Abstract
Peptide receptor radionuclide therapy (PRRT) has recently been established as an important treatment modality for somatostatin receptor (SSTR)-positive tumors. The purpose of this study was to evaluate the clinical response, side-effects as well as the quality of life following 90Y-DOTA-lanreotide (DOTALAN) and/or 90Y-DOTA-Tyr 3-DPhe1-octreotide (DOTATOC) therapy in patients with progressive metastatic disease during a 6-year follow-up period. Following dosimetric evaluation with 111In-DOTALAN and 111In-DOTATOC, 13 patients with estimated absorbed tumor doses of >5 Gy/GBq (carcinoid, n = 5; radioiodine-negative thyroid cancer, n = 4; gastrinoma, n = 1; insulinoma, n = 1; glucagonoma, n = 1; glomus jugularis tumor, n = 1) were assigned for PRRT. A dose of 925 MBq of 90Y-DOTALAN (four patients) or 1.85–3.7 GBq of 90Y-DOTATOC (10 patients) was administered intravenously and repeated every 4–8 weeks. Tumor dosimetry was performed prior to and under therapy, re-staging every 2–3 months. Pain intensity, Karnofsky score and general symptoms were evaluated in order to determine quality of life. Patients were followed until death. Altogether, 53 infusions of PRRT (1.85–14.1 GBq) were administered. After the first follow-up of 3 months of 90Y-DOTALAN therapy, stable disease (SD) was observed in one patient and progressive disease (PD) in three patients. With 90Y-DOTATOC therapy, SD was found in all 10 patients. During the re-evaluation period (4–27 months), one patient had to be shifted from 90Y-DOTALAN to 90Y-DOTATOC therapy due to reduced 111In-DOTALAN uptake after 5.5 GBq. In the first 6 months after PRRT with DOTATOC, SD was found in nine of 10 patients and PD in one patient. Thereafter, SD was observed in two patients and PD in eight patients. Nine of 13 patients after PRRT with either DOTALAN or DOTATOC died. None of the patients had experienced severe acute hematological side-effects. Transient thrombocytopenia or lymphocytopenia was seen in 10 patients after 3.7 GBq, and a skin reaction in one patient. Total accumulated kidney dose ranged between 4 and 64 Gy, with reduced creatinine clearance in two patients. Pain relief was achieved in three of three patients after ~3.7 GBq ERT within 4–6 months. Appetite, weight, Karnofsky score and general well-being had improved in patients with SD during and after therapy. Based on the results of this study conducted on a small group of patients, we conclude that PRRT may offer an alternative treatment option for SSTR-positive tumors, with only mild transient side-effects and a marked improvement in the quality of life.
Keywords: DOTA-octreotide, DOTA-lanreotide, peptide receptor radionuclide therapy, quality of life
Introduction
The high-level expression of peptide receptors (R) on various tumor cells as compared with normal tissues or blood cells has provided the molecular basis for the clinical use of radiolabelled peptides as tumor tracers in nuclear medicine.[1,2] In recent years, the use of radiolabelled somatostatin analogs as specific radiopharmaceuticals for the in vivo detection and treatment of somatostatin receptor-positive (SSTR) tumors has been implemented.[3–6] In contrast to 111In-DTPA-D-Phe 1-octreotide (OCTREOSCAN®), which binds to human (h)SSTR2 and hSSTR5 of the five known hSSTR subtypes with high affinity (Kd 0.1-5 nM), to hSSTR3 with moderate affinity (Kd 10-100 nM) and does not bind to hSSTR1 and hSSTR4; 111In/90Y-DOTA-lanreotide (DOTALAN) was found to bind to hSSTR2-5 with high affinity and to hSSTR1 with lower affinity (Kd 200 nM), and was therefore suggested to be a potential radioligand for tumor diagnosis and therapy.[5,7] Compared with OCTREOSCAN® and 111In-DOTA-Tyr3-DPhe1-octreotide (DOTATOC), discrepancies were found in the scintigraphic results with DOTALAN in about one-third of (neuroendocrine) the tumor patients concerning both the tumor uptake and detection. On a molecular level, this divergence seems to be based on the increased high-affinity binding of 111In-DOTATOC to hSSTR2.[8]
In the management of patients with SSTR-positive tumors, these new radiopeptides seem to be a potential alternate treatment option, but their exact role remains to be determined. The best radioligand should be carefully investigated because of the different binding behavior. At present, many treatment protocols exist with different study designs.[9–12] Side-effects, kidney protection and optimal dose of radiopeptide for successful treatment of cancer are still under considerable debate, and long-term results and survival rates are lacking. Furthermore, little attention has been given to the quality of life with these new therapies, which is perhaps the most important gain for patients with end-stage cancer. The aim of our study was to assess the clinical utility as well as side-effects of receptor-based 90Y-DOTALAN/DOTATOC therapy (PRRT) in patients with metastatic SSTR expressing cancer refractory to conventional treatment.
