Abstract
Background:
Relieving preoperative anxiety is an important concern for the pediatric anesthesiologist. Midazolam has become the most frequently used premedication in children. However, new drugs such as the α2 -agonists have emerged as alternatives for premedication in pediatric anesthesia.
Methods:
One hundred and twenty children scheduled for adenotonsillectomy were enrolled in this prospective, double-blind, randomized study. The children were divided into two equal groups to receive either intranasal dexmedetomidine 1 μg/kg (group D), or oral midazolam 0.5 mg/kg (group M) at approximately 60 and 30 mins, respectively, before induction of anesthesia. Preoperative sedative effects, anxiety level changes, and the ease of child-parent separation were assessed. Also, the recovery profile and postoperative analgesic properties were assessed.
Results:
Children premedicated with intranasal dexmedetomidine achieved significantly lower sedation levels (P=0.042), lower anxiety levels (P=0.036), and easier child-parent separation (P=0.029) than children who received oral midazolam at the time of transferring the patients to the operating room. Postoperatively, the time to achieve an Aldrete score of 10 was similar in both the groups (P=0.067). Also, the number of children who required fentanyl as rescue analgesia medication was significantly less (P=0.027) in the dexmedetomidine group.
Conclusion:
Intranasal dexmedetomidine appears to be a better choice for preanesthetic medication than oral midazolam in our study. Dexmedetomidine was associated with lower sedation levels, lower anxiety levels, and easier child-parent separation at the time of transferring patients to the operating room than children who received oral midazolam. Moreover, intranasal dexmedetomidine has better analgesic property than oral midazolam with discharge time from postanesthetic care unit similar to oral midazolam.
Keywords: Dexmedetomidine, midazolam, pediatric, sedation
INTRODUCTION
Relieving pre- and post-operative anxiety is an important concern for the pediatric anesthesiologist.[1] Anxiety can produce aggressive reactions, increase distress, and may make the control of postoperative pain difficult.[2,3] The benzodiazepine, midazolam, has become the most frequently used preanesthesia medication given to children scheduled for surgery.[1,2] Midazolam has a number of beneficial effects when used as premedication in children: sedation, fast onset, and limited duration of action.[4] Despite having a number of beneficial effects, it is far from an ideal premedicant having untoward side effects such as restlessness, paradoxical reaction, cognitive impairment, amnesia, and respiratory depression.[5,6]
However, new drugs such as the α2 -agonists have emerged as alternatives for premedication in pediatric anesthesia. Dexmedetomidine is a highly selective α2 -agonist with both sedative and analgesic properties and is devoid of respiratory depressant effect. These properties render it potentially useful for anesthesia premedication.[7]
Therefore, the purpose of this study was to evaluate the preoperative sedative effects, anxiety level changes and the ease of child-parent separation (as a primary end-point), and the recovery profile and postoperative analgesic properties (as a secondary end-point) of preoperative intranasal dexmedetomidine sedation compared with those of oral midazolam in children scheduled for adenotonsillectomy.
METHODS
After obtaining the approval from the Institutional Ethics Committee and written informed consent from the patients’ parents or legal guardian, 120 children of American Society of Anesthesiology (ASA) physical status I of both sexes, aged 4 to 12 years, scheduled for elective outpatient adenotonsillectomy surgery, were enrolled in this prospective, double-blind, randomized study. Exclusion criteria included ASA physical status higher than I, known allergy or hypersensitivity reaction to dexmedetomidine or midazolam, a history of central nervous system disorder, psychotropic medication use, and mental retardation. Also, the children who spit, vomited, sniffed or refused intranasal administration of medication were excluded from the study. This study was carried out in Magrabi Eye and Ear Hospital, Sultanate of Oman from January 2010 to March 2011.
