Abstract
Sister chromatid exchange (SCE) test is a sensitive, biomarker of genotoxic substances. The frequency of SCE in lymphocytes of ten pan chewing patients, oral submucous fibrosis (OSF) patients and age matched healthy controls were investigated. The frequency of mean SCE/cell was found to be 10.428 ± 0.755 in OSF patients, 8.752 ± 0.383 in case of pan chewers as compared to 5.912 ± 0.310 in controls. These values show a significant increase in frequency of SCE/cell in OSF patients and pan chewers when compared with that of healthy controls. There is a positive correlation co-efficient of SCE/cell with frequency, quantity, duration, intensity and period of exposure of pan-parag to oral mucosa in pan chewers and OSF patients indicating genotoxic effect of pan. Thus SCE could be used as a biomarker in chewers also to assess the level of genomic damage and to advocate efficient control measures.
Keywords: Genotoxic, sister chromatid exchange, biomarker
INTRODUCTION
Mutation refers to genetic damage or change brought about by environmental or chemical factors. A vast majority of the recognized human carcinogens are genotoxic. Sister chromatid exchange (SCE), right from the time when Taylor (1958) discovered an unexpected exchange of labeled DNA between sister chromatids,[1] has remained a good biomarker, possibly even a biodosimeter, for exposure to potential carcinogens.[2]
Mutation occurring either in a hereditary fashion or due to environmental factors like high-energy radiation, certain chemicals, viruses etc. has caused disturbances in the normal morphological patterns of mitosis. Recently, the different chemical constituents of pan, namely nicotine, arecholine, arecaidine, anatarbine and dimethyl sulfoxide, have gained acceleration for the fact of causing genotoxic effects. These genotoxic agents are capable of causing nicks in the DNA strands, and the nick in a single DNA strand frees the severed end, which then invades the exposed complementary helix to create a short pairing region. This initiates a general recombination event and DNA renaturation. DNA nicking agents induce reparative exchanges between sister chromatids during the cell cycle when replicate sisters are on hand. Therefore, this causes an exchange or crossover of genetic material between the two chromatids of a chromosome during mitosis. Thus, SCE is a mechanism to safeguard and repair the damaged DNA.
SCE is defined as a symmetrical exchange at one locus between sister chromatids, and appears to involve DNA breakage and repair mechanism during the S phase of the cell cycle, which does not alter the overall chromosomal morphology.
Normally, five to eight SCEs per cell are present, but this is greatly increased in patients with hereditary disorders like Bloom's syndrome, Xeroderma pigmentosum, Fanconi's anemia as well as malignancies and premalignancies.
Oral submucous fibrosis (OSF) is a chronic, insidious, fibrotic change affecting any part of the oral mucosa. When analyzed, it is found to have a possible correlation with tobacco and betel nut chewing habits, which have been proved to induce genotoxic effects in the initiation of OSF.[3] However, very little information is available regarding the use of pan chewers and OSF patients. The present study is aimed at evaluating chromosomal/DNA instability using SCE as a marker.
MATERIALS AND METHODS
Peripheral blood samples were obtained with informed consent from 10 male patients who had the habit of chewing pan for 5 or more years, 10 male OSF patients who had panparag chewing habit and 10 age-and sex-matched controls without any chewing habit, attending the Oral Pathology Department of Ragas Dental College, Chennai. All of them were males. Individuals who were not occupationally exposed to mutagens, under recent antibiotic therapy, had any recent viral diseases and had history of drug intake continuously for more than 2 months were included for the study.
Peripheral blood samples were collected in heparinised syringe under aseptic conditions. Whole blood lymphocytes from individuals were cultured in RPMI-1640 (Rosewell Park Memorial Institute), supplemented with fetal bovine serum and phytohemagglutinin. After 24 h, bromo deoxy uridine was added at a concentration of 5 μg/ml and cells were grown in dark at 37°C. At the 69th hour, Colchicine was added after hypotonic treatment by potassium chloride and fixation done in 3:1 methanol:acetic acid. Slides were prepared by air-drying. One-day-old slides were stained with Hoechst (Bisbenzimide) at a concentration of 5 μg/ml in the dark for 30 min, and then washed with distilled water mounted in 2×SSC with a coverslip and exposed to sunlight for 2 h. They were washed again with distilled water and stained in 4% Giemsa. SCE scoring was done at a rate of 25 metaphase plates per individual. Plates with less than 46 chromosomes, scattered or clumped chromosomes, overlapped chromosomes, improperly stained chromosomes and morphologically altered chromosomes were not taken into account for counting.
