Abstract
Objectives:
Spinal anesthesia has the advantage that profound nerve block can be produced in a large part of the body by the relatively simple injection of a small amount of local anesthetic. Intrathecal local anesthetics have limited duration. Different additives have been used to prolong spinal anesthesia. The effect of corticosteroids in prolonging the analgesic effects of local anesthetics in peripheral nerves is well documented. The purpose of this investigation was to determine whether the addition of dexamethasone to intrathecal bupivacaine would prolong the duration of sensory analgesia or not.
Methods:
We conducted a randomized, prospective, double-blind, case-control, clinical trial. A total of 50 patients were scheduled for orthopedic surgery under spinal anesthesia. The patients were randomly allocated to receive 15 mg hyperbaric bupivacaine 0.5% with 2 cc normal saline (control group) or 15 mg hyperbaric bupivacaine 0.5% plus 8 mg dexamethasone (case group) intrathecally. The patients were evaluated for quality, quantity, and duration of block; blood pressure, heart rate, nausea, and vomiting or other complications.
Results:
There were no signification differences in demographic data, sensory level, and onset time of the sensory block between two groups. Sensory block duration in the case group was 119±10.69 minutes and in the control group was 89.44±8.37 minutes which was significantly higher in the case group (P<0.001). The duration of analgesia was 401.92±72.44 minutes in the case group; whereas it was 202±43.67 minutes in the control group (P<0.001). The frequency of complications was not different between two groups.
Conclusion:
This study has shown that the addition of intrathecal dexamethasone to bupivacaine significantly improved the duration of sensory block in spinal anesthesia without any changes in onset time and complications.
Keywords: Bupivacaine, dexamethasone, intrathecal, sensory block, spinal anesthesia
INTRODUCTION
Spinal anesthesia is the most consistent block for lower abdomen and orthopedic surgery. Spinal anesthesia avoids the risks of general anesthesia such as aspiration of gastric contents and difficulty with airway management.[1] Bupivacaine is appropriate for procedures lasting up to 90-120 minutes.[1–3] Therefore, various additives such as epinephrine, phenylephrine, clonidine, opioids, etc. were added to local anesthetics.[2–5] The additions of epinephrine to local anesthesia cause tachycardia, pallor, and hypertension, which can be risky in patients with cardiovascular disease.[1] Intrathecal opioid administration has central and respiratory depression effects. Recently, some studies reported the effects of corticosteroids in quality and quantity of the sensory block in the peripheral nerves.[3–5]
Dexamethasone relieves pain by reducing inflammation and blocking transmission of nociceptive C-fibers and by suppressing ectopic neural discharge.[6] It has been shown that the duration of postoperative analgesia was prolonged when dexamethasone is given as an adjunct for peripheral nerve blocks.[7,8] Although dexamethasone has been used intrathecally for many years, it has not been evaluated when it was given in conjunction with bupivacaine intrathecally. The purpose of this investigation was to evaluate the effect of conjugation of dexamethasone with bupivacaine on the duration and onset time of spinal anesthesia.
METHODS
Fifty patients with class American Society of Anesthesiologist (ASA) I-II, between 18 and 55 years old, scheduled for orthopedic surgery under spinal anesthesia, were included in this prospective, randomized, double-blind clinical trial. The orthopedic procedures were on lower limbs with surgery duration around 30-70 minutes. After ethic committee approval, written informed consent was obtained from each patient preoperatively.
Patients with history of long-term steroid therapy, allergy to the drugs, uncontrolled hypertension, neurologic or psychological disorders, spinal column surgery, low back pain, alcohol abuse, opium addict or using any drug that modifies pain perception were excluded from the study.
Patients were randomly allocated into two groups, intrathecal bupivacaine- dexamethasone as the case group and intrathecal bupivacaine- normal saline as the control group.
After IV line preparation, a 5 cc/kg lactated ringers solution was infused to all patients. Patients received no premedication, and upon arrival of patients into the operating room, ECG, peripheral oxygen saturation (SPO2), and noninvasive arterial blood pressure (NIBP) were monitored and recorded at 5-minute intervals until the end of surgery and vital signs were recorded every 15 minutes in the Post Anesthesia Care Unit (PACU).
