Abstract
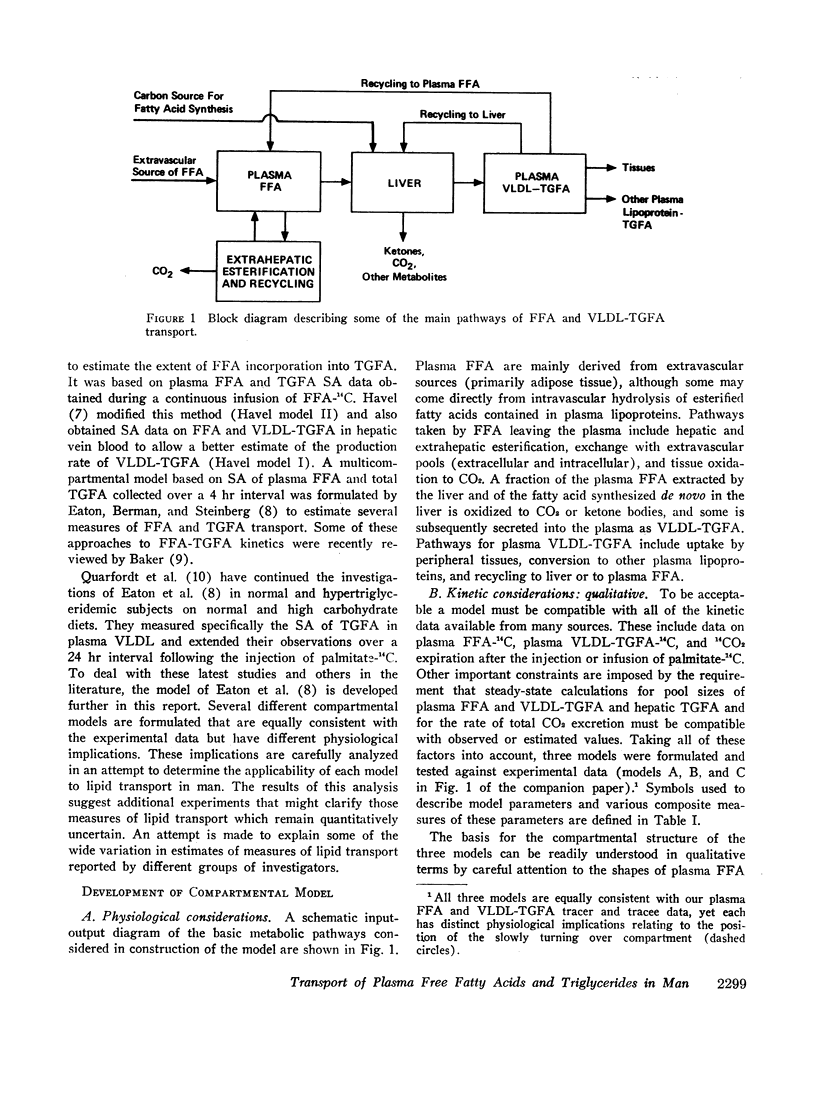
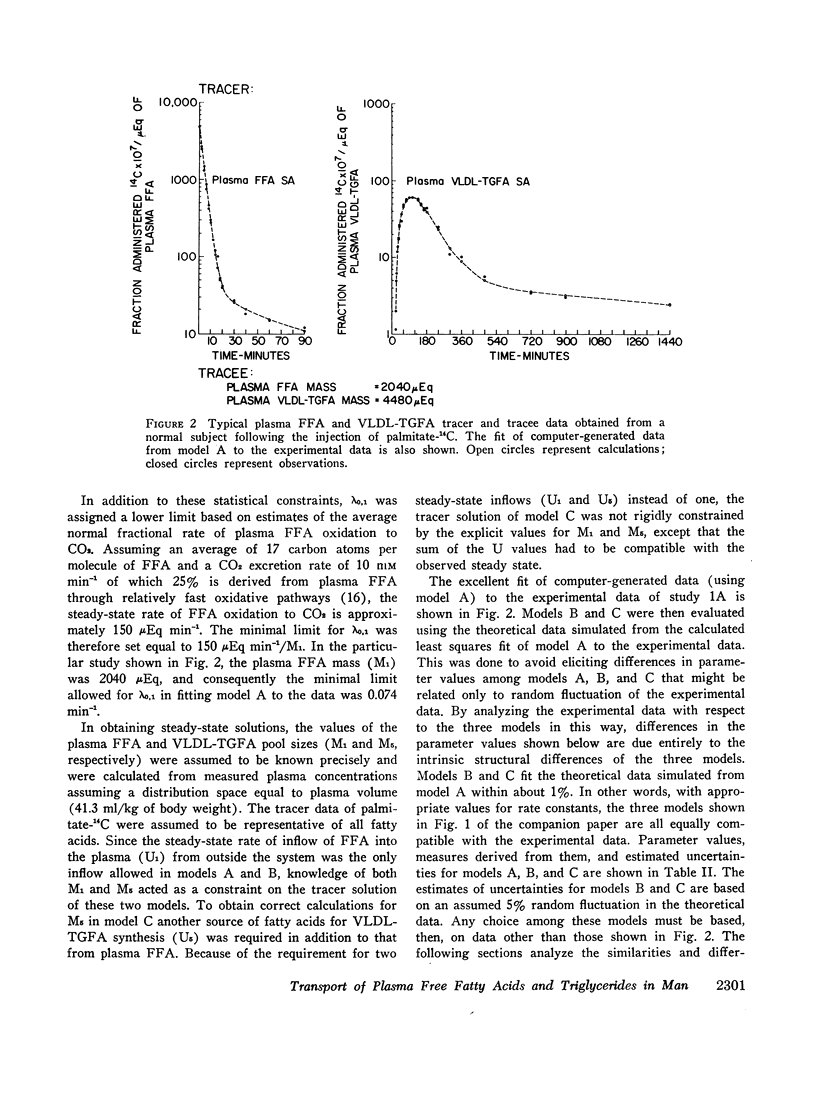
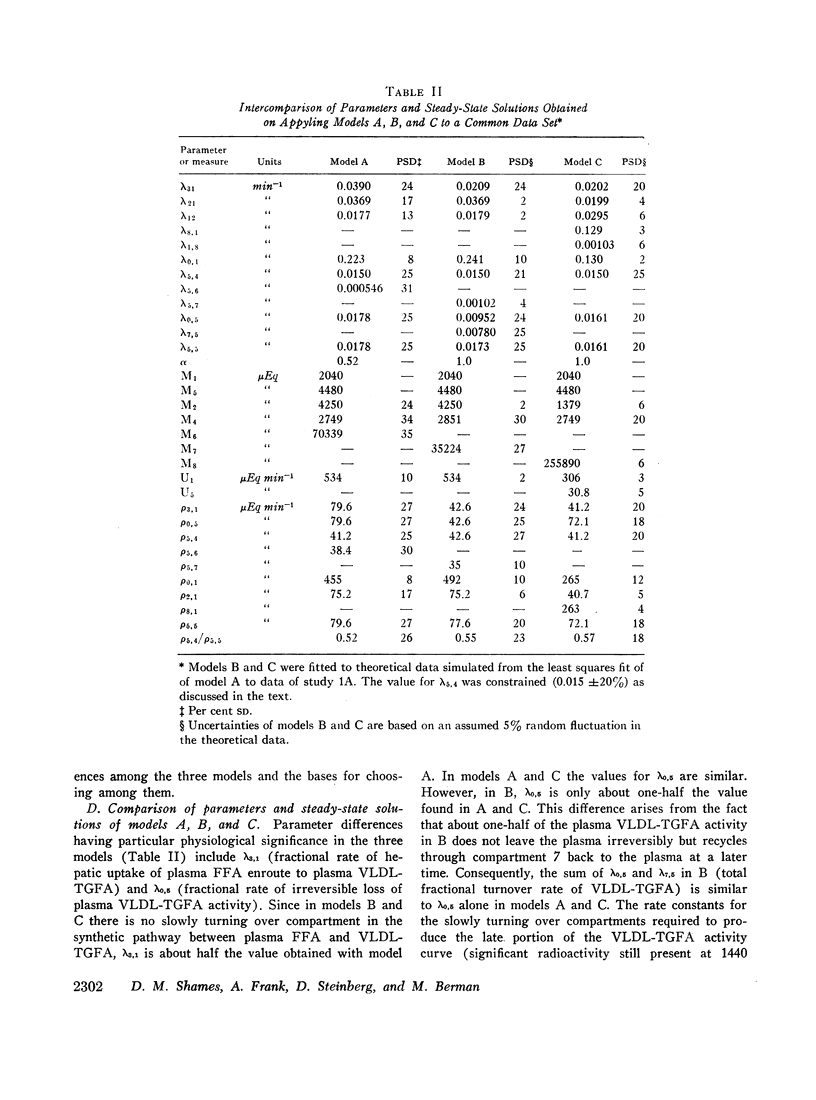
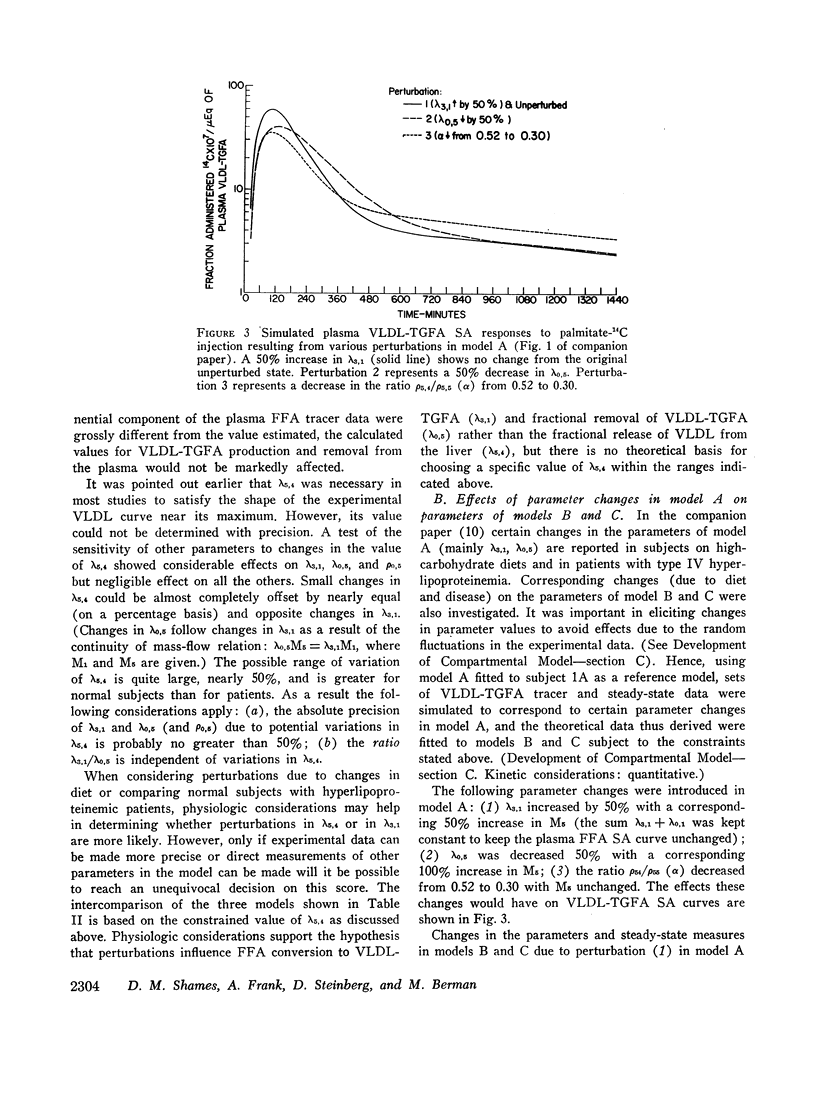
Three different multicompartmental models of free fatty acid (FFA) and very low density lipoprotein triglyceride fatty acid (VLDL-TGFA) transport in man are formulated from plasma FFA and VLDL-TGFA tracee and tracer data collected over a 24 hr interval after the injection of palmitate-14C. All modeling and data fitting were performed on a digital computer using the SAAM program. Structural differences in the three models relate to the position of the slowly turning over compartment required to generate the late portion of the plasma VLDL-TGFA tracer data. The positions of this slow compartment are along the hepatic pathway from FFA to VLDL-TGFA (model A) or in the distribution system of VLDL-TGFA (model B) or in the distribution system of FFA (model C). Although all three models are equally consistent with our experimental data and are supported by observations of others, each reveals inconsistency with some data obtained from the literature. Consequently, a combination model of FFA-TGFA transport, incorporating properties of models A, B, and C would be more consistent with all available data. Experiments that would help to determine the quantitative significance of each of the slow compartments in the combination model are suggested.