Preanesthesia management
All patients were fasted overnight with clear fluids allowed until 4 hours preoperatively. In the preoperative holding area and in the presence of one parent, children were randomly (the block randomization method was used with the block size determined to be six) allocated to one of two equal groups to receive intranasal dexmedetomidine (group D, n=60), or oral midazolam (group M, n=60). The patients and their guardians were masked to the treatment arms. All the study drugs were prepared and given by an independent investigator not involved in the observation or administration of anesthesia for the children. Group D received 1 μg/kg intranasal dexmedetomidine (Precedex, 200 μg per 2mL; Abbott, USA) in a total volume of 0.5 mL normal saline and 10 mL apple juice orally as a placebo at 60 and 30 min before induction of anesthesia, respectively. Intranasal dexmedetomidine was dripped into both the nostrils using a 1ml syringe with the child in the recumbent position. Group M received 0.5 ml normal saline intranasally as a placebo and 0.5 mg/kg oral midazolam (Dormicum®, 5 mg/mL; F. Hoffman La Roche Ltd, Basel, Switzerland) with a maximum dose of 15 mg in 10 ml apple juice at 60 and 30 min before induction of anesthesia, respectively.
Observers and attending anesthesiologists were blinded to the study drug given. The heart rate (HR), systolic blood pressure (SBP), and oxygen saturation (SpaO2) were measured before administration of the study drug (baseline values) and every 10 min after that until the children were transferred to the operating room (OR). Sedation and anxiety levels were assessed before administration of the study drug (baseline values) and at the time of transferring to the OR. Also, the ease of child-parent separation at the time of transferring to the OR was assessed. Sedation level was assessed using a 6-point sedation scale, which was modified from the observer assessment of alertness and sedation scale (MOAA/S) [Table 1].[8] Anxiety level was assessed using the modified Yale preoperative anxiety scale (mYPAS).[9] The mYPAS is an instrument developed from the yale preoperative anxiety scale (YPAS) and enables the evaluation of anxiety in children preoperatively and at induction of anesthesia. It contains 22 specific behaviors within five domains (activity, emotional expressivity, state of arousal, vocalization, and use of adults) that are reflective of an anxious state and can be performed by an observer in less than 1 min. The range is 23–100 with an increased score being indicative of greater anxiety. Also, the ease of separation of the child from the parents was evaluated using a 3 point scale, first described by Feld et al. [Table 1].[10]
Table 1.
The scoring system for assessments of premedication in children

Anesthesia and postoperative management
Anesthesia was induced by inhaled sevoflurane 2-5% in oxygen using a modified Jackson Rees anesthesia system. After the induction, each child received an intravenous cannula and was given 1 μg/kg of intravenous fentanyl. Endotracheal intubation was performed in all cases without the aid of muscle relaxation and anesthesia, thereafter maintained with sevoflurane (2-4%) in nitrous oxide (60%) and oxygen (40%) at normocapnia as judged by continuous end-tidal CO2 monitoring. During the surgery, each child received paracetamol 15 mg/kg as an intravenous infusion. A standardized intraoperative intravenous infusion was started using Ringer's lactate solution at a rate of 4 ml/kg/h. The children were extubated at the end of surgery when awake and were thereafter immediately placed in the recovery position and allowed to wake up naturally in the postanesthesia care unit (PACU). The time to achieve an Aldrete score of 10 (readiness to be discharged from the PACU) was recorded. Postoperative pain was assessed every 1 hour for the first 6 hours using the objective pain scale (OPS), first described by Hannallah and colleagues.[11] This method has been validated for the assessment of pain in children.[12] The OPS score is based on the assessment of the following five parameters: blood pressure compared to preinduction baseline; crying; movement; agitation; and complaints of pain. Each parameter is given a score of 0-2, resulting in a minimum OPS score of 0 (= no pain) and a maximum OPS score of 10 (= severe pain). Patients with OPS score ≥5 were given 0.5 μg/kg of intravenous fentanyl as rescue therapy.