Spinal anesthesia was performed in the sitting position at L4 -L5 level through a midline approach using a 25-gauge Quincke spinal needle. Patients of the control group received 15 mg (3 ml) of 0.5% hyperbaric bupivacaine diluted in preservative free normal saline (2 ml) and patients of the case group received 15 mg (3 ml) of 0.5% hyperbaric bupivacaine and 8 mg preservative free dexamethasone with the Dexadic brand name (2 ml), overall 5 ml volume intrathecally. To facilitate the double-blinding method, the medication was prepared and injected by an anesthesiologist who was not involved in the study. After performance of the spinal anesthesia patients were kept in supine position and oxygen 3-5 L min -1 was given through a face mask. The sensory block level was assessed by a pin prick test by a short bevel needle along the mid-axillary line bilaterally. The sensory block level was controlled every 30 seconds for 20 minutes; then it was evaluated every 5 minutes until a 4 sensory level regression from highest level or to the end of the surgery. Onset time was defined from the time of injection of drugs into the intrathecal space to the peak of sensory and motor block (highest dermatome level) and the duration of sensory block was defined from peak of sensory block up to 4 sensory level regressions or when the patients feel pain in the field of surgery.
Hypotension, a 30% decrease in systolic blood pressure from base line or systolic blood pressure <100 mm Hg and bradycardia, HR<50 beats/min was treated by IV ephedrine 5-10 mg plus crystalloid fluids; and IV atropine 0.5 mg respectively. Nausea and vomiting were also evaluated and were treated with 0.15 mg /kg IV metoclopramide.
After 4 dermatome block regression, pain assessment intraoperatively or in PACU was done using the visual analogue pain scale (VAS) between 0-10 (0 = no pain, 10 = the most severe pain) every 1 hour. If the postoperative VAS was higher than 6, it was treated by morphine 2 mg IV. Patients were observed at the time of discharge from hospital and 1 month later and asked about any neurologic deficit.
The demographic data of patients were studied for each of the two groups. Continues covariates such as age, weight, height, and BMI were compared using the analysis of variance T-test. Onset time, sensory block duration, and duration of analgesia were analyzed by a T-test as appropriate, with the P value reported at the 95% confidence interval. For categorical covariates (sex, nausea/vomiting, hypotension, bradycardia, use of ephedrine, the use of atropine), the comparison was studied using a chi-squared test or Fisher's exact test. Sensory level compared by Mann-Whitney test. The significance level was defined as a P value less than 0.05. To calculate the sample size, a power analysis of α=0.05 and β=0.80 showed that 25 patients per study group were needed to detect the difference between two groups.
RESULTS
All patients (n=50) completed the study; there was no statistical difference in patients’ demographics [Table 1]. The onset time of sensory block was 11.2±2.0 minutes for the case group and 10.9±1.8 minutes for the control Group (P=0.57). The maximum sensory level was between T4 and T10 in both groups and there was no significant difference (P=0.76) [Figure 1].
Table 1.
Patients’ characteristics
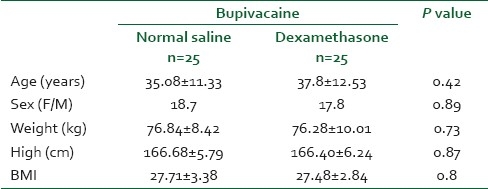
Figure 1.
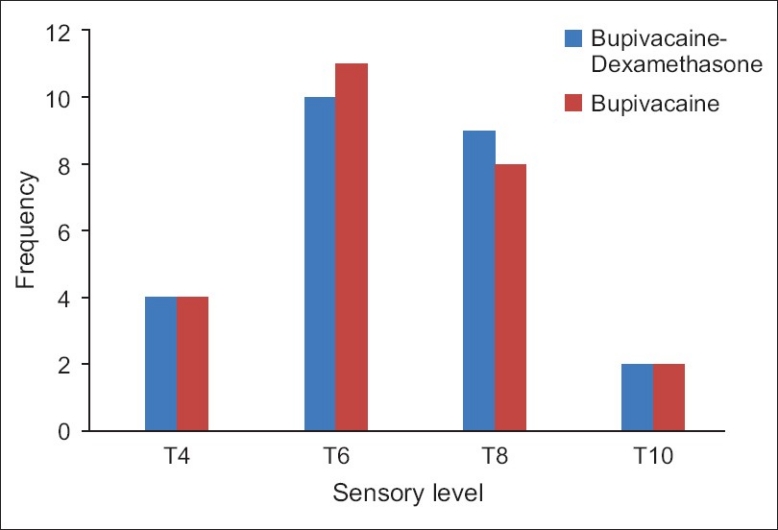
Sensory block level in the bupivacaine-dexamethasone versus bupivacaine-normal saline group
The duration of the sensory block was 119.1±10.6 minutes in the case group and 89.4±8.3 minutes in the control group with a P value less than 0.001; also pain-free period in the case group was more than that in the control group(P<0.001). Receiving time to VAS >6 and the first analgesic dose prescription in the case group was significantly longer than that in the control group (P<0.001) [Table 2]. Hypotension was mild to moderate in both groups and was not different; except one patient in the control group who had a mean arterial pressure less than 60 mmHg and required 20 mg IV ephedrine to restore his blood pressure [Table 3].
