IVM is not widespread in humans, although it has potential to become a viable alternative to traditional IVF in cases where the mother is susceptible to ovarian hyperstimulation syndrome (OHSS) or polycystic ovary syndrome (PCOS) [5–8]. IVM is however used routinely in ruminants such as cattle where management of reproduction is an important industry concern [1, 9]. The development of IVM systems that optimise the successful development of the embryo has long been identified as essential in both commercial and research settings [10]. However, the success of IVM suffers from differences between the nutrient composition in culture media compared with in vivo conditions [11, 12]; hence, an understanding of intrafollicular conditions is critical for successful oocyte selection and maturation regardless of species.
The avascular part of the follicle structure is shown schematically in the antral stage of development in Figure 1. This structure consists of the follicle wall (made up of mural granulosa cells), surrounding a region comprised of follicular fluid (the antrum). The oocyte and its surrounding layers of cumulus cells, known collectively as the cumulus-oocyte complex (COC), are eccentrically placed within the follicle. In some follicles (particularly smaller follicles), the COC sits partially within the follicle wall; however, in many follicles it rests within the antrum, either next to the follicle wall or attached to it by a “stem” of granulosa cells. Surrounding the avascular part of the follicle is the basal lamina and the theca externa and theca interna (not shown in Figure 1). The vascular supply to the follicle, and so the supply of nutrients to the oocyte, lies in theca layers around the perimeter of the follicle.
Figure 1.
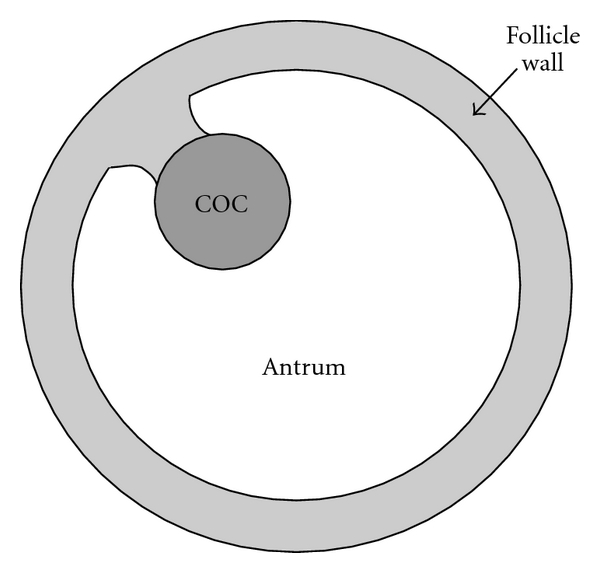
The antral follicle with the follicle wall, the antrum, and the COC labelled. In some follicles (particularly smaller follicles), the COC sits partially within the follicle wall; however, in many follicles it rests within the antrum, either next to the follicle wall or attached to it by a “stem” of granulosa cells as shown here.
The composition of follicular fluid varies between follicles and depends on their size and structure [4, 13]. Each antral follicle has a unique follicular fluid composition, and, despite several experimental studies, it is still not clear what constitutes a follicular environment that allows oocytes to develop successfully [2, 4]. Without a quantitive description of in vivo conditions the development of in vitro systems must rely to some extent on a “trial and error” approach. Mathematical modelling of nutrient transport in the follicle provides understanding of how the follicular environment affects the levels of nutrient seen by the oocyte. Previous mathematical models of nutrient transport in the antral follicle focus on investigation of oxygen (O2) transport [14, 15]. These studies treat the follicle wall as a homogeneous mass of cells and do not explicitly consider the COC, which is assumed to lie within, and behave in the same manner as, the follicle wall. We explicitly include the COC in our model under the hypothesis that the oocyte protrusion into the follicle may affect its nutrient environment. To our knowledge, this explicit inclusion of the COC within the follicle has not been considered in modelling studies.
Several experimental studies indicate that perifollicular vascularity is associated with oocyte developmental competence [2, 16]. This has in turn been associated with the dissolved O2 content of follicular fluid [16], although this association has been disputed [17]. While O2 is clearly essential for successful oocyte maturation, very high O2 levels can result in an increased presence of, for example, reactive oxygen species (ROS), which can be damaging to the oocyte [18]. Studies examining the effect of O2 concentration during in vitro maturation of oocytes across several species have not yielded a consensus on the optimal O2 concentration for oocyte maturation, due to conflicting results [19–24]. Here we present a mathematical model which relates perifollicular O2 concentration to follicular fluid O2 concentration and the O2 concentration reaching the COC in antral follicles.
Thus, this model is the first of its kind to explicitly include a COC, which allows prediction of the nutrient environment near the oocyte itself, as well as whole follicle measures. The model predicts that the O2 levels within the antral follicle are dependent on the size and structure of the follicle and that the average level of dissolved O2 in follicular fluid does not necessarily correspond to that reaching the COC.
2. Mathematical Model
2.1. Model Geometry
The model proposed here considers the follicle wall, the antrum, and the COC as three separate regions. The antral follicle is approximated as an axially symmetric structure, with rotational symmetry about the line that passes through both the centre of the follicle and the centre of the COC. The model geometry is shown in Figure 2 with the COC in two different positions within the follicle. The origin is chosen as the centre of the COC (labelled O in Figure 2) and is distance d from the centre of the follicle. An appropriate coordinate system is (ρ, θ), where ρ ≥ 0 is radial distance from the origin and θ is the angle shown in Figure 2(a). The axially symmetric geometry is described by considering 0 ≤ θ ≤ π. As the origin is at the centre of the COC, the boundary at the edge of the COC is described by ρ = r C, where r C is the constant radius of the COC. The boundaries of the antrum, ρ A(θ), and the follicle, ρ F(θ), are
Figure 2.