Materials and Methods
Patients
We enrolled 13 patients (median: 66 years, male:female = 7:6) in this study with metastatic tumor disease (five carcinoids, one glomus jugularis tumor, four radioiodine-negative thyroid carcinomas, one gastrinoma, one insulinoma and one glucagonoma) during a period of 6 years. Before PRRT, all 13 patients had surgery. Four patients had undergone chemotherapy, four patients had external radiotherapy and four other patients had radioiodine therapy. Three patients were treated with 90Y-DOTALAN, nine patients with 90YDOTATOC and one patient with both radiopeptides.
Treatment protocols and therapy monitoring
Before starting therapy, 10 patients underwent diagnostic and dosimetric evaluation with 111In-DOTALAN and 111In-DOTATOC. Three patients started therapy with 90Y-DOTALAN without diagnostic 111In-DTATOC scintigraphy because DOTATOC was not yet available at that time. Inclusion criteria were as follows:
Positive 111In-DOTALAN/-DOTATOC scintigraphy with tumor uptake > 5-10 Gy/GBq.
Progressive tumor disease under conventional therapy.
Life expectancy > 3 months.
Karnofsky score > 60, age >18 years.
Laboratory tests: granulocytes > 1500/mm3, platelets > 100,000/mm3, liver and kidney function < Grade I toxicity according to the WHO-criteria.
Exclusion criteria of the study were pregnancy and severe concomitant illness, including severe psychiatric disorder.
There were two different treatment protocols, one for DOTALAN and one for DOTATOC. For DOTALAN therapy, patients started with two infusions of 0.9 GBq 90Y-DOTALAN each with a time interval of 4-weeks apart. After a further 4 weeks, restaging was performed. If no kidney and/or hematological toxicity and no severe side-effects were found, a further two infusions of 925 MBq 90Y-DOTALAN each with a time interval of 4 weeks apart was administered. The patients were restaged after a further 4 weeks. The end point of the therapy was severe adverse side-effects according the WHO standard criteria, kidney dose > 30 Gy/GBq (with two exceptions), no therapeutic success (progressive disease) and/or reduction of 111In-DOTALAN tumor uptake. For DOTATOC therapy, patients were started with two infusions (1.85 GBq each) with a time interval of 4 weeks apart. After a further 4 weeks, restaging was performed. After exclusion of kidney and/or hematological toxicity and severe side-effects, patients with large tumors (>3 cm in diameter) received one infusion of 1.85 GBq/m2. Two infusions of 1.85 GBq each in a time interval of 4 weeks were applied to patients with small tumors. The re-staging was done after 4 weeks and, depending on the patients′ situation, further therapy with one infusion of 1.85 GBq/m2 for patients with large tumors or two infusions of 1.85 GBq each for patients with small lesions were administered. Laboratory tests (blood count, renal and liver function parameter) were controlled prior to and weekly after starting therapy, tumor markers, which were positive in the pre-study investigation every 4 weeks. The end points of the DOTATOC therapy were similar to the DOTALAN therapy protocol already described.