Statistical analysis
We chose the mean sedation score at the time of transfer to the OR to calculate the required sample size for this study. The required sample size was calculated to be 60 patients per group with α=0.05 and a power of 90% to detect a difference of at least 20% in the mean sedation score. The statistical analysis of our results was conducted using the computer program Statistical Package for the Social Sciences (SPSS) version 15.0 for Windows (SPSS, Chicago, IL). Data were expressed as mean±SD or number (percentages). The two way repeated measures analysis of variance was used to compare the interval data, and Tukey test was used as the post hoc test to determine the differences between and within the groups. Fisher's exact test was used to compare nominal data or percentages. Bonferroni correction for repeated comparisons was applied if necessary. P<0.05 was considered significant.
RESULTS
The two groups were comparable with respect to the following variables; age, gender, weight, and duration of surgery [Table 2]. The baseline HR and SBP values were similar in both groups. At the time of transferring patients to the OR, the HR was significantly less in the dexmedetomidine group compared to the midazolam group (P=0.036) and the SBP was significantly less in the dexmedetomidine group compared to the midazolam group (P=0.032). However, there were no episodes of bradycardia, hypotension, or hypertension in both groups. On the subject of SpaO2 values, there were similar baseline values in both groups. At the time of transferring patients to the OR, there was no statistically significant difference (P=0.087) between the two groups. No SpaO2 reduction below 95% could be detected in both groups. There were no child had a reduction of SpaO2 to below 95% [Table 2].
Table 2.
Patients’ characteristics and peri-operative assessments
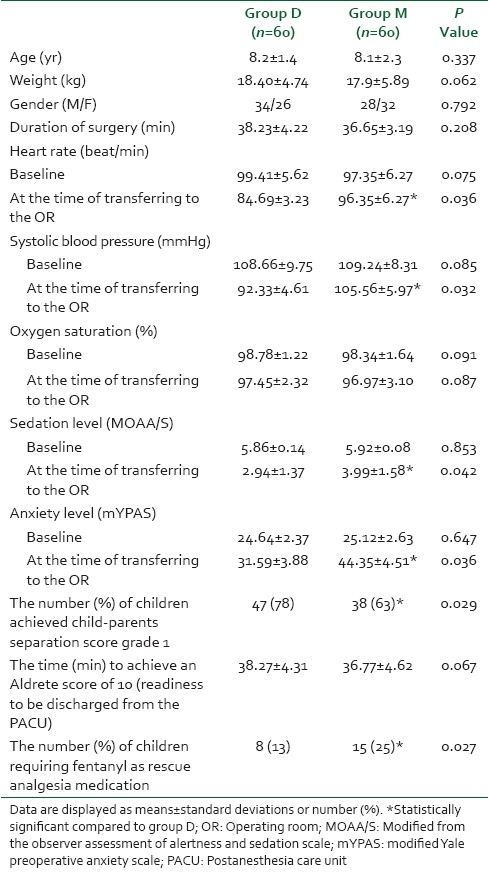
The baseline sedation and anxiety levels were comparable in both groups. At the time of transferring patients to the OR, children premedicated with intranasal dexmedetomidine achieved significantly lower sedation levels (P=0.042), and lower anxiety levels (P=0.036). Furthermore, the number and percentages of children achieved child-parents separation score grade 1 were significantly higher (P=0.029) in the dexmedetomidine group compared with the midazolam group [Table 2]. In the recovery room and postoperatively, the time to achieve an Aldrete score of 10 was similar (P=0.067) in both groups [Table 2]. Children in the dexmedetomidine group had significantly lower levels of pain in the first two hours postoperatively compared with the same values in the midazolam group [Figure 1] and the number of children who required fentanyl as rescue analgesia medication was significantly less (P=0.027) in the dexmedetomidine group compared with the children in the midazolam group [Table 2].
Figure 1.