Statistical analysis was done using SPSS 10.05 version. Test for statistical significance (P-value) and correlation co-efficient was done using this version for period of exposure, duration of the habit intensity and mouth opening to SCE/cell. P<0.05 indicates statistical significance. Positive r-value indicates that comparative values are directly proportional and negative r-value indicates that comparative values are inversely proportional.
RESULTS
A total of 30 patients were taken for this study, comprising of 10 controls, 10 pan chewers and 10 OSF patients.
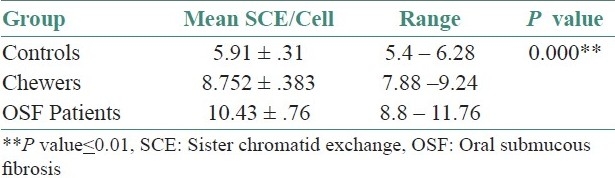
The mean age for all the three groups of people taken was 37 years. The SCE values from 25 metaphase plates per individual were analyzed and the mean SCE/cell for controls, pan chewers and OSF patients were 5.912 ± 0.310, 8.752 ± 0.383 and 10.428 ± 0.755, respectively.
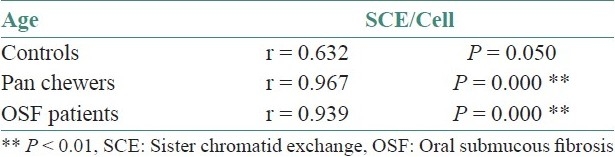
The mean SCE/cell of pan chewers and OSF patients had a statistically significant elevation compared with that of the controls (P=0.000). The mean SCE/cell of OSF had a statistically significant elevation compared with that of the pan chewers (P=0.000) [Table 1 and Graph 1].
Table 1.
Comparison of mean sister chromatid exchange/cell among controls, pan chewers and oral submucous fibrosis patients
Graph 1.
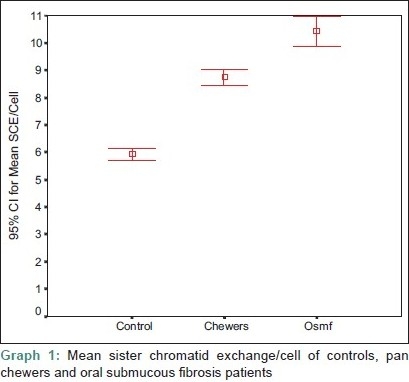
Mean sister chromatid exchange/cell of controls, pan chewers and oral submucous fibrosis patients
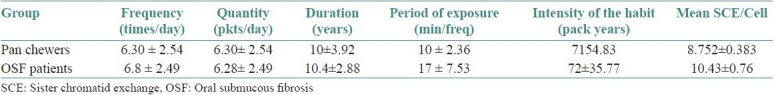
The mean frequency of the habit, quantity/day, period of exposure/frequency, total duration of exposure and intensity were 6.30 ± 2.54 times/day, 6.30 ± 2.54 packs/day, 10 ± 3.92 min/frequency, 10 ± 3.92 years and 71 ± 54.837 pack-years, respectively, in pan chewers and, in OSF patients, the same were 6.80 ± 2.49 times/day, 6.80 ± 2.49 packs/day, 17 ± 7.53 min/frequency, 10.40 ± 2.88 years and 72.1 ± 35.77 pack-years, respectively [Table 2].
Table 2.
