Abstract
Several unstable mutant hemoglobins have alterations which affect areas of the molecule involved in the attachment of heme to globin. Loss of heme from globin has been demonstrated during the denaturation of some of these unstable mutants. The importance of heme ligands for the stability of hemoglobin was illustrated in the present experiments on the denaturation of several hemoglobins and hemoglobin derivatives by heat, oxidative dyes, and alkali. Heating of normal hemolysates diluted to 4 g of hemoglobin per 100 ml at 50°C for 20 hr in 0.05 M sodium phosphate, pH 7.4, caused precipitation of 23-54% of the hemoglobin. Dialysis against water or dilution of the sample decreased denaturation to 12-20%. Precipitation was decreased to less than 3.5% by the presence of 0.015 M potassium cyanide. Increasing the ionic strength of the medium increased precipitation. Cyanide prevented the formation of inclusion bodies when red cells containing unstable hemoglobin Philly, β35 tyr → phe, were incubated with the redox dye new methylene blue. Conversion to methemoglobin increased the rate of alkali denaturation of hemoglobin but the presence of potassium cyanide returned the denaturation rate to that of ferrohemoglobin. The ability of cyanide to decrease heat precipitation of hemoglobin may depend on a dimeric or tetrameric state of the hemoglobin molecule. Purified β-chains, which exist as tetramers, were stabilized but purified monomeric α-chains were not rendered more heat resistant by the ligand. Stabilization of hemoglobin by cyanide required binding of the ligand to only one heme of an αβ-dimer. Hemoglobin Gun Hill, an unstable molecule with heme groups present only on the α-chains was quite heat stable in the presence of cyanide. The binding of cyanide to the iron atom in