Abstract
Aim:
This study aims to evaluate the cytotoxicity of a new fast set highly viscous conventional glass ionomer cement (GIC) with L929 fibroblasts.
Materials and Methods:
The cement capsule was mixed and introduced into a paraffin wax mould. After setting, the cement was incubated in Dulbecco's Modified Eagle's Medium. Six replicates of the material extract were added to the culture medium in 96-well plates. L929 mouse fibroblast cells were added into the wells and then incubated for 48 h. Dimethylthiazol diphenyltetrazolium bromide test was performed for cytotoxicity evaluation.
Results:
The results showed that this GIC brand did not yield a half-maximal inhibitory concentration value, IC50, as the cell viability was above 50% at all concentrations. Cell viability over 90% was observed at the concentrations of 3.125 and 1.5625 mg/ml. Maximum concentration of the material showed cell viability of 59.4%.
Conclusions:
This new fast set highly viscous conventional GIC showed low cytotoxicity to mouse fibroblast cells, and it can be suggested as a substitute for dental cements exhibiting a long setting time.
Keywords: Cell viability, dental cements, fibroblast cells, glass ionomer cement
INTRODUCTION
Since 1960, the idea of adhesion to dental hard tissues resulted in the invention of many dental cements including glass ionomer cements (GICs).[1] GIC is basically a product of acid base reaction between ion leachable glass and aqueous solution of polyacrylic acid.[2] This cement exhibits several desirable properties including good biocompatibility, physicochemical bonding to enamel and dentin, low coefficient of thermal expansion and anticariogenic activity.[3,4] Following many improvements, the GICs became one of the most successful dental cements in clinical dentistry.[4]
Beside all the developments and evolution of resin-modified GICs and compomers, conventional GICs have been subjected to many substantial modifications in their composition and powder/liquid ratio in order to overcome their compromised physical and mechanical properties.[5–12] These improved formulations, provided with fast set applications, would significantly reduce the probability of moisture contamination. In addition, the chair time will be reduced thus making the material more acceptable for both the patient and the clinician.[13]
Fast set, highly viscous conventional GICs were also introduced to extend the indication for occlusal restorations, which require high-strength materials. Even though they did not show an improvement in the mechanical and wear properties in the initial stages, the long term wear resistance and hardness was found to be high, and was suggested to compete with that of composite resins.[14,15]
As a result of this considerable heterogeneity in the composition of new conventional GICs, it is essential to evaluate their cytotoxic activity and host tissue response.[10,16] This preliminary study aims to investigate the cytotoxicity of a new fast set highly viscous radiopaque conventional GIC with the L929 mouse fibroblast cell line.
MATERIALS AND METHODS
A Paraffin wax mould, with a diameter 1.5 cm and depth 2 mm, supported by sterile stainless steel mould was prepared as mentioned by Min et al.[17] (with modification) [Figure 1a]. The fast set, highly viscous radiopaque conventional glass ionomer capsule (Ionofil Molar AC Quick, Voco, Germany) was activated and mixed immediately in a capsule mixer for 10 s as recommended by the manufacturer. The mix was then introduced into the paraffin wax mould [Figure 1b].
Figure 1.
(a) Paraffin wax mould supported by sterile stainless steel mould. (b) Glass ionomer cement after application. (c) Sterilization of the set cement by ultraviolet radiation
After setting of the cement (setting time 2.5 min), it was sterilized using ultraviolet radiation for 30 min [Figure 1c]. The cement was weighed and then introduced into sterile glass bottles containing Dulbecco's Modified Eagle's Medium (200 mg/ml). Following this, it was incubated for 72 h at 37°C.
After incubation, the material extract was passed through a filter (20 μm) into another sterile glass bottle. Six replicates of the material extract were added into 96-well plate. L929 mouse fibroblast cell culture was applied into the wells containing full and diluted concentrations of the material extract, and then incubated for 48 h at 37°C and 5% CO2. Dimethylthiazol diphenyltetrazolium bromide was added to each well for 4 h. The wells were evacuated and Dimethyl sulphoxide was then introduced into each well and the optical density (wavelength 570 nm) was determined using an enzyme-linked immunosorbent assay reader.