Comparison of the mean frequency, quantity, duration of pan chewing habit, period of exposure, intensity of the habit and sister chromatid exchange/cell in pan chewers and oral submucous fibrosis patients
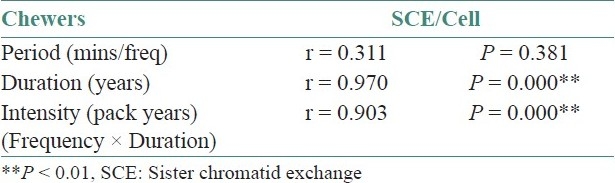
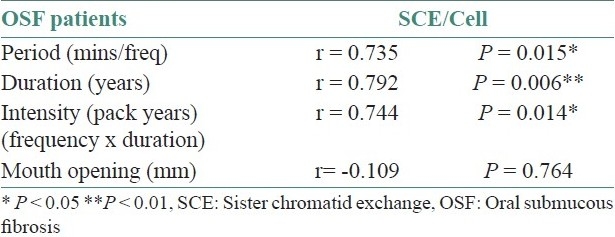
The correlation co-efficient values (r-values) of quantity of the chewing material, frequency/day, period of exposure, duration of the habit, intensity of the habit and mouth opening to SCE/cell in pan chewers is given in Table 3. There is a positive correlation co-efficient for quantity of the chewing material, frequency/day, duration of the habit and intensity of the habit to SCE/cell. All have statistically significant values to SCE/cell, except for period of exposure.
Table 3.
Co-relation of sister chromatid exchange/cell with period, duration, intensity of pan chewing habit in pan chewers
The correlation co-efficient values (r-value) of quantity of the chewing material, frequency/day, period of exposure, duration of the habit, intensity of the habit and mouth opening to SCE/cell in OSF patients is given in Table 4. There is a positive correlation co-efficient for period of exposure, duration of the habit and intensity of the habit to SCE/cell, and they all have a statistical significance except for the quantity of the chewing material and frequency/day. There is a negative correlation for mouth opening to SCE/cell in OSF patients.
Table 4.
Co-relation of sister chromatid exchange/cell with period, duration, intensity of pan chewing habit and mouth opening in oral submucous fibrosis patients
The relationship between intensity of the habit, age and SCE is given in Graph 2.
Graph 2.
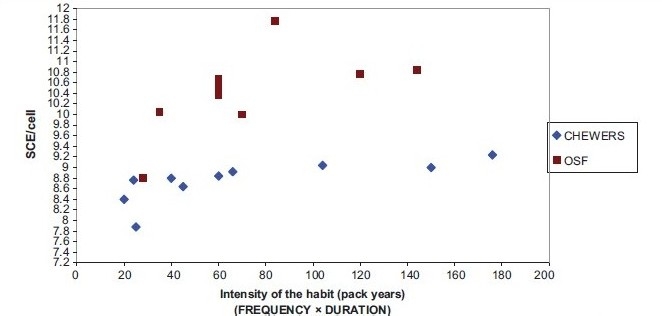
Comparison of intensity of the habit of pan chewers, oral submucous fibrosis patients and sister chromatid exchange/cell
An increase in SCE/cell with age in controls [Figure 1], pan chewers [Figure 2] and OSF patients [Figure 3] is seen. The increase in SCE/cell in relation to age in case of controls is not statistically significant, but is statistically significant in pan chewers and OSF patients [Table 5 and Graph 3].
Figure 1.
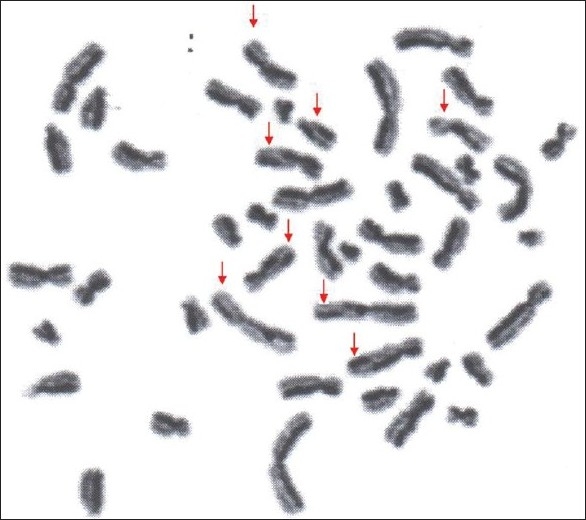
Photograph showing metaphase plate with sister chromatid exchange in controls
Figure 2.