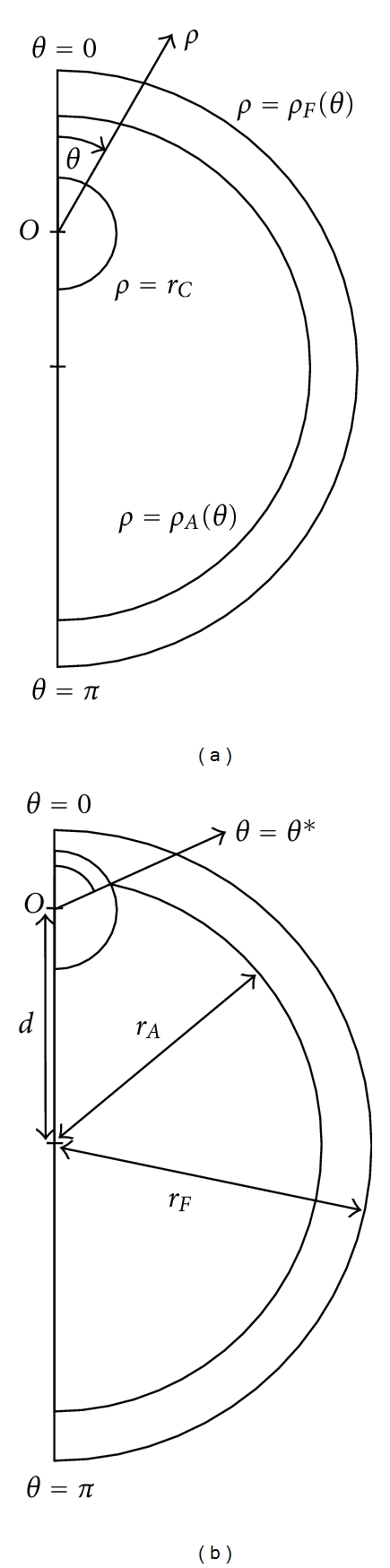
The model geometry used to represent the antral follicle. This geometry is described using polar coordinates (ρ, θ) with axial symmetry. The origin of the geometry is taken to be the centre of the COC, and thus the origin varies with respect to the antrum and the follicle wall. The geometry is shown for (a) the COC entirely within the antrum and (b) the COC partially enclosed within the antrum and partially within the follicle wall, intersecting the inner boundary of this layer at θ = θ*.
| (1) |
where r A is the radius of the antrum and r F the radius of the follicle as measured from the center of the follicle. When the COC is partially embedded in the follicle wall, θ* is the value of θ at which the COC and the antrum intersect and
| (2) |
If the COC is completely within the antrum, then θ* = 0 in (1).
The rate of follicle growth is assumed to be slow compared with the rate of O2 transport within the follicle. Thus, a steady-state model at each stage of follicle development is appropriate. The antral fluid is formed from transudate from blood plasma and also secretions from granulosa cells, cumulus cells, and the oocyte [25]; this may result in fluid motion and hence mixing. It is assumed that any fluid motion is negligible and so the dominant mechanism of O2 transport throughout the follicle is by diffusion. In the antrum itself, it is assumed that O2 consumption is negligible; therefore, O2 concentration in the antrum, c A, satisfies Laplace's equation
| (3) |
In the follicle wall we assume that the cumulus cells can be treated as a homogenous layer of intra- and extracellular matter, as in previous models of O2 transport in the antral follicle [14, 15]. Therefore, the O2 concentration in the follicle wall, c G, satisfies
| (4) |
where D G is the diffusion coefficient of O2 in the follicle wall, α G is the volume fraction of extracellular material in the follicle wall (the ratio of extracellular to total volume), and q G is the rate of O2 uptake by granulosa cells (amount per unit volume of granulosa cell mass per unit time). Experimental determination of oxygen consumption by ovine granulosa cells and murine embryos has indicated little dependence on O2 concentration, except at very low concentrations [26, 27]. The function q G is therefore assumed to be independent of O2 concentration as in previous studies of O2 transport in granulosa cells, embryos, and follicles [14, 15, 26, 27].
Consumption of O2 by the COC is incorporated into this model via a flux condition at the COC boundary
| (5) |
where D A is the diffusion coefficient of O2 in the antrum and q COC is the rate of O2 uptake by the COC (amount per unit COC surface area per unit time). As with the rate of O2 uptake by granulosa cells, the rate of O2 uptake by the COC is assumed constant.
At the boundary between the follicle wall and the antrum continuity of flux and concentration hold. Symmetry conditions apply at θ = 0 and θ = π in both the follicle wall and the antrum.
The flux of O2 into the follicle at the boundary ρ F(θ), the edge of the follicle, is represented by
| (6) |
where c P is the O2 concentration in the blood vessels surrounding the follicle and ∂c G/∂n F is the concentration gradient in the direction of the outward unit normal vector to the follicle surface. The parameter h p is the transfer coefficient for this problem and quantifies the ease of O2 transport from the vascular network of the ovary into the follicle.