To determine the quality of life, we evaluated the subjective pain intensity by means of the visual analog score (VAS; 0 = severe pain, 10 = painless), immediately after and every 4 weeks following therapy and by recording the intake of analgesics. The Karnofsky score, general symptoms such as appetite, weight, bowel movement, micturition and sleep pattern were recorded by interviewing the patient before and every 4 weeks under therapy. The overall general well being was rated by 5-point VAS (1 = very poor, 5 = excellent).
Diagnostic and dosimetric evaluation
For diagnostic and dosimetric evaluation, serial whole body scans (anterior and posterior views, matrix 256 × 1024, 15-min each) up to 48 h after intravenous injection of 5.5 GBq 111In-DOTALAN/-DOTATOC were performed as described before by Virgolini et al. and Traub et al.[6,7]
Preparation and labeling of 111In-/90Y-DOTALAN and 111In-/90Y-DOTATOC
The chemical synthesis, preparation and labeling of 111In-/90Y-DOTALAN and 111In/90Y-DOTATOC were performed according to the procedures described by Virgolini et al.[8]
Results
Therapeutic response of 90Y-DOTALAN
Altogether, 14 infusions of the radiolabelled peptide were administered (1.85-5.5 GBq) to four patients [Table 1]. Patients no. 1 and no. 2 receiving 1.85 GBq presented progressive disease (PD) under therapy and died 4 months after starting treatment. In the case of the gastrinoma patient, 5.5 GBq was administered. First, stable disease (SD) over 6 months, then regressive disease (after 5 infusions of 90YY-DOTALAN) were observed.[13] Fourteen months after starting therapy, PD in the liver and reduced 111In-DOTALAN tumor uptake were found. Because of the high tumor uptake of 111In-DOTATOC, the patient afterwards received 90Y-DOTATOC therapy ([Table 2], patient no. 9). Patient no.4 presented SD for 10 months with reduction of lung metastases (cumulative activity: 3.7 GBq). However, the patient died 12 months after starting therapy due to pulmonary embolism. An improved quality of life was recorded in the gastrinoma patient with improved appetite, weight gain and overall general sense of well being.
Table 1.
Response to therapy with 90Y-DOTALAN
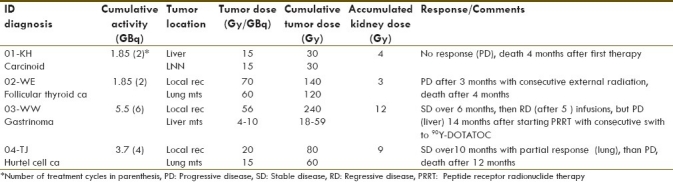
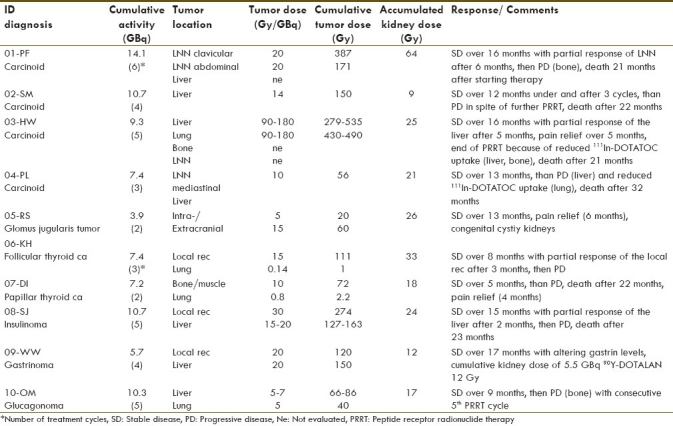
Table 2.