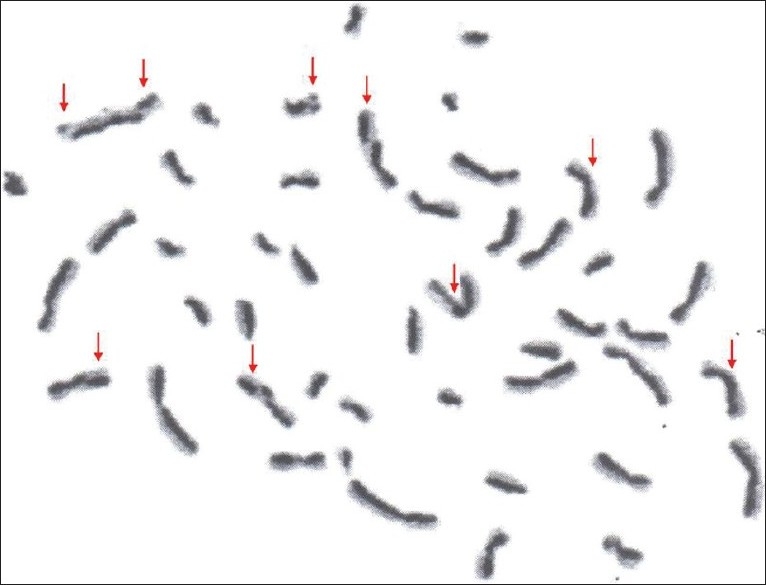
Photograph showing metaphase plate with sister chromatid exchange in pan chewers
Figure 3.
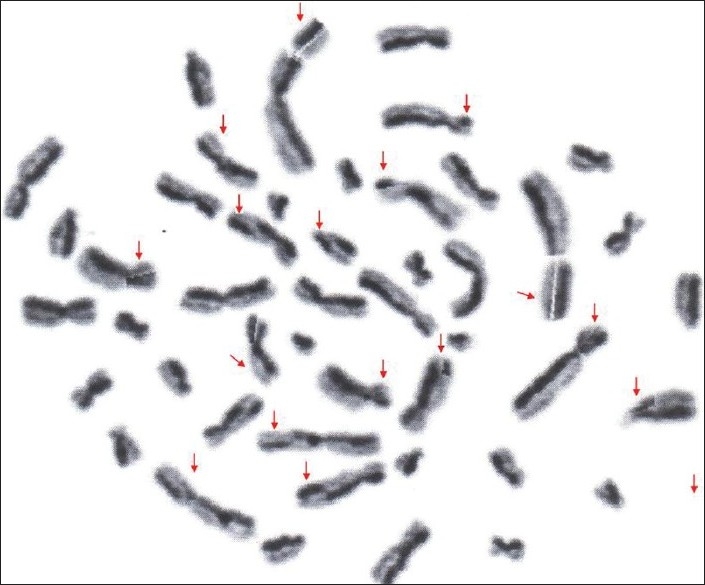
Photograph showing metaphase plate with sister chromatid exchange in osf patients.
Table 5.
Correlation co-efficient of sister chromatid exchange/cell to the age of the controls, pan chewers, oral submucous fibrosis patients
Graph 3.