RESULTS
The results showed that this GIC brand did not yield a half-maximal inhibitory concentration (IC50) as the cell viability was above 50% at all concentrations [Figure 2]. Cell viability over 90% was observed at the concentrations of 3.125 and 1.5625 mg/ml. At the concentration of 6.25, 12.5 and 25 mg/ml, the cell viability was above 80%. At the concentration 50 mg/ml, the cell viability was 68%. Maximum concentration of the material showed cell viability of 59.4%.
Figure 2.
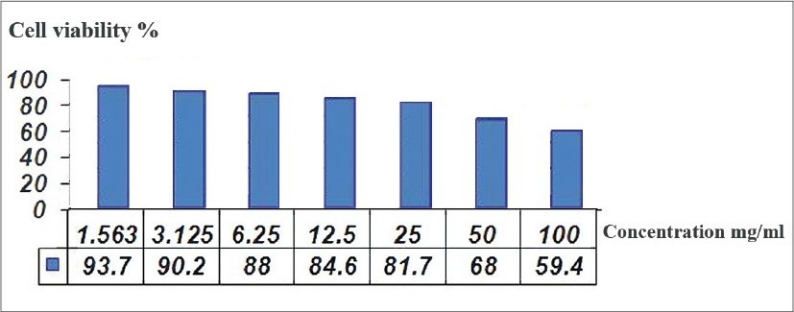
Cell viability at different concentrations of the material
DISCUSSION
GIC is widely used in clinical dentistry as a luting cement, cavity base or liner and for the restoration of primary and permanent teeth.[18] In endodontic treatment, it can be used for root end filling, repair of perforation areas, filling of resorption defects and temporary filling during endodontic therapy.[4] Despite these wide clinical indications, the imperfect physical and mechanical properties of conventional GIC were the main cause for the resistance to its adoption in wider clinical applications.[3,4]
Resin-modified GICs were introduced in the late 1980s as an attempt to improve the physical and mechanical properties while maintaining the basic features of the conventional GIC.[4,7] However, many studies found them to be cytotoxic.[19,20] Furthermore, they also showed strong genotoxic effects.[21] These unfavorable properties are mainly attributed to their ability to release the monomer HEMA (2 hydroxethyl methacrylate) which can lead to a variety of adverse biological effects.[20]
The low cytotoxic activity observed in this fast set, highly viscous conventional GIC brand is consistent with other conventional types that show good biocompatibility due to their rapid neutralization, release of generally benign ions from the set cement and low setting exothermic reaction.[20]
Some clinical situations, like root end surgery, warrant the use of fast set dental cement, as this will prevent its solubility, disintegration and dislodgement thus, maintaining its dimensional stability.[22,23] The presence of these fast set conventional GICs in pre-proportioned, mechanically mixed capsules will provide an easier procedure with a consistent mix, which can be effectively applied to the area of interest.
The concurrent existence of low cytotoxicity along with favorable physical properties including simpler handling properties, suitable working and setting time, is highly advantageous when the material is going to be introduced in areas where the moisture is difficult to control, and it will also be in direct contact with vital tissues, like the periapical tissues.[24] This would appreciably reduce the host tissue response and maintain the dimensional stability of the cement.