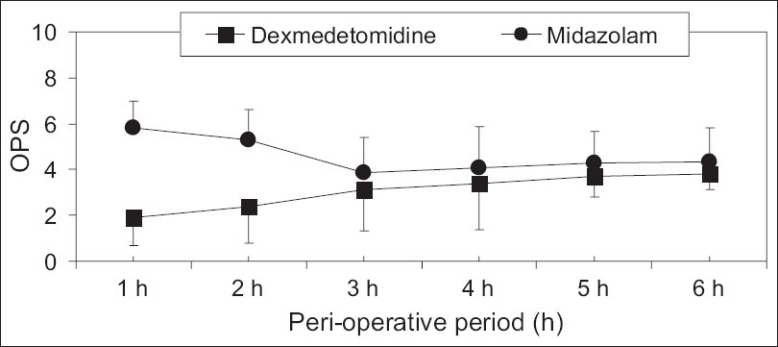
The objective pain scale (OPS) levels during the postoperative period. Data are displayed as means±standard deviations. *Statistically significant compared to group D
DISCUSSION
This study demonstrated that children premedicated with intranasal dexmedetomidine achieved significantly lower sedation levels, lower anxiety levels, and easier child-parent separation at the time of transferring to the OR than children who received oral midazolam. Benzodiazepines produce its effect by facilitating gamma amino butyric acid (GABA)–receptor binding in the cerebral cortex which enhances the membrane conductance of chloride ions. This leads to a change in membrane polarization that inhibits normal neuronal function. Midazolam produces sedation, anxiolysis, and some antegrade amnesia.[4] The site of action of the α2 receptors agonists is primarily in the locus coeruleus in the central nervous system. Dexmedetomidine induces electroencephalogram activity similar to natural sleep. It affords sedative, anxiolytic, and analgesic effects without causing excessive drowsiness.[13]
Previous studies comparing intranasal dexmedetomidine with oral midazolam, reported to some extent similar results. Yuen et al.[14] reported that intranasal dexmedetomidine produces more sedation than oral midazolam. The behavior of the children at parental separation and at induction of anesthesia with intranasal dexmedetomidine sedation was comparable to children who received oral midazolam.
On the other hand, Schmidt et al.[15] found no difference in preanesthesia levels of sedation and response to separation from parents between intranasal dexmedetomidine and oral midazolam. The discrepancy in the results could have resulted from the different scales used for assessment of sedation and anxiety level changes. They used a 4-point sedation scale and a 4-point behavior scoring system which were less sensitive than the scales used in the current study. In the current study, sedation level changes were assessed using a 6-point sedation scale, which was modified from the MOAA/S and proved to be sensitive in the assessment of sedation level changes over time in children.[8] Anxiety level changes were assessed using the mYPAS which is sensitive to changes in anxiety over time and has been shown to have good inter-observer reliability and high construct and concurrent validity.[9]
In addition, this study demonstrated that intranasal dexmedetomidine reduced HR and SBP during the preoperative sedation period compared with the oral midazolam. Children in both groups maintained normal SpaO2 values. Similar results were reported by Yuen et al.[14] Dexmedetomidine is known to decrease sympathetic outflow and circulating catecholamine levels and would therefore be expected to cause a decrease in HR and SBP[16]
Another significant finding was that the time to achieve an Aldrete score of 10 was similar in both groups and dexmedetomidine showed better analgesic properties than midazolam (lower OPS). The number of children who required fentanyl as rescue analgesia medication was significantly less in the dexmedetomidine group compared with the children in the midazolam group. It is now well described that dexmedetomidine has analgesia sparing effects when used for sedation in the intensive care unit (ICU).[17] The half-life of dexmedetomidine has been described as two hours and this would likely explain as to why the analgesic sparing properties persisted postoperatively.[13] This interesting characteristic of dexmedetomidine (persistent analgesic effects with preserving the patient arousability) in the postoperative period resulted in significantly more analgesia with equivalent discharge times when compared with midazolam. Similar results were reported in a previous study by Schmidt et al.[15]
In fact, there were certain limitations to this study. In order not to interfere with the normal OR schedule, we did not measure the sedation effect changes overtime of the two studied drugs to detect the onset time and peak sedation effect or the plasma concentrations of the drugs. We relied on the results of previous studies which detected the mean times of the peak sedation effect of the studied drugs. Future studies are supposed to consider that the child-parent separation should be adjusted to meet the peak sedation effect.