Table 2.
Comparison of onset time, duration of sensory block and pain free period between two groups
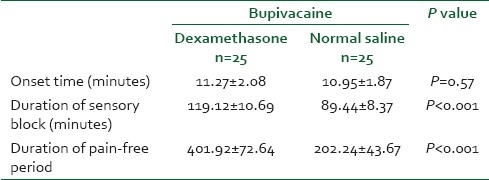
Table 3.
Incidence of adverse events between two groups during the study period
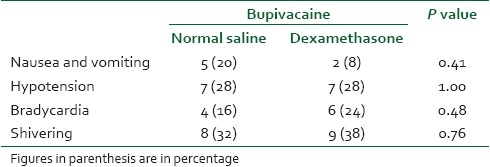
Two patients in the case group and three patients in the control group complained of postdural puncture headache which was treated by hydration and simple analgesia. Other complications such as bradycardia, nausea, and vomiting were not different between the two groups [Table 3] and no neurologic deficit was observed in any patients.
DISCUSSION
Present results in this study showed that the supplementation of spinal bupivacaine with 8 mg dexamethasone significantly prolonged sensory block and postoperative analgesia compared with intrathecal bupivacaine, without any effects on the onset time of sensory block in orthopedic surgery.
Several experiments demonstrated analgesic effects of steroids in neuroaxial and peripheral block.[9–12] Movafegh et al. reported that the addition of dexamethasone (8 mg) to lidocaine for spinal anesthesia provided significant prolongation of sensory and motor block in comparison with plain lidocaine and there is no difference between dexamethasone-lidocaine 5% and epinephrine (0.2 mg) - lidocaine 5% in sensory and motor block duration. Consequently, the onset time of sensory and motor blockade were similar among these three groups.[13]
Corticosteroids cause skin vasoconstriction on topical application. The vasoconstriction effects of topical steroids are mediated by occupancy of classical glucocorticoid receptors.[14,15] In our study, dexamethasone produced a significant prolonged sensory block which can be explained by vasoconstriction mechanism, in contrast with traditional theory of steroid action; steroids bind to intracellular receptors and modulate nuclear transcription.[16]
Mirzaie et al. reported that corticosteroids and bupivacaine can diminish the incidence of back pain after laminectomy in the immediately postoperative period.[17] Kotani et al. administered methylprednisolone with bupivacaine intrathecally in patients with postherpetic neuralgia. They concluded that this combination induced excellent and long-lasting analgesia.[18]
Taguchi et al. reported that intrathecal injection of betamethasone successfully decreased the pain score in three patients with intractable cancer pain[19] Another study reported that epidural dexamethasone (5 mg) reduces postoperative pain score and morphine consumption following laparoscopic cholecystectomy with no apparent side effects[20] Atsuhrio reported that intrathecal or epidural methylprednisolone decreased continuous pain and allodynia in patients with postherpetic neuralgia. The analgesia was much greater in the intrathecal group compared to the epidural group. Interleukin 8 in the CSF decreased significantly in the intrathecal group as compared to the epidural group.[21]
Steroids have a powerful anti inflammatory as well as analgesic property but the mechanism of the analgesia induced by corticosteroid is not fully understood.[22,23] Epidural steroids were used for back pain treatment. Intrathecal dexamethasone may influence intraspinal prostaglandin production. Acute noxious stimulation of peripheral tissues leads to sensitization of dorsal horn neurons of the spinal cord by the release of substances such as glutamate and aspartate. These amino acids activate N-methyl-D-Aspartate receptors resulting in calcium ion influx which leads to activation of phospholipase A2, which converts membrane phospholipase to arachidonic acid. Corticosteroids are capable of reducing prostaglandin synthesis by inhibition of phospholipase A2 through the production of calcium-dependent phospholipid binding proteins called annexins and by the inhibition of cyclooxygenases during inflammation.[24]
Some authors also believe that analgesic properties of corticosteroids are the results of their systemic effects.[25] The block prolonging effect may be due to its local action on nerve fibers.[26] Previous works demonstrated that addition of dexamethasone to local anesthetics prolonged duration of blockade of peripheral nerves. A study in supra-clavicular block suggests that the addition of dexamethasone to bupivacaine significantly prolonged duration of analgesia.[6] Another study in axillary block reported that dexamethasone when added to lidocaine significantly prolongs duration of analgesia without any change in onset.[27]