methemoglobin is thought to be associated with increased planarity of the heme group and increased stability of the heme-globin complex. The stabilizing effect of cyanide in the above experiments suggests that Heinz body formation, heat precipitation of hemoglobin, and the increased alkali denaturation of methemoglobin depend on changes of heme-globin binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
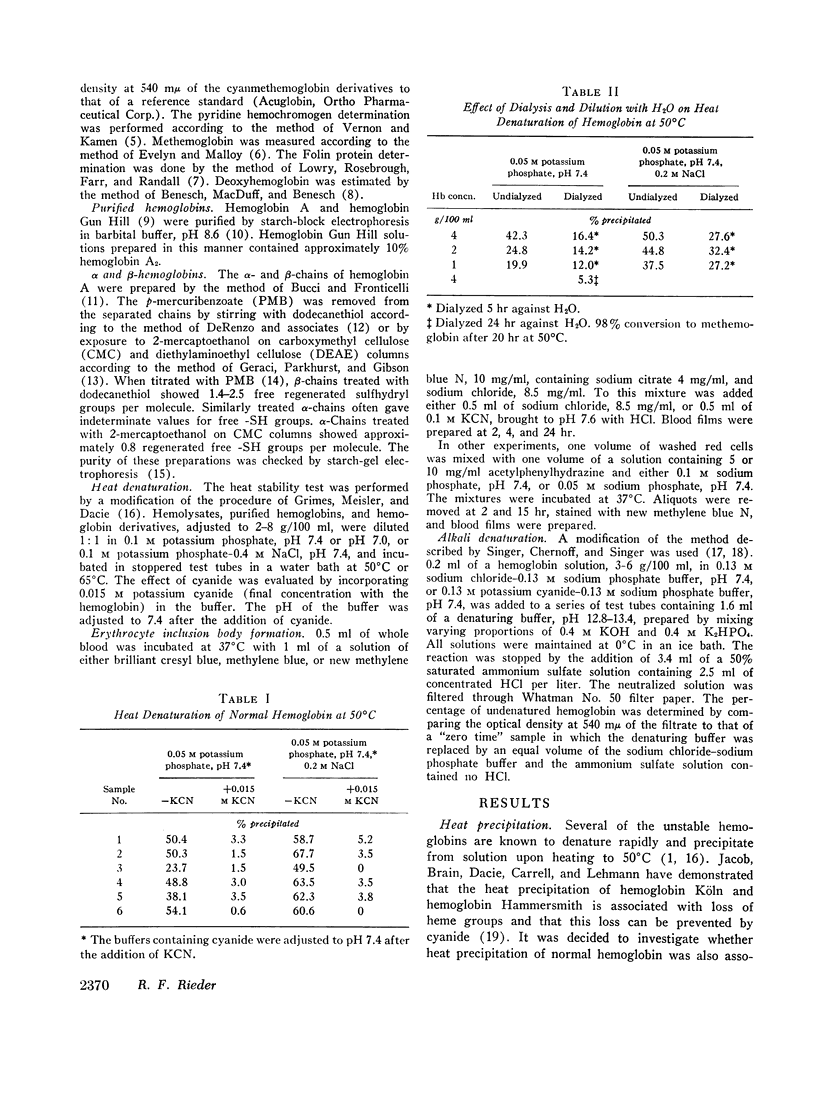
- BENESCH R., BENESCH R. E. Determination of--SH groups in proteins. Methods Biochem Anal. 1962;10:43–70. doi: 10.1002/9780470110270.ch2. [DOI] [PubMed] [Google Scholar]
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
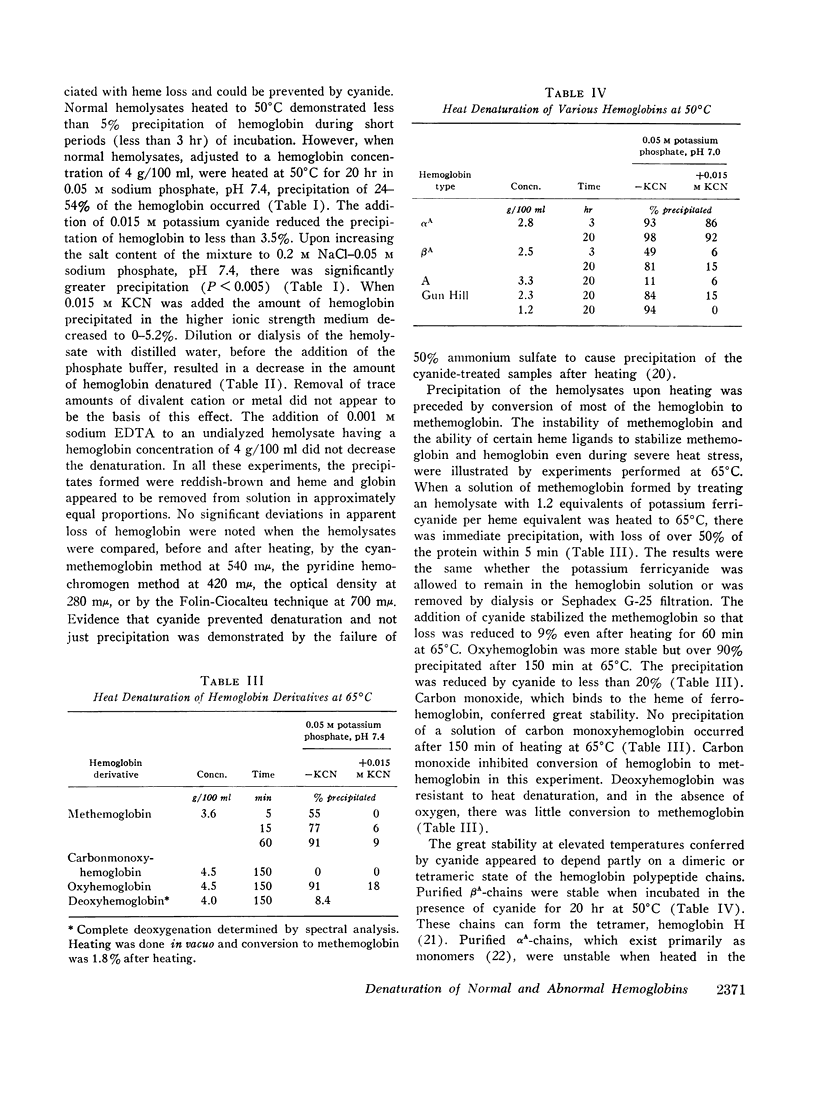
- Bonaventura J., Riggs A. Hemoglobin Kansas, a human hemoglobin with a neutral amino acid substitution and an abnormal oxygen equilibrium. J Biol Chem. 1968 Mar 10;243(5):980–991. [PubMed] [Google Scholar]
- Bradley T. B., Jr, Wohl R. C., Rieder R. F. Hemoglobin Gun Hill: deletion of five amino acid residues and impaired heme-globin binding. Science. 1967 Sep 29;157(3796):1581–1583. doi: 10.1126/science.157.3796.1581. [DOI] [PubMed] [Google Scholar]
- Brimhall B., Jones R. T., Baur E. W., Motulsky A. G. Structural characterization of hemoglobin Tacoma. Biochemistry. 1969 May;8(5):2125–2129. doi: 10.1021/bi00833a051. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H. The unstable haemoglobin haemolytic anaemias. Semin Hematol. 1969 Apr;6(2):116–132. [PubMed] [Google Scholar]
- De Renzo E. C., Ioppolo C., Amiconi G., Antonini E., Wyman J. Properties of the alpha and beta chains of hemoglobin prepared from their mercuribenzoate derivatives by treatment with 1-dodecanethiol. J Biol Chem. 1967 Nov 10;242(21):4850–4853. [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- FESSAS P., LOUKOPOULOS D. ALPHA-CHAIN OF HUMAN HEMOGLOBIN: OCCURRENCE IN VIVO. Science. 1964 Feb 7;143(3606):590–591. doi: 10.1126/science.143.3606.590. [DOI] [PubMed] [Google Scholar]