CONCLUSION
Intranasal dexmedetomidine appears to be a better choice for preanesthetic medication than oral midazolam in our study. Dexmedetomidine was associated with lower sedation levels, lower anxiety levels, and easier child-parent separation at the time of transferring patients to the OR than children who received oral midazolam. Moreover, intranasal dexmedetomidine has better analgesic property than oral midazolam with discharge time from PACU similar to oral midazolam.
ACKNOWLEDGMENTS
We thank Dr. Mahmoud M. Ibrahim, Assistant Professor of educational psychology (statistical education), Sultan Quaboos University, Oman, for his statistical assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: A comparison of four routes of administration. Paediatr Anaesth. 2002;12:685–9. doi: 10.1046/j.1460-9592.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 2.Egan KJ, Ready LB, Nessly M, Greer BE. Self-administration of midazolam for postoperative anxiety: A double blinded study. Pain. 1992;49:3–8. doi: 10.1016/0304-3959(92)90180-J. [DOI] [PubMed] [Google Scholar]
- 3.Gil KM, Ginsberg B, Muir M, Sykes D, Williams DA. Patientcontrolled analgesia in postoperative pain: The relation of psychological factors to pain and analgesic use. Clin J Pain. 1990;6:37–42. doi: 10.1097/00002508-199006000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Kain ZN, Hofstadter MB, Mayes LC, Krivutza DM, Alexander G, Wang SM, et al. Midazolam: Effects on amnesia and anxiety in children. Anesthesiology. 2000;93:676–84. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Bergendahl H, Lönnqvist PA, Eksborg S. Clonidine: An alternative to benzodiazepines for premedication in children. Curr Opin Anaesthesiol. 2005;18:608–13. doi: 10.1097/01.aco.0000191891.44314.36. [DOI] [PubMed] [Google Scholar]
- 6.Bergendahl H, Lönnqvist PA, Eksborg S. Clonidine in paediatric anaesthesia: Review of the literature and comparison with benzodiazepines for premedication. Acta Anaesthesiol Scand. 2006;50:135–43. doi: 10.1111/j.1399-6576.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- 7.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, et al. AGA Institute Review of Endoscopic Sedation. Gastroenterology. 2007;133:675–701. doi: 10.1053/j.gastro.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Kain ZN, Mayes LC, Cicchetti DV, Bagnall AL, Finley JD, Hofstadter MB. The Yale Preoperative Anxiety Scale: How does it compare with a “gold standard”? Anesth Analg. 1997;85:783–8. doi: 10.1097/00000539-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Feld LH, Champeau MW, van Steennis CA, Scott JC. Preanesthetic medication in children: A comparison of oral transmucosal fentanyl citrate versus placebo. Anesthesiology. 1989;71:374–7. [PubMed] [Google Scholar]
- 11.Norden J, Hannallah R, Getson P, O’Donnell R, Kelliher G, Walker N. Reliability of an objective pain scale in children. Anesth Analg. 1991;72:S199. [Google Scholar]
- 12.Watcha MF, Jones MB, Lagueruela RG, Schweiger C, White PF. Comparison of ketorolac and morphine as adjuvants during paediatric surgery. Anesthesiology. 1992;76:368–72. doi: 10.1097/00000542-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists.Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–65. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: A double-blinded randomized controlled trial. Anesth Analg. 2008;106:1715–21. doi: 10.1213/ane.0b013e31816c8929. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt AP, Valinetti EA, Bandeira D, Bertacchi MF, Simões CM, Auler JO., Jr Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17:667–74. doi: 10.1111/j.1460-9592.2006.02185.x. [DOI] [PubMed] [Google Scholar]
- 16.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine-a novel alpha 2-adrenoceptor agonist-in healthy volunteers. Pain. 1991;46:281–5. doi: 10.1016/0304-3959(91)90111-A. [DOI] [PubMed] [Google Scholar]