In animal experiments, triamcinolone[28] did not induce spinal neurotoxicity, whereas repeated high-dose intrathecal injections of betamethasone[29] caused histopathological changes of the spinal cord. Intrathecal injection of steroids was frequently used for the treatment of mumps meningitis, chronic lymphocytic leukemia and central nervous involvement in lupus erythematosus.[30] Like our finding, some of the studies did not reported any considerable complication for intrathecal dexamethasone. Kotani et al. found no complication in patients who received intrathecal methyl prednisolone.[18] Also Sugita did not find complication after intrathecal injections of 8 mg dexamethasone in patients with posttraumatic visual disturbance.[31]
CONCLUSION
In our investigation, we utilized the combination of bupivacaine and 8 mg dexamethasone intrathecally. We found that the addition of dexamethasone significantly prolongs the duration of sensory block and decreases opioid requirements in postoperative management. Further studies are needed to evaluate the optimal dose of dexamethasone to be used in spinal anesthesia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Brown D. Spinal, Epidural and caudal anesthesia. In: Miller RD, editor. Miller's Anesthesia. 7th ed. Philadelphia: Churchill living stone; 2010. pp. 1611–38. [Google Scholar]
- 2.Murali KT, Panda NB, Batra YK, Rajeev S. Combination of low doses of intrathecal Ketamine and midazolam with bupivacaine improves postoperative analgesia in orthopedic surgery. Eur J Anesthesiol. 2008;25:299–306. doi: 10.1017/S0265021507002645. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty S, Chakrabarti J, Bhattacharya D. Intrathecal tramadol added to bupivacaine as spinal anesthetic increases analgesic effect of the spinal blockade after major gynecological surgeries. Indian J pharmacol. 2008;40:180–2. doi: 10.4103/0253-7613.43166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu SC, Ngan Kee WD, Kwan AS. Addition of meperidine to bupivacaine for spinal anesthesia for cesarean section. Br j Anesth. 2002;88:379–83. doi: 10.1093/bja/88.3.379. [DOI] [PubMed] [Google Scholar]
- 5.Alhashemi JA, Kaki AM. Effect of intrathecal tramadol administration post operative pain after transurethral resection of prostate. Br J Anesth. 2003;91:536–40. doi: 10.1093/bja/aeg213. [DOI] [PubMed] [Google Scholar]
- 6.Golwala MP, Swadia VN, Aditi A, Dhimar, Sridbar NV. Pain relief by dexamethasone as an adjuvant to local anesthetics in supraclavicular brachial plexus block. J Anesth Clin Pharmacol. 2009;25:285–8. [Google Scholar]
- 7.Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27:285–8. doi: 10.1097/EJA.0b013e3283350c38. [DOI] [PubMed] [Google Scholar]
- 8.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anesthesiol Scand Suppl. 1965;16:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 9.Glasser RS, Knego RS, Delashaw JB, Fessler RG. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. J Neurosurg. 1993;78:383–7. doi: 10.3171/jns.1993.78.3.0383. [DOI] [PubMed] [Google Scholar]
- 10.Castillo J, Curley J, Hotz J, Uezono M, Tigner J, Chasin M, et al. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85:1157–66. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Droger C, Benziger D, Gao F, Berde CB. Prolonged intercostals nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology. 1998;89:969–74. doi: 10.1097/00000542-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Kopacz DJ, Lacouture PG, Wu D, Nandy P, Swanton R, Landau C. The dose response and effects of dexamethasone on bupivacaine microcapsules for intercostals blockade (T9 to T11) in healthy volunteers. Anesth Analg. 2003;96:576–82. doi: 10.1097/00000539-200302000-00050. [DOI] [PubMed] [Google Scholar]
- 13.Movafegh A, Ghafari MH. A comparison of the sensory and motor blockade duration of intrathecal lidocaine 5% plus epinephrine and lidocaine 5% plus dexamethasone. Int J pharmacol. 2005;1:346–9. [Google Scholar]