Model parameters for the bovine antral follicle and a parameter sensitivity analysis are given in Table 1. The diffusion coefficients of O2 in the antrum and the follicle wall are assumed to be that of O2 in physiological saline [28]. An average bovine preovulatory follicle diameter is 15–18 mm [29]. However, bovine ovulatory follicle radius is highly variable, depending on the number of follicles ovulated, breed, age, and any hormones administered. A study using ultrasonography classified follicles by animal age, the number of follicles ovulated, and the year (2004–2006) and showed bovine preovulatory follicle diameters ranging from 8 to 30 mm (radii of 4–15 mm), with a mean in each group considered ranging from 13.9 to 17.1 mm (radius 6.95–8.55 mm) [29]. Therefore, a range of follicle radius values, r F, up to 15 mm were considered to allow consideration of antral follicles through development to ovulation. There is no apparent relationship between the radius of a follicle and the thickness of its follicle wall in bovine antral follicles [30, 31], and there appears to be considerable variation in granulosa cell numbers between follicles [15]. However, a relationship between granulosa cell number (n G) and follicle radius has been determined in humans by Redding et al. [15] as
Table 1.
Nominal model parameters and sensitivity analysis. The sensitivity analysis is carried out by considering the nominal parameter values as the baseline (with the COC centrally located within the follicle) and calculating the change in the mean O2 concentration at the COC boundary (r C) for a 10% increase or decrease in each parameter from the baseline.
| Parameter | Value | Reference | +10% | −10% |
|---|---|---|---|---|
| αG | 0.3 (no units) | [14, 15] | −1.5% | +1.4% |
| CP | 0.128 (mol m−3) | [14] | +41% | −41% |
| DA | 2.5 × 10−9 (m2 s−1) | [28] | +25% | −30% |
| DG | 2.5 × 10−9 (m2 s−1) | [28] | +3.1% | −3.7% |
| hP | 0.1 (m s−1) | Variable (see text) | See discussion | See discussion |
| qG | 0.0363 (mol m−3 s−1) | [14] | −3.1% | +3.1% |
| q COC | 1.62 × 10−6 (mol m−2 s−1) | From [44], units converted using r C | −28% | +28% |
| rF | 1 × 10−3 (m) | See text | +1.9% | −4.5% |
| rC | 160 × 10−6 (m) | [45] | +30% | −37% |
| vG | 1.14 × 10−15 (m3) | [15] | −6.9% | +6.2% |
| a | 43.1 × 106 | [15] | −6.9% | +6.2% |
| b | 281.6 | [15] | +5.6% | −7.6% |
| c | −387.3 | [15] | −5.4% | +4.6% |
| (7) |
where a, b, and c are constants. The antrum radius, r A, is then calculated using
| (8) |
where v G is the volume of a granulosa cell. The volume fraction of extracellular material in the follicle wall of a bovine follicle, α G, is not well determined. A value of α G = 0.3 is thought to be typical of volume fractions in the follicle wall of mammalian follicles [14, 15] and is used in previous studies of O2 transport in the antral follicle. However, as discussed in those studies, accurate experimental determination of this volume fraction would benefit models of this type. The magnitude of the parameter h p is unknown and so a range of values for this parameter was considered; model solutions were insensitive to h p for h P > 0.01 m/s.
In the special case that the COC lies at the centre of the follicle the model described by (3)–(6) is analytically solvable. Although this is an unlikely physical scenario, especially in large follicles, it provides validation of numerical solution procedures. Also, as will be discussed in Section 3 of this study, it provides a “worst case” solution where predicted O2 concentrations reaching the COC are lowest. The model of Redding et al. [14, 15], which incorporates the COC mass into the follicle wall is also considered here, holding total cell mass (COC plus granulosa cells) constant between models. This model is also analytically solvable and provides a “best case” scenario where predicted O2 concentrations reaching the COC are highest. When numerical solutions were necessary, they were obtained using the finite element solver COMSOL Multiphysics (Version 3.3, COMSOL AB, 2006). The solutions are shown here in two cases: (1) with the COC resting on the inner surface of the follicle wall, and (2) with the COC within the antrum one COC radius (r C) away from the follicle wall.
3. Results
When the COC cell mass is incorporated into the follicle wall, the predicted O2 concentration in the antrum is constant and equal to that at the inner boundary of the follicle wall, which is little less than the perifollicular concentration c P. However, when the COC is explicitly included in modeling, there is a significant decrease in O2 concentration in the vicinity of it. Figure 3 shows O2 concentrations (normalized by c P) in a follicle of 1 mm radius for the COC in two different positions in the antrum.
Figure 3.