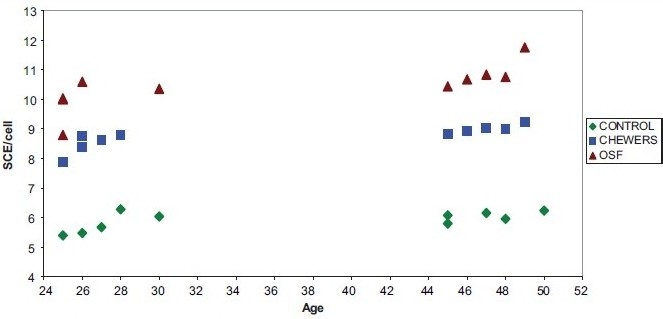
Comparison of age of the pan chewers, oral submucous fibrosis patients and sister chromatid exchange/cell
DISCUSSION
Oral OSF is strongly advocated as a precancerous condition that renders the mucosa more vulnerable to the action of carcinogens.[3] Since the time of Schwartz's report of OSF in East African women of Indian origin, this condition has evoked the interest of dental professionals. The peculiarity of this disease is that it is confined to a particular geographic region, i.e. it is predominantly seen among the Indians and those who have settled in other countries and, to a lesser extent, in other Asiatic people.[4]
In India, tobacco, areca nut, chewing is very popular and has been strongly implicated in the causation of oral precancer and cancer. In the recent years, the consumption of commercial preparations like panparag with areca nut as the main ingredient has become a fashionable habit, especially in the younger age groups. As a result, OSF has become more prevalent in the younger age group people, who have started the habit very recently.
Arecoline (the major areca nut alkaloid), nicotine (the major tobacco alkaloid) and many of tobacco (areca nut)-specific nitrosamines detected in the saliva of chewers are all potent mutagens and carcinogens. It is also proposed that chewing of areca nut with tobacco may have a synergistic effect and accelerate the promotion of tobacco-related carcinogenesis.[5]
It was Taylor in 1958 who introduced assessment of the SCE in peripheral blood lymphocytes to evaluate few toxic potentials of chemical mutagens and carcinogens.[6] Chromosomal instability is always associated with neoplastic disorders.[7] Because OSF is considered as a precancerous condition, and the rate of malignant transformation is as high as 7.6% in the Indian subcontinent, the present study was designed to utilize a quantitative assay of SCE in OSF patients.
Earlier studies on SCE as an indicator of mutagenesis[8] were confined to betel and tobacco chewers and among betel-chewing pregnant women with or without the use of oral contraceptives.[9] Later studies on SCE frequencies included betel nut and tobacco chewers,[5,7] oral leukoplakias,[10] oral cancer[11] and OSF.[3,11] Increase in SCE was reported in the lymphocytes of patients with betel nut and pan chewers (10.61 ± 2.85), oral leukoplakia (7.95 ± 1.63) and oral cancer (9.38 ± 1.28).
Adhvaryu et al. (1986)[1] were the pioneers in estimating increased SCE frequencies in lymphocytes of tobacco/betel nut chewers (7.40 ± 0.271) and patients with OSF (7.89 ± 0.279) than their controls (6.16 ± 6.167). Similarly, Ghosh et al. (1990)[7] showed an increase in the frequency of SCE in OSF patients (8.12 ± 1.69). Again, in 1991, Adhvaryu et al.[11] estimated the SCE frequencies in tobacco/areca nut chewers and OSF patients and reconfirmed that there is an increase in SCE/cell in tobacco chewers (7.219 ± 0.0221) and in OSF patients (7.617 ± 0.183) than their controls (6.185 ± 0.088).
In our study, the SCE in controls ranged from 5.4 to 6.28 SCE/cell, with a mean of 5.91 ± 0.31 in pan chewers. There was an increase in the SCE/cell, which ranged from 7.88 to 9.24, with a mean of 8.752 ± 0.383. The increase in SCE/cell in pan chewers was statistically significant (P=0.000). In OSF patients, there was an increase of SCE/cell; the range was from 8.8 to 11.76, with a mean of 10.43 ± 0.76, which is statistically significant (P=0.000). The higher value recorded in the present study compared with previous observations could be attributed to the use of panparag, the main ingredient being areca nut, whereas earlier workers’ estimation reflected the use of betel leaf, betel nut, lime and tobacco. The probability of an increase in the quantity of areca nut in panparag when compared with the conventional chewing accounts for the difference.