- 14.Marks R, Barlow JW, Funder JW. Steroid-induced vasoconstriction: Glucocorticoid antagonist studies. J Clin Endocrinol Metab. 1982;54:1075–7. doi: 10.1210/jcem-54-5-1075. [DOI] [PubMed] [Google Scholar]
- 15.Seidenari S, Di Nardo A, Mantovani L, Giannetti A. Parallel intraindividual evaluation of the vasoconstrictory action and the anti-allergic activity of topical corticosteroids. Exp Dermatol. 1997;6:75–80. doi: 10.1111/j.1600-0625.1997.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 16.Taguchi H, Shingu K, Okuda H, Matsumoto H. Analgesia for pelvic and perineal cancer pain by intrathecal steroid injection. Acta Anaesthesiol Scand. 2002;46:190–3. doi: 10.1046/j.0001-5172.2001.00000.x-i1. [DOI] [PubMed] [Google Scholar]
- 17.Mirzai H, Tekin I, Alincak H. Perioperative use of corticosteroid and bupivacaine combination in lumbar disc surgery. Spine. 2002;27:343–6. doi: 10.1097/00007632-200202150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M, et al. Intrathecal methylprednisolone for intractable postherpetic neuralgia. N Engl J Med. 2000;343:1514–9. doi: 10.1056/NEJM200011233432102. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi H, Shingu K, Okuda H, Matsumoto H. Analgesia for pelvic and perineal cancer pain by intrathecal steroid injection. Acta Anaesthesiol Scand. 2002;46:190–3. doi: 10.1046/j.0001-5172.2001.00000.x-i1. [DOI] [PubMed] [Google Scholar]
- 20.Thomas S, Beevi S. Epidural dexamethasone reduces postoperative pain and analgesic requirements. Can J Anaesth. 2006;53:899–905. doi: 10.1007/BF03022833. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi A, Kotani N, Sato T, Takamura K, Sakai I, Matsuki A. Comparative therapeutic evaluation intrathecal versus epidural methylprednisolone for long Term analgesia in patients with intractable post herpetic neuralgia. Reg Anesth Pain Med. 1999;24:287–93. doi: 10.1016/s1098-7339(99)90101-3. [DOI] [PubMed] [Google Scholar]
- 22.McCormack K. The spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effects. Drugs. 1994;47:28–45. doi: 10.2165/00003495-199400475-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ahlgren SC, Wang JF, Levine JD. C-fiber mechanical stimulusresponse functions are different in inflammatory versus neuropathic hyperalgesia in the rat. Neuroscience. 1997;76:285–90. doi: 10.1016/s0306-4522(96)00290-4. [DOI] [PubMed] [Google Scholar]
- 24.Yao XL, Cowan MJ, Glawin MT, Lawrence MM, Angus CW, Shelhamer JH. Dexamethasone alters arachidonate release from human epithelial cells by induction of P11 protein synthesis and inhibition of phospholipas A2 activity. J Biol Chem. 1999;274:17202–8. doi: 10.1074/jbc.274.24.17202. [DOI] [PubMed] [Google Scholar]
- 25.Baxendale BR, Vater M, Lavery KM. Dexamethasone reduces pain and swelling following extraction of third molar teeth. Anesthesia. 1993;48:961–4. doi: 10.1111/j.1365-2044.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- 26.Kopacz DJ, Lacouture PG, WU D, Nandy P, Swanton R. The dose response and effects of dexamethasone on bupivacaine microcapsules for intercostals blockade in healthy volunteers. Anesth Analg. 2003;96:576–82. doi: 10.1097/00000539-200302000-00050. [DOI] [PubMed] [Google Scholar]
- 27.Movafegh A, Razazian M, Hajimaohamadi F, Mysamie A. Dexamethasone added to lidocaine prolongs axillary bracial plexus blockage. Anesth Analg. 2006;102:263–7. doi: 10.1213/01.ane.0000189055.06729.0a. [DOI] [PubMed] [Google Scholar]
- 28.Abram SE, Marsala M, Yaksh TL. Analgesic and neurotoxic effects of intrathecal corticosteroids in rats. Anesthesiology. 1994;81:1198–205. doi: 10.1097/00000542-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Latham JM, Fraser RD, Moore RJ, Blumbergs PC, Bogduk N. The pathologic effects of intrathecal betamethasone. Spine (Phila Pa 1976) 1997;22:1558–62. doi: 10.1097/00007632-199707150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Dong Y, Zhang X, Tang F, Tian X, Zhao Y, Zhang F. Intrathecal injection with methotrexate plus dexamethasone in the treatment of central nervous system involvement in systemic lupus erythematosus. Chin Med J. 2001;114:764–6. [PubMed] [Google Scholar]
- 31.Sugita K, Kobayashi S, Yokoko A, Inoue T. Intrathecal steroid therapy for post traumatic visual disturbance. Neuronchirurgia (Stuttg) 1983;26:112–7. doi: 10.1055/s-2008-1053622. [DOI] [PubMed] [Google Scholar]