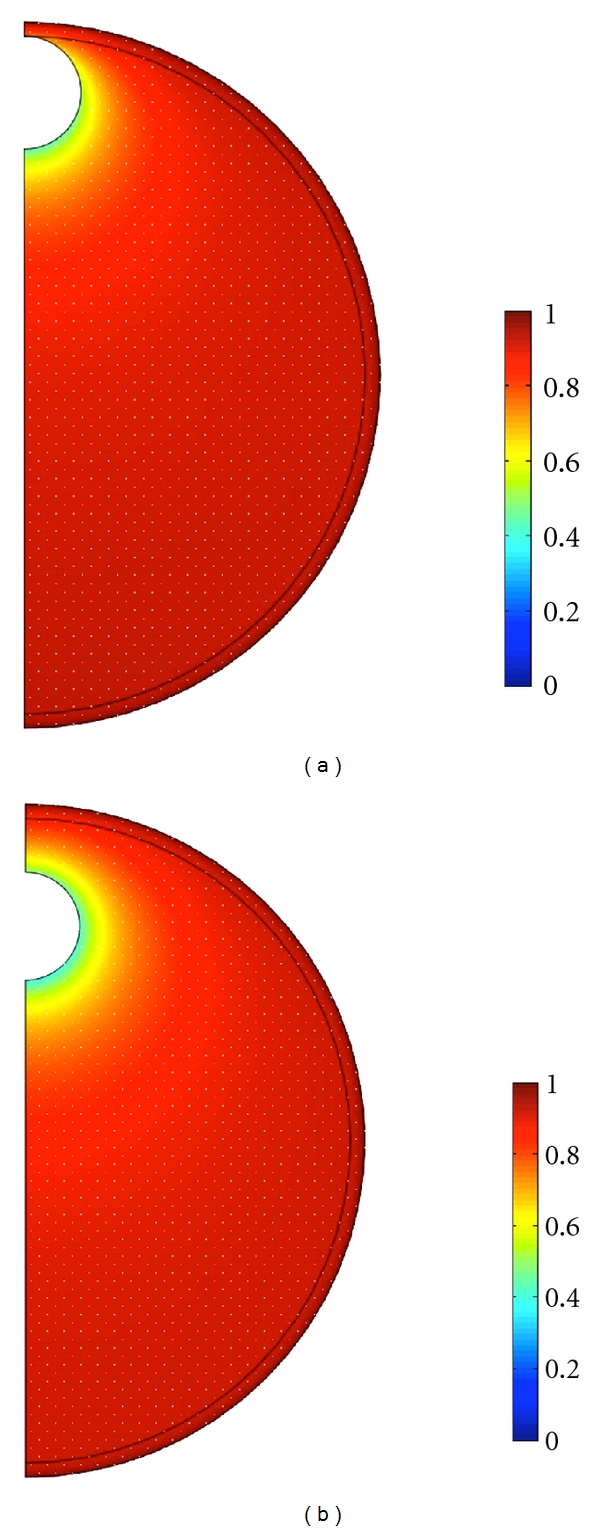
The O2 concentrations (normalized by c P) in a follicle of 1 mm radius when (a) the COC rests on the inner boundary of the follicle wall and (b) the COC is a distance of one radius (r C) away from the follicle wall. Explicit inclusion of the COC in the model shows a decrease in O2 concentration within the antrum in the vicinity of the COC.
It is straightforward for experimentalists to measure the mean dissolved O2 concentration in follicular fluid, whereas it is very difficult to measure the O2 concentration in the vicinity of the COC, particularly in vivo. This mean O2 concentration, as predicted by our model, is plotted versus follicle radius in Figure 4 for (A) the COC incorporated into the follicle wall, (B) the COC resting on the inner boundary of the follicle wall, (C) the COC a distance of one radius (rC) away from the follicle wall, and (D) the COC at the centre of the follicle. Case (A) is the model considered by Redding et al. [14, 15] for human follicles, and our results correspond to that model when the human parameter values from those studies are used. Cases (A) and (D), respectively, provide a maximum and a minimum for predictions of mean follicular fluid O2 concentration and solutions do not differ substantially in this case. A typical ovulatory follicle radius range [29] is shown, and this corresponds to a local minimum in O2 concentration.
Figure 4.
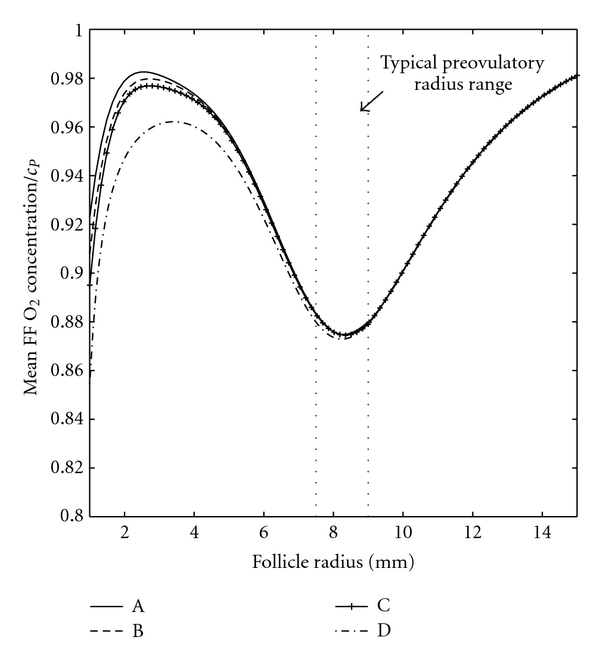
Mean follicular fluid (FF) O2 concentration versus follicle radius for (A) the COC incorporated into the follicle wall, (B) the COC resting on the inner boundary of the follicle wall, (C) the COC a distance of one radius (r C) away from the follicle wall, and (D) the COC at the centre of the follicle wall.
However, model predictions of the mean O2 concentration at the COC surface in each case differ substantially from predictions of mean O2 concentration in follicular fluid (Figure 5(a)). It is clear that the closer the COC to the centre of the antrum, the lower the O2 concentration at its surface. Figure 5(b) shows the ratio of the mean O2 concentration in follicular fluid to the mean O2 concentration at the COC surface in the cases where the COC is explicitly included in modelling (cases B, C, D). When the COC rests on the follicle wall (case B), the level of O2 reaching the COC is just 60% of the follicular fluid average, and this ratio decreases as the COC gets closer to the centre of the antrum.
Figure 5.