- GRIMES A. J., MEISLER A., DACIE J. V. CONGENITAL HEINZ-BODY ANAEMIA. FURTHER EVIDENCE ON THE CAUSE OF HEINZ-BODY PRODUCTION IN RED CELLS. Br J Haematol. 1964 Jul;10:281–290. doi: 10.1111/j.1365-2141.1964.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Hartridge H. Heat coagulation of haemoglobin compounds. J Physiol. 1912 Mar 29;44(1-2):34–42. doi: 10.1113/jphysiol.1912.sp001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollender A., Lorkin P. A., Lehmann H., Svensson B. New unstable haemoglobin borås: beta 88 (F4) leucine-arginine. Nature. 1969 Jun 7;222(5197):953–955. doi: 10.1038/222953a0. [DOI] [PubMed] [Google Scholar]
- JONXIS J. H., VISSER H. K. Determination of low percentages of fetal hemoglobin in blood of normal children. AMA J Dis Child. 1956 Dec;92(6):588–591. doi: 10.1001/archpedi.1956.02060030582007. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V. Altered sulfhydryl reactivity of hemoglobins and red blood cell membranes in congenital Heinz body hemolytic anemia. J Clin Invest. 1968 Dec;47(12):2664–2677. doi: 10.1172/JCI105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Effect of drying upon the absorption spectra of haemoglobin and its derivatives. Nature. 1952 Jul 26;170(4317):161–162. doi: 10.1038/170161a0. [DOI] [PubMed] [Google Scholar]
- KEILIN J. Nature of the haem-binding groups in native and denatured haemoglobin and myoglobin. Nature. 1960 Jul 30;187:365–371. doi: 10.1038/187365a0. [DOI] [PubMed] [Google Scholar]
- KRISTOFFERSEN K. FETAL HEMOGLOBIN; THE INFLUENCE OF CARBON MONOXIDE WHEN ESTIMATING HB-F AS ALKALI-RESISTANT HEMOGLOBIN. Scand J Clin Lab Invest. 1965;17:151–161. [PubMed] [Google Scholar]