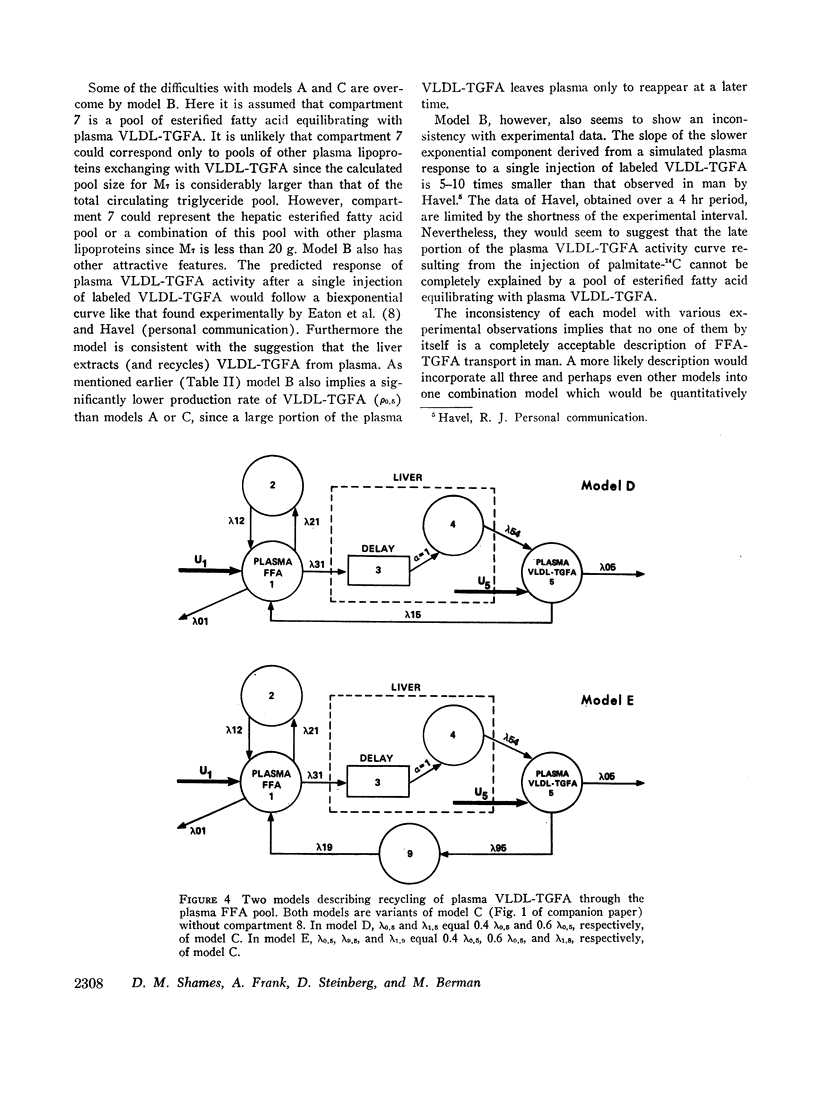
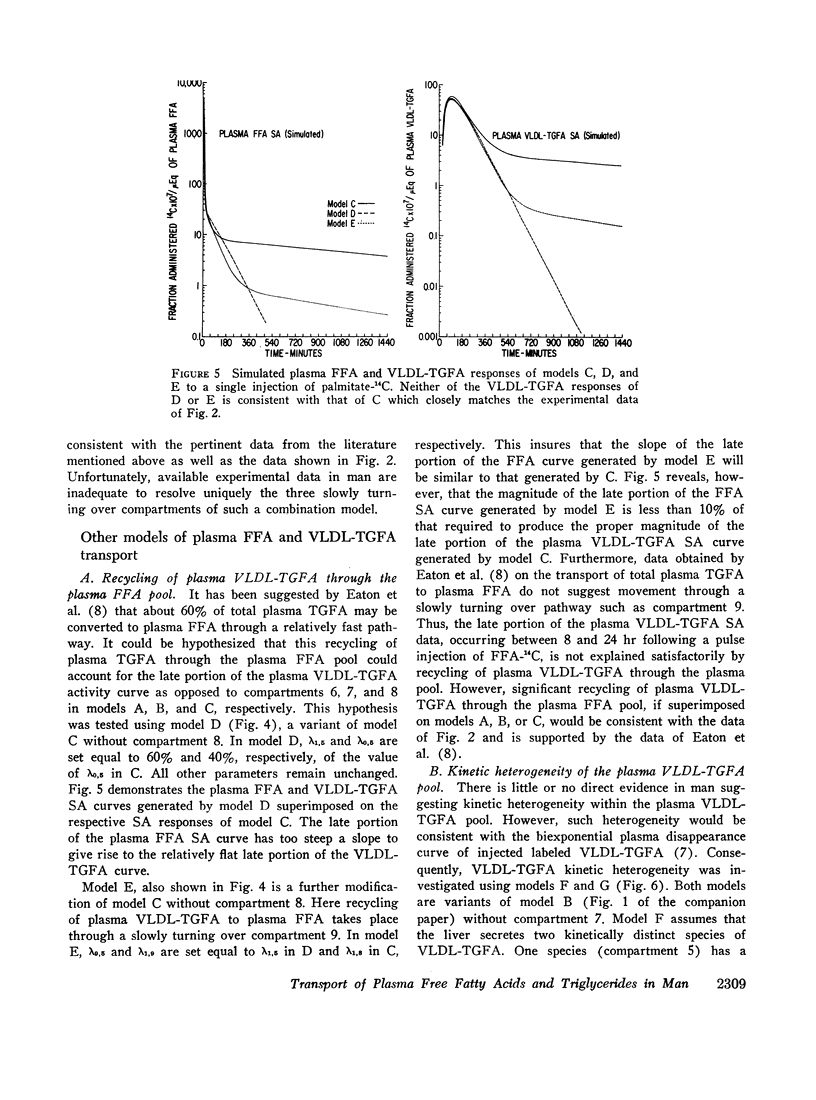
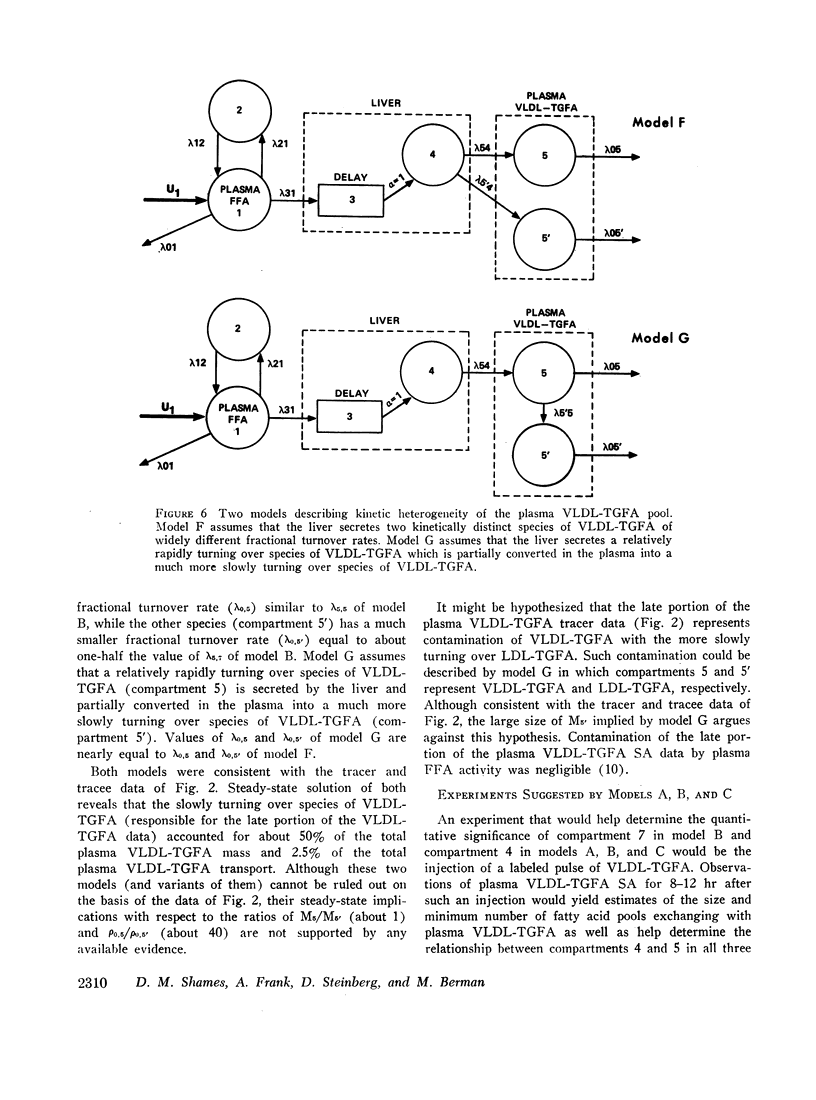
Several other models suggesting recycling of plasma VLDL-TGFA through the plasma FFA pool, kinetic heterogencity of the plasma VLDL-TGFA pool, and contamination of plasma VLDL-TGFA radioactivity with low density lipoprotein (LDL) TGFA radioactivity were tested. The first model does not explain the late portion of the plasma VLDL-TGFA tracer data. The second and third models, while consistent with our tracee and tracer data, have steady-state implications with respect to the extent of kinetic heterogeneity and size of the LDL-TGFA contaminant that make them unlikely.
Assumptions underlying other investigator's models of FFA and TGFA transport in man are reviewed within the logical framework of our models. Quantitative differences among the various models are shown by evaluating all of the models with respect to a common set of plasma FFA and VLDL-TGFA data.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER N., SCHOTZ M. C. USE OF MULTICOMPARTMENTAL MODELS TO MEASURE RATES OF TRIGLYCERIDE METABOLISM IN RATS. J Lipid Res. 1964 Apr;5:188–197. [PubMed] [Google Scholar]
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN M. The formulation and testing of models. Ann N Y Acad Sci. 1963 May 10;108:182–194. doi: 10.1111/j.1749-6632.1963.tb13373.x. [DOI] [PubMed] [Google Scholar]