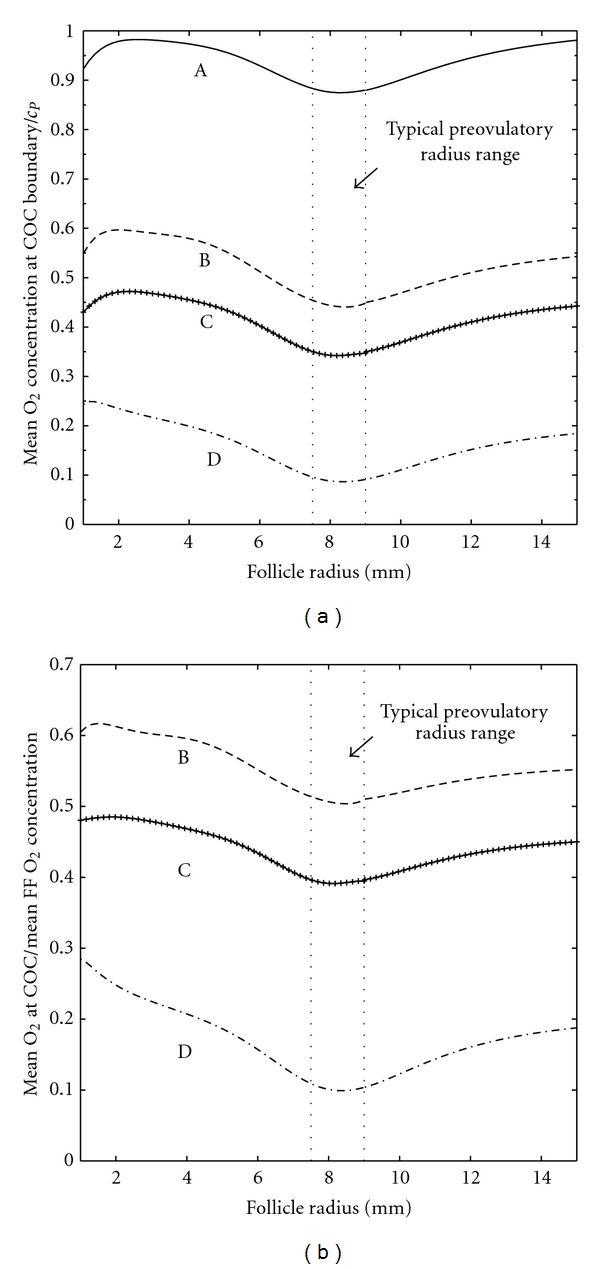
(a) Mean O2 concentration at the surface of the COC versus follicle radius for (A) the COC incorporated into the follicle wall, (B) the COC resting on the inner boundary of the follicle wall, (C) the COC a distance of one radius (rC) away from the follicle wall, and (D) the COC at the centre of the follicle wall. (b) The ratio of mean O2 concentration at the surface of the COC to mean follicular fluid (FF) O2 concentration plotted against follicle radius for the cases when the COC is explicitly included in modelling (cases B–D).
The model is highly sensitive to the diffusion coefficients D A and D G. There is some discussion in the literature about the appropriate diffusion coefficient for oxygen in tissues (see [32] for a thorough discussion of the literature regarding this matter). If it were the case that either diffusion coefficient was lower than that in physiological saline, this would reduce O2 concentrations throughout the antrum and at the COC surface but would not change the qualitative nature of results. Similarly changes to q COC and r C, properties of the COC to which the model is sensitive, do not change the qualitative nature of results.
4. Discussion
Here we have described the construction of a mathematical model to describe O2 transport in the antral follicle which explicitly includes a COC which may protrude into, or lie entirely within, the antrum. This model enables linking of follicular O2 concentration, a measurable quantity which has been proposed as a prognostic parameter in assisted reproduction technologies, and the O2 environment of the oocyte. Model results show that the structure of the follicle is important in determining O2 availability to the oocyte, and so this structure is also likely to affect developmental competence, a factor which may be important when selecting oocytes for assisted reproduction.
Model predictions of mean O2 concentration in follicular fluid show the same form as those obtained using the model of O2 transport in the antral follicle described by Redding et al. [14, 15], which did not explicitly include a COC. Results from these studies, and the work described in this paper, give understanding of the level of dissolved O2 in an antral follicle at different stages of development. At the start of the antral stage predicted mean O2 concentration rises rapidly with follicle radius to a local maximum at a radius of 2-3 mm (Figure 4). At this stage follicle growth is predominantly due to antrum formation, rather than granulosa cell proliferation. An increasing follicular surface area and decreasing follicle wall thickness (as a fraction of follicle radius) allow more O2 to enter the follicle resulting in the observed increase in O2 concentration with follicle radius. Later in follicle growth, granulosa cell proliferation increases the thickness of the follicle wall resulting in a decrease in O2 concentration within the antrum.
There is a local minimum in O2 concentration predicted by the model at around 8 mm radius (16 mm diameter) which falls within the range of typical diameters for bovine ovulatory follicles. Beyond that radius the O2 concentration within the follicle steadily rises toward a global maximum which, in the case of mean follicular fluid O2 concentration, is close to plasma O2 concentration. This implies that larger than average preovulatory follicles, provided that they are sufficiently vascularised, will see a larger than average O2 concentration. Whether this is beneficial or detrimental to their subsequent development when fertilised is likely to depend on the associated level of ROS, which is known to increase at high O2 concentrations. In bovine IVM it has been shown that the developmental potential of an oocyte increases with follicle (and oocyte) diameter in follicles up to 15 mm in diameter [33]. In studies using hormonal treatment to induce ovulation before artificial insemination, the pregnancy rate increased with follicle diameter to, approximately, 12–15 mm in diameter and then decreased as follicle size increased further [34, 35]. However, changes in pregnancy rate with follicle diameter were not detected in spontaneously ovulating cows [34].
An important difference between our model and previous models is the explicit inclusion of the COC. This allows the position of the COC within the follicle to be altered to determine the effects of follicle geometry on the O2 environment within the follicle. Average O2 concentrations in follicular fluid are higher the closer the COC sits to the follicle wall, and neglecting the COC from models will necessarily overestimate the O2 seen by the COC, unless the COC is completely incorporated into the follicle wall. Our model suggests that the position of the COC within the follicle plays a significant role in determining the O2 concentration seen by the oocyte and that follicle structure should be taken into account, along with average dissolved follicular fluid O2 concentration, in order to understand the effect of O2 concentration on the developmental competence of the oocyte. The influence of follicle geometry on the O2 availability to the COC may give some indication as to why the dissolved O2 content of follicular fluid has not been conclusively linked to developmental competence of oocytes [16, 17].