CONCLUSIONS
The new fast set highly viscous conventional GIC showed low cytotoxicity to mouse fibroblast cells and, in some clinical situations, it can be suggested as a substitute for dental cements exhibiting long setting time
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998;19:467–78. doi: 10.1016/s0142-9612(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AD, Kent BE. The glass-ionomer cement, a new translucent dental filling material. J Appl Chem Biotechnol. 1971;21:313. [Google Scholar]
- 3.Naasan MA, Watson TF. Conventional glass ionomers as posterior restorations.A status report for the American Journal of Dentistry. Am J Dent. 1998;11:36–45. [PubMed] [Google Scholar]
- 4.De Bruyne MA, De Moor RJ. The use of glass ionomer cements in both conventional and surgical endodontics. Int Endod J. 2004;37:91–104. doi: 10.1111/j.0143-2885.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- 5.Guggenberger R, May R, Stefan KP. New trends in glass-ionomer chemistry. Biomaterials. 1998;19:479–83. doi: 10.1016/s0142-9612(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 6.Saito S, Tosaki S, Hirota K. Characteristics of Glass-Ionomer cements. In: Davidson CL, Mjör IA, editors. Advances in glass ionomer cements. 1st ed. Illinois: Quintessence Publishing Co. Inc; 1999. pp. 15–50. [Google Scholar]
- 7.Culberston BM. Glass-ionomer dental restoratives. Prog Polym Sci. 2001;26:577–604. [Google Scholar]
- 8.Davidson CL. Advances in glass-ionomer cements. J Appl Oral Sci. 2006;14(Suppl):3–9. doi: 10.1590/s1678-77572006000700002. [DOI] [PubMed] [Google Scholar]
- 9.Moshaverinia A, Ansari S, Movasaghi Z, Billington RW, Darr JA, Rehman IU. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent Mater. 2008;24:1381–90. doi: 10.1016/j.dental.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Moshaverinia A, Roohpour N, Ansari S, Moshaverinia M, Schricker S, Darr JA, et al. Effects of N-vinylpyrrolidone containing polyelectrolytes on surface properties of conventional glass-ionomer cements. Dent Mater. 2009;25:1240–7. doi: 10.1016/j.dental.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Hammouda IM. Reinforcement of conventional glass-ionomer restorative material with short glass fibers. J Mech Behav Biomed Mater. 2009;2:73–81. doi: 10.1016/j.jmbbm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Alireza M, Nima R, Winston WLC, Scott RS. A review of powder modifications in conventional glass-ionomer dental cements. J Mater Chem. 2011;21:1319–28. [Google Scholar]
- 13.Towler MR, Bushby AJ, Billington RW, Hill RG. A preliminary comparison of the mechanical properties of chemically cured and ultrasonically cured glass ionomer cements, using nano-indentation techniques. Biomaterials. 2001;22:1401–6. doi: 10.1016/s0142-9612(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 14.Yap AU, Pek YS, Cheang P. Physico-mechanical properties of a fast-set highly viscous GIC restorative. J Oral Rehabil. 2003;30:1–8. doi: 10.1046/j.1365-2842.2003.01006.x. [DOI] [PubMed] [Google Scholar]
- 15.van Duinen RN, Kleverlaan CJ, de Gee AJ, Werner A, Feilzer AJ. Early and long-term wear of ‘fast-set’ conventional glass-ionomer cements. Dent Mater. 2005;21:716–20. doi: 10.1016/j.dental.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Six N, Lasfargues JJ, Goldberg M. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J Dent. 2000;28:413–22. doi: 10.1016/s0300-5712(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 17.Min KS, Lee SI, Lee Y, Kim EC. Effect of radiopaque Portland cement on mineralization in human dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e82–6. doi: 10.1016/j.tripleo.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Berg JH. Glass ionomer cements. Pediatr Dent. 2002;24:430–8. [PubMed] [Google Scholar]
- 19.Costa CA, Giro EM, do Nascimento AB, Teixeira HM, Hebling J. Short-term evaluation of the pulpo-dentin complex response to a resin-modified glass-ionomer cement and a bonding agent applied in deep cavities. Dent Mater. 2003;19:739–46. doi: 10.1016/s0109-5641(03)00021-6. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson JW, Czarnecka B. The biocompatibility of resin-modified glass-ionomer cements for dentistry. Dent Mater. 2008;24:1702–8. doi: 10.1016/j.dental.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Heil J, Reifferscheid G, Waldmann P, Leyhausen G, Geurtsen W. Genotoxicity of dental materials. Mutat Res. 1996;368:181–94. doi: 10.1016/s0165-1218(96)90060-9. [DOI] [PubMed] [Google Scholar]
- 22.Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32:569–72. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Bortoluzzi EA, Broon NJ, Bramante CM, Consolaro A, Garcia RB, de Moraes IG, et al. Mineral Trioxide Aggregate with or without Calcium Chloride in Pulpotomy. J Endod. 2008;34:172–5. doi: 10.1016/j.joen.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Stropko JJ, Doyon GE, Gutmann JL. Root-end management: Resection, cavity preparation, and material placement. Endod Top. 2005;11:131–51. [Google Scholar]