- Baker N. The use of computers to study rates of lipid metabolism. J Lipid Res. 1969 Jan;10(1):1–24. [PubMed] [Google Scholar]
- Basso L. V., Havel R. J. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970 Mar;49(3):537–547. doi: 10.1172/JCI106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell G. L., Berman M., Robertson J. S. Nomenclature for tracer kinetics. Int J Appl Radiat Isot. 1968 Mar;19(3):249–262. doi: 10.1016/0020-708x(68)90022-7. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A., EKELUND L. G. Splanchnic production and uptake of endogenous triglycerides in the fasting state in man. J Clin Invest. 1963 May;42:714–720. doi: 10.1172/JCI104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. P., Berman M., Steinberg D. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest. 1969 Aug;48(8):1560–1579. doi: 10.1172/JCI106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR J. W., GROSS R. C., WAGNER R. M., REAVEN G. M. VALIDATION OF AN INCOMPLETELY COUPLED TWO-COMPARTMENT NONRECYCLING CATENARY MODEL FOR TURNOVER OF LIVER AND PLASMA TRIGLYCERIDE IN MAN. J Lipid Res. 1965 Jan;6:119–134. [PubMed] [Google Scholar]
- FINE M., MICHAELS G., SHAH S., CHAI B., FUKAYAMA G., KINSELL L. The incorporation of C14 from uniformly labeled glucose into plasma triglycerides in normals and hyperglyceridemics. Metabolism. 1962 Aug;11:893–911. [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr The metabolism of albumin-bound C14-labeled unesterified fatty acids in normal human subjects. J Clin Invest. 