In terms of cattle breeding or even human IVM systems that aim to replicate the in-vivo environment, the finding that average follicular fluid oxygen concentration is likely to be considerably higher than that reaching the COC should be taken into account when considering optimal incubator settings. The geometry of an in vitro environment may also influence the availability of O2 to the COC—for example, the volume of fluid in which the COC is matured may alter O2 availability—and this may need to be accounted for when determining incubator settings for IVM.
Figure 3 shows that the drop in oxygen concentration across the mural granulosa follicle wall is small while there is a significant decrease in follicular oxygen concentration in the vicinity of the COC. This implies that the granulosa cells do not play a large role in determining follicular fluid oxygen concentrations so sparing oxygen for use by the maturing oocyte. The parameter describing oxygen uptake by mural granulosa is a factor of ten lower than that describing oxygen consumption by cumulus cells (the cells directly surrounding the oocyte) given by Clark et al. [32]. There is in vitro evidence from murine [36, 37] and bovine [38] studies that, in the absence of follicle stimulating hormone (FSH), the metabolism of cumulus and granulosa cells differs significantly. This suggests differing roles for the granulosa and cumulus cells in supporting the developing oocyte. Models show that both the cumulus cells [32] and the granulosa cells (modelled here) appear to spare oxygen, which is necessary for ATP production within the oocyte. However, cumulus cells must provide glucose metabolites such as pyruvate to the oocyte to support its metabolism (as the immature oocyte has limited capability for metabolising glucose [39]), so that a fine balance of sparing oxygen for the oocyte and utilising oxygen to produce pyruvate must be met in these cells. On the other hand the metabolism of the mural granulosa is able to be largely anaerobic which allows as much oxygen as possible through to the developing follicle. It is known that removal of the cumulus cell matrix from the oocyte is detrimental to oocyte development [40, 41] and the metabolic support that cumulus cells provide to the oocyte makes inclusion of the whole COC in IVM systems desirable [5]. On the other hand, it seems that mural granulosa cells are important for folliculogenesis rather than providing the oocyte with nutrients [36]. Mathematical models of this type applied to other important nutrients, for example, may provide further insight into the differing roles of these two cell types in oocyte development that may lead to improvements in in vitro handling of oocytes and COCs.
Neither this model, nor previous models of O2 transport in the antral follicle [14, 15, 26], account explicitly for angiogenesis. Angiogenesis occurs around the follicle in response to the release of stimulating chemicals released from follicular cells [42, 43]. One of these chemicals, vascular endothelial growth factor (VEGF), is known to have increased expression as a response to hypoxia [43]. In addition, at least in pigs, angiogenesis appears to begin in medium-sized follicles [43]. This coincides with a stage of development when models of nutrient transport in the follicle suggest a significant drop in both dissolved follicular fluid O2 concentrations and O2 availability to the oocyte. This lends support to the suggestion that follicular growth is limited by both the nutrient environment within the follicle [14] as well as the development of an adequate vascular supply [43]. An unevenly distributed vascular supply to the follicle is not expected to have much effect on the uniformity of O2 concentration within the follicular fluid, unless there are large “gaps” in the vascular supply. This is due to the small size of O2 molecules meaning that they diffuse readily across membranes and through extracellular material. However, the distribution of vascularisation may have implications for the transport of larger molecules in the follicle.
The data for cell number versus follicle radius used in this study is for human antral follicles, whilst other model parameters are determined for bovine follicles. The location of the local maximum for O2 concentrations in follicular fluid and at the COC could be predicted more accurately with data on bovine granulosa cell number versus follicle radius should this become available. Similarly, with accurate parameterization, this model can be applied to humans or other mammalian species, as well as for other substrates that diffuse easily across cell membranes.
The model represents an idealised spherical follicle containing a spherical COC and does not account for O2 consuming cells in any “stem” attaching the COC to the follicle wall, nor the structural and functional changes in the COC prior to ovulation. A “stem” of cumulus cells will slightly reduce the O2 concentration reaching the COC. Therefore, this idealised geometry will slightly overestimate the O2 environment seen by the COC. In the final stages of maturation, immediately before ovulation, the cumulus cell mass expands, so the space between cumulus cells increases and the extracellular material surrounding these cells becomes mucinous [40]. Computation of oxygen concentrations at this final stage of maturation may require appropriate changes to the model geometry and, probably, the rate of oxygen diffusion through the cumulus matrix toward the oocyte, that is, the diffusion coefficient in this region. Finally the model assumes that COC oxygen consumption is constant throughout maturation. An increase in oxygen demand by the COC, for example, late in development, would lower follicular oxygen levels significantly (see Table 1) which may restrict oxygen availability to the oocyte.
5. Summary
We have described a mathematical model of O2 transport in the antral follicle so as to link the O2 concentration in follicular fluid (measured using fluid aspirated from the ovary) with the O2 environment of the COC itself, taking into account the structure of the follicle. This model differs from previous models of nutrient transport in the ovarian follicle [14, 15, 26] in its explicit inclusion of the COC. It predicts the O2 concentration seen by the oocyte to differ significantly from the mean concentration in the follicle antrum showing that follicle structure as well as perifollicular O2 supply can affect the ability of an oocyte to successfully mature. Our model enables a better informed use of follicular fluid O2 concentration as an indicator of developmental competence of oocytes. Hence, it enables better informed selection of oocytes for use in assisted reproduction technology programs and better choice of culture conditions for in vitro maturation of oocytes.