Response to therapy with 90Y-DOTATOC
Therapeutic response of 90Y-DOTATOC
Thirty-nine infusions of the 90Y-labelled peptide DOTATOC were administered to a total of 10 patients (3.9-14.1 GBq). Eight of 10 patients were treated following the protocol for large tumors. Under therapy, SD was observed in all 10 patients, with tumor volume reduction in four patients [Figure 1]. Reduced 111in-DOTATOC uptake was seen in three of 10 patients after administration of more than 7.4 GBq of 90Y-DOTATOC [Figure 2]. The follow-up period ranged between 7 and 27 months. Over the first 6 months, SD in nine patients and PD in one patient was observed. Further follow-up recorded SD in two patients and PD in eight patients. In the case of two patients with PD during the follow-up period, we decided to give further therapy as palliative care (patient no. 2 with rapid growth of liver metastases, patient no. 10 with a metastatic glucagonoma after resection of a bone metastasis). Six of 10 patients died Table 2.
Figure 1.
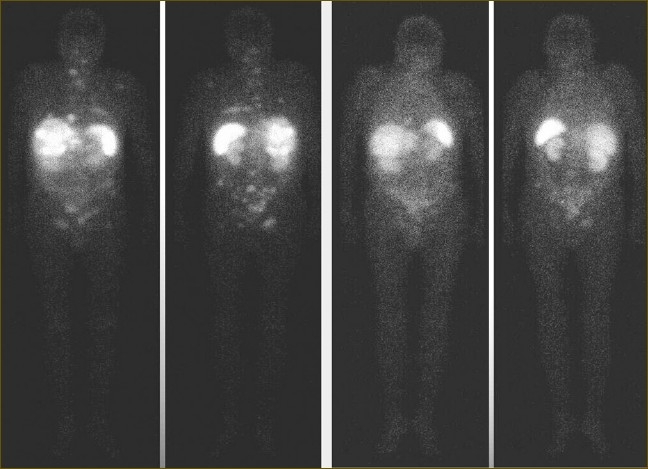
111In-DOTATOC whole-boy scans of patient no. 3 prior to (left panel) and after (right panel) 9.3 GBq of 90Y-DOTATOC. Reduced radiopeptide uptake in the liver and bone was consistent with decreased size of metastatic tumor lesions
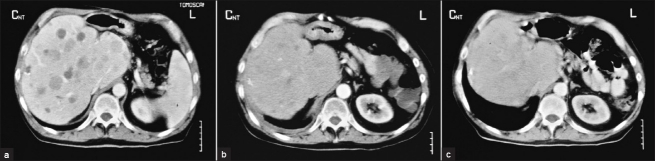
Figure 2.
Liver computed tomography images of patient no. 3 with multiple liver metastases: prior to (a), after 9.3 GBq of 90Y-DOTATOC (b) and after 6 months follow-up (c) with response to the liver metastases
Side-effects under therapy
None of the 13 patients developed any acute hematological side-effect. In all four 90Y-DOTALAN patients, we recorded transient drop of thrombocytes, while in six of 10 90Y-DOTATOC patients transient drop of lymphocytes was observed in the first 2 weeks after dose administration. One patient developed skin rash 1 week after the 5th dose (cumulative activity: 10.4 GBq), which was not considered as a side-effect. However, immediately after the 6th infusion, the skin rash appeared again and, hence, therapy was terminated.
In 11 of 13 patients, we did not observe any renal dysfunction under therapy. Three patients presented cumulated kidney dose of more than 30 Gy. The patient with the glomus jugularis tumor having congenital cystic kidneys received 3.9 GBq 90Y-DOTATOC with a cumulated kidney dose of 26 Gy, and did not show any impairment of kidney function under therapy as well as during follow-up. A reduced creatinine clearance was seen in two patients after doses of 7 and 14.1 GBq (accumulative kidney dose: 18 Gy in patient no. 7 and 64 Gy in patient no. 1).