1958 Nov;37(11):1504–1515. doi: 10.1172/JCI103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDBERG S. J., KLEIN R. F., TROUT D. L., BOGDONOFF M. D., ESTES E. H., Jr The incorporation of plasma free fatty acids into plasma triglycerides in man. J Clin Invest. 1961 Oct;40:1846–1855. doi: 10.1172/JCI104409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. C., Eigenbrodt E. H., Farquhar J. W. Endogenous triglyceride turnover in liver and plasma of the dog. J Lipid Res. 1967 Mar;8(2):114–125. [PubMed] [Google Scholar]
- HAVEL R. J. Conversion of plasma free fatty acids into triglycerides of plasma lipoprotein fractions in man. Metabolism. 1961 Dec;10:1031–1034. [PubMed] [Google Scholar]
- Jones A. L., Ruderman N. B., Herrera M. G. Electron microscopic and biochemical study of lipoprotein synthesis in the isolated perfused rat liver. J Lipid Res. 1967 Sep;8(5):429–446. [PubMed] [Google Scholar]
- LAURELL S. Recycling of intravenously injected palmitic acid-1-C14 as esterified fatty acid in the plasma of rats and turnover rate of plasma triglycerides. Acta Physiol Scand. 1959 Nov 15;47:218–232. doi: 10.1111/j.1748-1716.1960.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., Spritz N. Effects of prolonged ethanol intake in man: role of dietary adipose, and endogenously synthesized fatty acids in the pathogenesis of the alcoholic fatty liver. J Clin Invest. 