Acknowledgments
A. R. Clark was supported in this work by a University of Adelaide scholarship (Adelaide Scholarships International, ASI). The authors are thankful for helpful comments from Associate Professor J. G. Thompson (Obstetrics and Gynaecology, The University of Adelaide) and an anonymous referee.
References
- 1.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67(1):6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 2.van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular enothelial growth factor levels and perifollicular blood flow characteristics. Human Reproduction. 1997;12(5):1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Mammalian graafian follicle. http://www.microscopy.fsu.edu/primer/anatomy/brightfieldgallery/mammaliangraafianfollicle40xsmall.html.
- 4.Andersen CY. Characteristics of human follicular fluid associated with successful conception after in vitro fertilization. Journal of Clinical Endocrinology and Metabolism. 1993;77(5):1227–1234. doi: 10.1210/jcem.77.5.7521343. [DOI] [PubMed] [Google Scholar]
- 5.Child TJ, Phillips SJ, Abdul-Jalil AK, Gulekli B, Tan SL. A comparison of in vitro maturation and in vitro fertilization for women with polycystic ovaries. Obstetrics and Gynecology. 2002;100(4):665–670. doi: 10.1016/s0029-7844(02)02193-2. [DOI] [PubMed] [Google Scholar]
- 6.Piquette GN. The in vitro maturation (IVM) of human oocytes for in vitro fertilization (IVF): is it time yet to switch to IVM-IVF? Fertility and Sterility. 2006;85(4):833–835. doi: 10.1016/j.fertnstert.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 7.Chian R-C. In-vitro maturation of immature oocytes for infertile women with PCOS. Reproductive BioMedicine Online. 2004;8(5):547–552. doi: 10.1016/s1472-6483(10)61101-7. [DOI] [PubMed] [Google Scholar]
- 8.Son WY, Park SJ, Hyun CS, Lee WD, Yoon SH, Lim JH. Successful birth after transfer of blastocysts derived from oocytes of unstimulated woman with regular menstrual cycle after IVM approach. Journal of Assisted Reproduction and Genetics. 2002;19(11):541–543. doi: 10.1023/A:1020920104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calado AM, Rocha E, Colaço A, Sousa M. A stereological study of medium antral follicles during the bovine estrous cycle. Tissue and Cell. 2003;35(5):313–323. doi: 10.1016/s0040-8166(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 10.Leibfried-Rutledge ML, Critser ES, Parrish JJ, First NL. In vitro maturation and fertilization of bovine oocytes. Theriogenology. 1989;31(1):61–74. [Google Scholar]
- 11.Roberts R, Franks S, Hardy K. Culture environment modulates maturation and metabolism of human oocytes. Human Reproduction. 2002;17(11):2950–2956. doi: 10.1093/humrep/17.11.2950. [DOI] [PubMed] [Google Scholar]
- 12.Sutton ML, Gilchrist RB, Thompson JG. Effect of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Human Reproduction Update. 2003;9(1):35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- 13.Orsi NM, Gopichandran N, Leese HJ, Picton HM, Harris SE. Fluctuations in bovine ovarian follicular fluid composition throughout the oestrous cycle. Reproduction. 2005;129(2):219–228. doi: 10.1530/rep.1.00460. [DOI] [PubMed] [Google Scholar]
- 14.Redding GP, Bronlund JE, Hart AL. Mathematical modelling of oxygen transport limited follicle growth. Reproduction. 2007;133(6):1095–1106. doi: 10.1530/REP-06-0171. [DOI] [PubMed] [Google Scholar]
- 15.Redding GP, Bronlund JE, Hart AL. Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling. Reproduction, Fertility and Development. 2008;20(3):408–417. doi: 10.1071/rd07190. [DOI] [PubMed] [Google Scholar]
- 16.Van Blerkom J. Epigenetic influences in oocyte developmental competence: perifollicular vascularity and intrafollicular oxygen. Journal of Assisted Reproduction and Genetics. 1998;15(5):226–234. doi: 10.1023/A:1022523906655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huey S, Abuhamad A, Barroso G, et al. Perifollicular blood flow Doppler indices, but not follicular pO2, pCO2, or pH, predict oocyte developmental competence in in vitro fertilization. Fertility and Sterility. 1999;72(4):707–712. doi: 10.1016/s0015-0282(99)00327-1. [DOI] [PubMed] [Google Scholar]
- 18.Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51(1):57–64. doi: 10.1080/15216540119253. [DOI] [PubMed] [Google Scholar]
- 19.Eppig JJ, Wigglesworth K. Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Molecular Reproduction and Development. 1995;42(4):447–456. doi: 10.1002/mrd.1080420412. [DOI] [PubMed] [Google Scholar]
- 20.Gwatkin RBL, Haidri AA. Oxygen requirements for the maturation of hamster oocytes. Journal of Reproduction and Fertility. 1974;37(1):127–129. doi: 10.1530/jrf.0.0370127. [DOI] [PubMed] [Google Scholar]