Quality of life
Three of 13 patients experienced pain relief after ~3.7 GBq of 90Y-radiolabeled peptide within a period of 4-6 months. In the case of the carcinoid patient (patient no. 3 of 90Y-DOTATOC therapy) with pain from multiple bone metastases, pain relief lasted for 5 months. During this time, the patient significantly reduced his intake of analgesics, was able to take longer walks and was feeling generally better, with reduced flush and diarrheal symptoms. The Karnofsky score changed from 60 to 70, and the general well being from 3 to 1 during this time [Table 3]. The glomus jugularis tumor patient with extra- and intracranial tumor lesions showed pain relief for 6 months. Symptoms such as headache and pressure from the tumor manifestation were reduced. In the case of patient no. 7 of 90Y-DOTATOC therapy with bone- and muscle-infiltrating metastasis of the hip, pain relief was observed for 4 months with concomitant reduction of pain medication. She reported deeper and longer sleep, longer and more painless walks as well as weight gain and improved appetite.
Table 3.
General well being, Karnovsky score, appetite and weight of patients treated with Y-90-DOTATOC
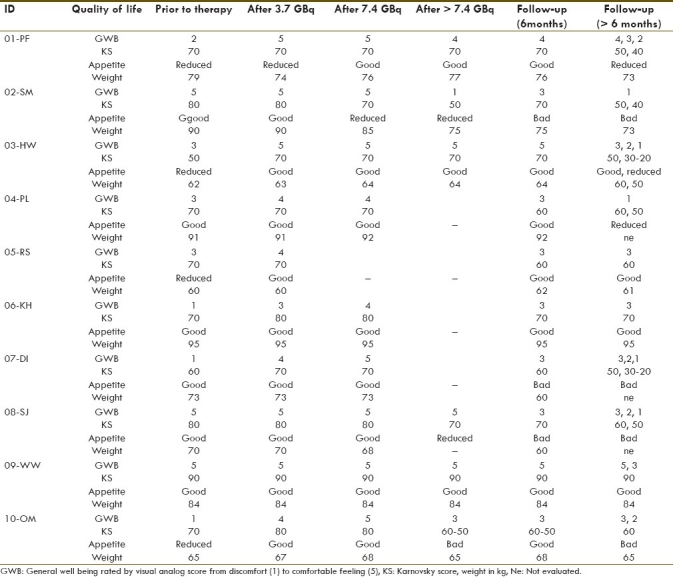
Therefore, all patients with SD under PRRT and in the follow-up period recorded improved appetite, weight gain and an overall general sense of well being. One patient with carcinoid (patient no. 1) started 90Y-DOTATOC therapy in a reduced general health because of his PD and gastric symptoms (pain). After treatment of his gastritis, and after the first two cycles of PRRT, re-evaluation revealed significant improvement in appetite, gain in weight, greater feeling of well being and SD. Later, PD was accompanied by reduced appetite, weight loss and discomfort [Table 3]. Similar results were also obtained for the Karnofsky score, which was higher in two patients of the 90YY-DOTALAN protocol and in four patients of the 90Y-DOTATOC protocol during the course of therapy compared with prior results.
Discussion
The fact that malignant cells express a high number of peptide receptors has provided the basis for new therapeutic approaches in nuclear medicine. The discovery of somatostatin receptor subtypes on various neuroendocrine tumors has stimulated the development of somatostatin analog-based scintigraphy and therapy.[3,4,7] However, the exact role of this novel therapeutic approach remains to be determined. Also, its ability to improve quality of life has not been addressed so far. In our study, two different somatostatin analogs were used: DOTALAN in the first phase, when it was the only available somatostatin analog and DOTATOC thereafter. In the first phase, little experience with dose application, kidney toxicity, blood abnormalities and other side-effects existed. This is why we administered only a single dose of 925 MBq of 90Y-DOTALAN per cycle to the patients. After introduction of the DOTATOC protocol, all patients underwent dosimetric evaluation with both radiolabelled peptides prior to PRRT. Therefore, the administered activity was adapted to the tumor manifestation with higher dose per cycle of radiolabelled peptide for larger tumors. Although our study group is small and heterogeneous in regard to tumor entity and tumor manifestation, we could observe partial tumor response or SD under therapy with either 90Y-DOTALAN or 90Y-DOTATOC.