1966 Sep;45(9):1400–1411. doi: 10.1172/JCI105448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINSSON A., SUNZEL H., HOOD B. Nitrogen, lipid, glycogen and deoxyribonucleic acid content of human liver. The effect of brief starvation and intravenous administration of glucose. Acta Med Scand. 1963 Jun;173:745–752. doi: 10.1111/j.0954-6820.1963.tb17461.x. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Bortz W. M., Durham B. C. The rate of appearance of FFA in plasma triglyceride of normal and obese subjects. Metabolism. 1968 Jun;17(6):515–521. doi: 10.1016/0026-0495(68)90043-7. [DOI] [PubMed] [Google Scholar]
- Quarfordt S. H., Frank A., Shames D. M., Berman M., Steinberg D. Very low density lipoprotein triglyceride transport in type IV hyperlipoproteinemia and the effects of carbohydrate-rich diets. J Clin Invest. 1970 Dec;49(12):2281–2297. doi: 10.1172/JCI106448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. G., Schwartz T. B. Dynamics of plasma triglyceride turnover in man. Metabolism. 1965 Dec;14(12):1243–1254. doi: 10.1016/s0026-0495(65)80004-x. [DOI] [PubMed] [Google Scholar]
- STEIN Y., SHAPIRO B. Uptake and metabolism of triglycerides by the rat liver. J Lipid Res. 1960 Jul;1:326–331. [PubMed] [Google Scholar]
- Sailer S., Sandhofer F., Braunsteiner H. Umsatzraten für freie Fettsäuren und Triglyceride im Plasma bei essentieller Hyperlipämie. Klin Wochenschr. 1966 Sep 1;44(17):1032–1036. doi: 10.1007/BF01878782. [DOI] [PubMed] [Google Scholar]