- 21.Haidri AA, Miller IM, Gwatkin RBL. Culture of mouse oocytes in vitro, using a system without oil or protein. Journal of Reproduction and Fertility. 1971;26(3):409–411. doi: 10.1530/jrf.0.0260409. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto S, Minami N, Takakura R, Yamada M, Imai H, Kashima N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocye complexes. Molecular Reproduction and Development. 2000;57(4):353–360. doi: 10.1002/1098-2795(200012)57:4<353::AID-MRD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Park JI, Hong JY, Yong HY, Hwang WS, Lim JM, Lee ES. High oxygen tension during in-vitro oocyte maturation improves in vitro development of porcine oocytes after fertilization. Animal Reproduction Science. 2005;87(1-2):133–141. doi: 10.1016/j.anireprosci.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Yeoman RR, Williams LE, Abee CR. Low oxygen inhibits but complex high-glucose medium facilitates maturation of squirrel monkey oocyte-granulosa cell complexes. Journal of Assisted Reproduction and Genetics. 1999;16(2):102–107. doi: 10.1023/A:1022525108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biology of Reproduction. 2010;82(6):1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 26.Gosden RG, Byatt-Smith JG. Oxygen concentration gradient across the ovarian follicular epithelium: model, predictions and implications. Human Reproduction. 1986;1(2):65–68. doi: 10.1093/oxfordjournals.humrep.a136362. [DOI] [PubMed] [Google Scholar]
- 27.Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Molecular Reproduction and Development. 1996;44(4):476–485. doi: 10.1002/(SICI)1098-2795(199608)44:4<476::AID-MRD7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Jones DP. Effect of mitochondrial clustering on O2 supply in hepatocytes. The American Journal of Physiology. 1984;247(1):C83–C89. doi: 10.1152/ajpcell.1984.247.1.C83. [DOI] [PubMed] [Google Scholar]
- 29.Gordon I. Laboratory Production of Cattle Embryos. 2nd edition. Oxford, UK: CABI Publishing; 2003. [Google Scholar]
- 30.Clarke HG, Hope SA, Byers S, Rodgers RJ. Formation of ovarian follicular fluid may be due to the osmotic potential of large glycosaminoglycans and proteoglycans. Reproduction. 2006;132(1):119–131. doi: 10.1530/rep.1.00960. [DOI] [PubMed] [Google Scholar]
- 31.van Wezel IL, Krupa M, Rodgers RJ. Development of the membrana granulosa of bovine antral follicles: structure, location of mitosis and pyknosis, and immunolocalization of involucrin and vimentin. Reproduction, Fertility and Development. 1999;11(1):37–48. doi: 10.1071/rd98069. [DOI] [PubMed] [Google Scholar]
- 32.Clark AR, Stokes YM, Lane M, Thompson JG. Mathematical modelling of oxygen concentration in bovine and murine cumulus-oocyte complexes. Reproduction. 2006;131(6):999–1006. doi: 10.1530/rep.1.00974. [DOI] [PubMed] [Google Scholar]
- 33.Arlotto T, Schwartz JL, First NL, Leibfried-Rutledge ML. Aspects of follicle and oocyte stage that affect in virtro maturation and development of bovine oocytes. Theriogenology. 1996;45(5):943–956. doi: 10.1016/0093-691x(96)00024-6. [DOI] [PubMed] [Google Scholar]
- 34.Perry GA, Smith MF, Lucy MC, et al. Relationship between follicle size at insemination and pregnancy success. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5268–5273. doi: 10.1073/pnas.0501700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry GA, Smith MF, Roberts AJ, MacNeil MD, Geary TW. Relationship between size of the ovulatory follicle and pregnancy success in beef heifers. Journal of Animal Science. 2007;85(3):684–689. doi: 10.2527/jas.2006-519. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura K, Eppig JJ. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reproduction, Fertility and Development. 2005;17(7):667–674. doi: 10.1071/rd05071. [DOI] [PubMed] [Google Scholar]
- 37.Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and their companion granulosa cells. Developmental Biology. 2005;279(1):20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 38.Clark AR, Stokes Y, Thompson J. Estimation of glucose uptake by ovarian follicular cells. Annals of Biomedical Engineering. 2011;39(10):2654–2667. doi: 10.1007/s10439-011-0353-y. [DOI] [PubMed] [Google Scholar]
- 39.Leese HJ, Barton AM. Production of pyruvate by isolated mouse cumulus cells. Journal of Experimental Zoology. 1985;234(2):231–236. doi: 10.1002/jez.1402340208. [DOI] [PubMed] [Google Scholar]
- 40.Eppig JJ, O’Brien M, Wigglesworth K. Mammalian oocyte growth and development in vitro. Molecular Reproduction and Development. 1996;44(2):260–273. doi: 10.1002/(SICI)1098-2795(199606)44:2<260::AID-MRD17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Tanghe S, Van Soom A, Nauwynck H, Coryn M, De Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation and fertilisation. Molecular Reproduction and Development. 2002;61(3):414–424. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- 42.Neeman M, Abramovitch R, Schiffenbauer YS, Tempel C. Regulation of angiogenesis by hypoxic stress: from solid tumours to the ovarian follicle. International Journal of Experimental Pathology. 1997;78(2):57–70. doi: 10.1046/j.1365-2613.1997.d01-247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamanini C, De Ambrogi M. Angiogenesis in the developing follicle and corpus luteum. Reproduction in Domestic Animals. 2004;39(4):206–216. doi: 10.1111/j.1439-0531.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 44.Sutton ML, Cetica PD, Beconi MT, Kind KL, Gilchrist RB, Thompson JG. Influence of oocyte-secreted factors and culture duration on the metabolic activity of bovine cumulus oocyte complexes. Reproduction. 2003;126(1):27–34. doi: 10.1530/rep.0.1260027. [DOI] [PubMed] [Google Scholar]
- 45.Sutton-McDowall ML, Gilchrist RB, Thompson JG. Cumulus expansion and glucose utilisation by bovine cumulus-oocyte complexes during in-vitro maturation: the influence of glucosamine and follicle-stimulating hormone. Reproduction. 2004;128(3):313–319. doi: 10.1530/rep.1.00225. [DOI] [PubMed] [Google Scholar]