These findings are remarkable because all patients had PD when entering the treatment phase and had already undergone chemotherapy, surgery and radiotherapy. Our results of SD and partial tumor response under therapy and during the first 6 months of follow-up are comparable with results of other studies reported in the literature.[9,10,12. 14]
When we started PRRT in the late 90s, no information existed about radiolabelled peptide therapy. From the long-term follow-up of our patients, it can be inferred that both the tumor load, especially that of the liver, as well as the performance status influence the outcome of receptor radionuclide therapy. Although preclinical studies in rats have shown the dependence of treatment response on tumor size,[15] 90Y-DOTATOC seems to give a higher response rate of larger tumor lesions, while Auger-emitting analogs, such as 177Lu-Tyr3-octreotate, act on smaller lesions.[15] Earlier introduction of therapy and/or a “cocktail therapy” may thus potentially influence the outcome of PRRT.
Concerning the side-effects in our study, neither acute nor severe hematological ones were observed. The reported nephrotoxicity after more than 7.4 GBq in two patients, who died ~2 years after stating PRRT because of PD, did not require further treatment.
When directly compared, discrepancies concerning both the tumor uptake and the detection of the tumor lesions were found between 111In-DOTALAN and 111In-DTPA-DPhe 1-octeotide or 111In-DOTATOC in about one-third of the neuroendocrine tumors patients.[8] In this study, we could observe a change of the SSTR profile on the tumor cells following therapy. In case of the gastrinoma patient, we found a higher tracer uptake for 111In-DOTATOC compared with 111In-DOTALAN after 90Y-DOTALAN therapy (cumulative dose: 5.5 GBq). This change of SSTR subtype expression profile caused the shift from 90Y-DOTALAN to 90Y-DOTATOC.
The patients started PRRT when disease was progressive and refractory to conventional treatment options. After stabilization of metastatic disease, patients reported weight gain, improved appetite, sleep and general well being for several months. The Karnofsky score was higher during the course of therapy than prior therapy in six of 13 cases. Pain as a physical component of quality of life was closely related to the psychological component of general well being. Pain relief with reduction of analgesic medication led to fewer side-effects, to more physical activity, improved appetite, better sleep and weight gain. This symptomatic benefit from the PRRT was judged by the patients as an improvement in quality of life. Recently, other working groups with different study designs of receptor-mediated radionuclide therapy reported on the clinical benefit.[9,12,14] Waldherr et al.[9] described improvement of clinical signs such as flush, diarrhea, vomiting and pain, with an overall clinical benefit of 63%. However, they did not describe the general well being of the patients. Similar results have also been reported by Bombardieri et al.[14]
In cancer patients, sources of satisfaction and self-esteem can be compromised. Fearfulness, therapeutic side-effects and the possibility of treatment failure and death are always present. These aspects may affect health-related quality of life, which includes both physical and psychological components. Thus, improvement in quality of life is an important goal of oncological treatment beyond, and perhaps independent of, the curative one. The main finding of our small study is that PRRT may result in a substantial improvement of quality of life parameters, even when there is only minor effects on tumor shrinkage.