We observed a slight increase in SCE/cell with age in controls, which is not statistically significant (P=0.05), but there was an increase in SCE/cell with age in pan chewers and OSF patients (P=0.000). It can be noted that as the age of the patient increases, there is an increase in the SCE/cell, and this increase can be attributed to the increased levels of damaged DNA, possibly accompanied by a decreased efficiency in the repair of induced damage. SCE/cell in relation to frequency, quantity, duration and intensity in pan chewers showed a high statistical significance. Similarly, SCE/cell in relation to intensity, period and duration of the habit in OSF patients showed a statistically significant elevation. This observation is similar to that by Ghosh et al., who reported a dose-response relationship between degree of exposure to betel nut and tobacco chewing to the level of chromosomal damage, as reflected by SCE/cel1.[5] In pan chewers SCE/cell in relation to period of exposure and in OSF patients SCE/cell in relation to quantity and frequency is not statistically significant in our study. This variation can be attributed to the small sample size taken. We observed an inverse correlation of mouth opening to SCE frequency. This indicates that increased genomic instability is associated with progression of OSF. Although this parameter could be utilized to assess clinical staging of OSF, our sample size is very small to suggest it for practical applications.
ACKNOWLEDGEMENTS
I whole heartedly acknowledge Dr. Kalpana Gourishankar, Dr. Chandra, Dr. Uma Devik Rao and Dr. Elizabeth Joshua for their valuable guidance throughout the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Sönmez S, Kaya M, Aktaş A, Ikbal M, Senel K. High frequency of SCE in lymhocytes of patients with Behcet's disease. Mutat Res. 1998;397:235–8. doi: 10.1016/s0027-5107(97)00218-2. [DOI] [PubMed] [Google Scholar]
- 2.Labbauf A, Klopman G, Rosenkranz HS. Dichotomous relationship between DNA reactivity and their induction of SCE in vivo and in vitro. Mutat Res. 1997;377:37–52. doi: 10.1016/s0027-5107(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 3.Adhvaryu SG, Bhatt RG, Dayal PK, Trivedi AH, Dave BJ, Vyas RC, et al. SCE frequencies in lymphocytes of tobacco/betel nut chewers and patients with OSF. Br J Cancer. 1986;53:141–3. doi: 10.1038/bjc.1986.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeng JH, Chang MC, Huhn LJ. Role of Areca nut in betel quid associated chemical carcinogenesis. Current awareness and future prospectives. Oral Oncol. 2001;37:477–92. doi: 10.1016/s1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh R, Ghosh PK. SCE in betel and tobacco chewers. Mutat Res. 1984;139:79–81. doi: 10.1016/0165-7992(84)90107-6. [DOI] [PubMed] [Google Scholar]
- 6.Wolff S, Perry P. Differential Giemsa staining of sister chromatids and the study of SCEs without autoradiography. Chromosoma. 1974;48:341–53. doi: 10.1007/BF00290991. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh PK, Madhavi R, Guntur M, Ghosh R. SCE in patients with OSF. Cancer Genet Cytogenet. 1990;44:197–201. doi: 10.1016/0165-4608(90)90047-e. [DOI] [PubMed] [Google Scholar]
- 8.Carrano AV, Thompson LH, Lindl PA. SCE as an indicator of mutagenesis. Nature. 1978;271:351–3. doi: 10.1038/271551a0. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh PK, Ghosh R. Effect of betal chewing on the frequency of SCE in pregnant women and women using oral contraceptives. Cancer Genet Cytogenet. 1988;32:2115. doi: 10.1016/0165-4608(88)90283-x. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh R, Sharma JK, Ghosh PK. SCE in lymphocytes of patients with oral leukoplakia. Cancer Genet Cytogenet. 1988;36:177–82. doi: 10.1016/0165-4608(88)90142-2. [DOI] [PubMed] [Google Scholar]
- 11.Adhvaryu SG, Dave BJ, Trivedi AH. Cytogeneic surveillance of tobacco areca nut (mava) chewers including patients with oral cancers and premalignant conditions. Mutat Res. 1991;261:41–9. doi: 10.1016/0165-1218(91)90096-5. [DOI] [PubMed] [Google Scholar]