Finally, we conclude that the benefit from PRRT in patients with SSTR-positive tumor disease refractory to conventional therapy may extend to an important improvement in quality of life. Further studies are warranted to prospectively establish the role of PRRT in this regard.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Reubi JC. In vitro identification of vasoactive peptide receptors in human tumors: Implications for tumor imaging. J Nucl Med. 1995;36:1846–53. [PubMed] [Google Scholar]
- 2.Virgolini I, Yang Q, Li S, Angelberger P, Neuhold N, Niederle B, et al. Cross-competition between vasoactive intestinal peptide and somatostatin for binding to tumor cell membrane receptors. Cancer Res. 1994;54:690–700. [PubMed] [Google Scholar]
- 3.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin-receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 4.Otte A, Jermann E, Behe M, Goetze M, Bucher HC, Roser HW, et al. DOTATOC – a powerful new tool for receptor-mediated radionuclide therapy. Eur J Nucl Med. 1997;24:792–5. doi: 10.1007/BF00879669. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Jones PM, Bischof C, Leimer M, Gludovacz D, Angelberger P, Pangerl T, et al. DOTA-lanreotide: a novel somatostatin analog for tumor diagnosis and therapy. Endocrinology. 1999;140:5136–48. doi: 10.1210/endo.140.11.7126. [DOI] [PubMed] [Google Scholar]
- 6.Traub T, Petkov V, Ofluoglu S, Pangerl T, Raderer M, Fueger BJ, et al. Indium-111-DOTA-lanreotide scintigraphy in patients with tumors of the lung. J Nucl Med. 2001;42:1309–15. [PubMed] [Google Scholar]
- 7.Virgolini I, Szilvasi I, Kurtaran A, Angelberger P, Raderer M, Havlik E, et al. Indium-111-DOTA-lanreotide: biodistribution safety and tumor dose in patients evaluated by somatostatin receptor-mediated radiotherapy. J Nucl Med. 1998;39:1928–36. [PubMed] [Google Scholar]
- 8.Virgolini I, Traub T, Novotny C, Leimer M, Füger B, Li SR, et al. Experience with Indium-111 and Yttrium-90-labeled somatostatin analogs. Curr Pharm Design. 2002;8:1781–807. doi: 10.2174/1381612023393756. [DOI] [PubMed] [Google Scholar]
- 9.Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, et al. Tumor response and clinical bebefit in neuroendocrine tumors after 7.4 GBq 90Y-DOTATOC. J Nucl Med. 2002;43:610–6. [PubMed] [Google Scholar]
- 10.Buscombe R, Caplin ME, Hepplewhite J, Johnson G, Bouloux PL, Meyer T, et al. Treating somatostatin receptor positive tumors with radiolanreotide. Eur J Nucl Med. 2001;28:253. [Google Scholar]
- 11.Chinol M, Bodei L, Cremonesi M, Paganelli G. Receptor-mediated radiotherapy with Y-90-DOTA-Dphe-Tyr-octretide: The experience of the european institute of oncology group. Semin Nucl Med. 2002;32:141–7. doi: 10.1053/snuc.2002.31563. [DOI] [PubMed] [Google Scholar]
- 12.Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med. 2002;32:148–55. doi: 10.1053/snuc.2002.31565. [DOI] [PubMed] [Google Scholar]
- 13.Leimer M, Kurtaran A, Smith-Jones P, Raderer M, Havlik E, Angelberger P, et al. Response to treatment with Yttrium 90-DOTA-lanreotide of a patient with metastatic gastrinoma. J Nucl Med. 1998;39:2090–3. [PubMed] [Google Scholar]
- 14.Bombardieri E, Seregni E, Savelli G, Villano C, Castellani MR, Cirillo F, et al. Radiolabeled somatostaitin analogs in the treatment of neuroendocrine tumors: Experience of the National Cancer Institute of Milano using high dose of 111In-pentetreotide in metastatic neuroendocrine gastroenteropancreatic tumors. J Endocrinol Invest. 2003;26(8 Suppl):63–9. [PubMed] [Google Scholar]
- 15.De Jong M, Valkeman R, Jamar F, Kvols LK, Kwekkeboom DJ, Breeman WA, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med. 2002;32:133–40. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]