- KUNKEL H. G., CEPPELLINI R., MULLER-EBERHARD U., WOLF J. Observations on the minor basic hemoglobin component in the blood of normal individuals and patients with thalassemia. J Clin Invest. 1957 Nov;36(11):1615–1625. doi: 10.1172/JCI103561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Variations in the structure of human haemoglobin. With particular reference to the unstable haemoglobins. Br Med Bull. 1969 Jan;25(1):14–23. doi: 10.1093/oxfordjournals.bmb.a070664. [DOI] [PubMed] [Google Scholar]
- Opfell R. W., Lorkin P. A., Lehmann H. Hereditary non-spherocytic haemolytic anaemia with post-splenectomy inclusion bodies and pigmenturia caused by an unstable haemoglobin Santa Ana-beta-88 (F4) leucine--proline. J Med Genet. 1968 Dec;5(4):292–297. doi: 10.1136/jmg.5.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- RIGAS D. A., KOLER R. D. Decreased erythrocyte survival in hemoglobin H disease as a result of the abnormal properties of hemoglobin H: the benefit of splenectomy. Blood. 1961 Jul;18:1–17. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Bradley T. B., Blumberg W. E. Role of haemichromes in the formation of inclusion bodies in haemoglobin H disease. Nature. 1969 Apr 19;222(5190):248–250. doi: 10.1038/222248a0. [DOI] [PubMed] [Google Scholar]
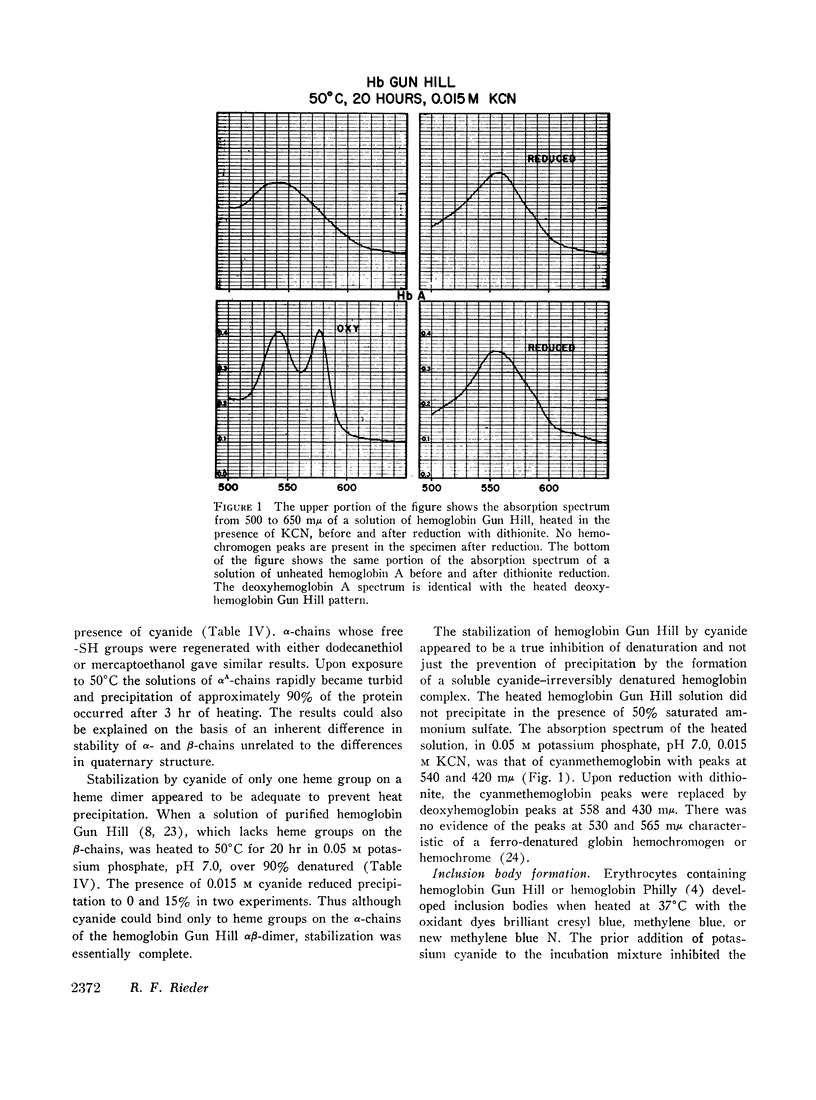
- Rieder R. F., Bradley T. B., Jr Hemoglobin Gun Hill: an unstable protein associated with chronic hemolysis. Blood. 1968 Sep;32(3):355–369. [PubMed] [Google Scholar]
- Rieder R. F., Oski F. A., Clegg J. B. Hemoglobin Philly (beta 35 tyrosine phenylalanine): studies in the molecular pathology of hemoglobin. J Clin Invest. 1969 Sep;48(9):1627–1642. doi: 10.1172/JCI106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
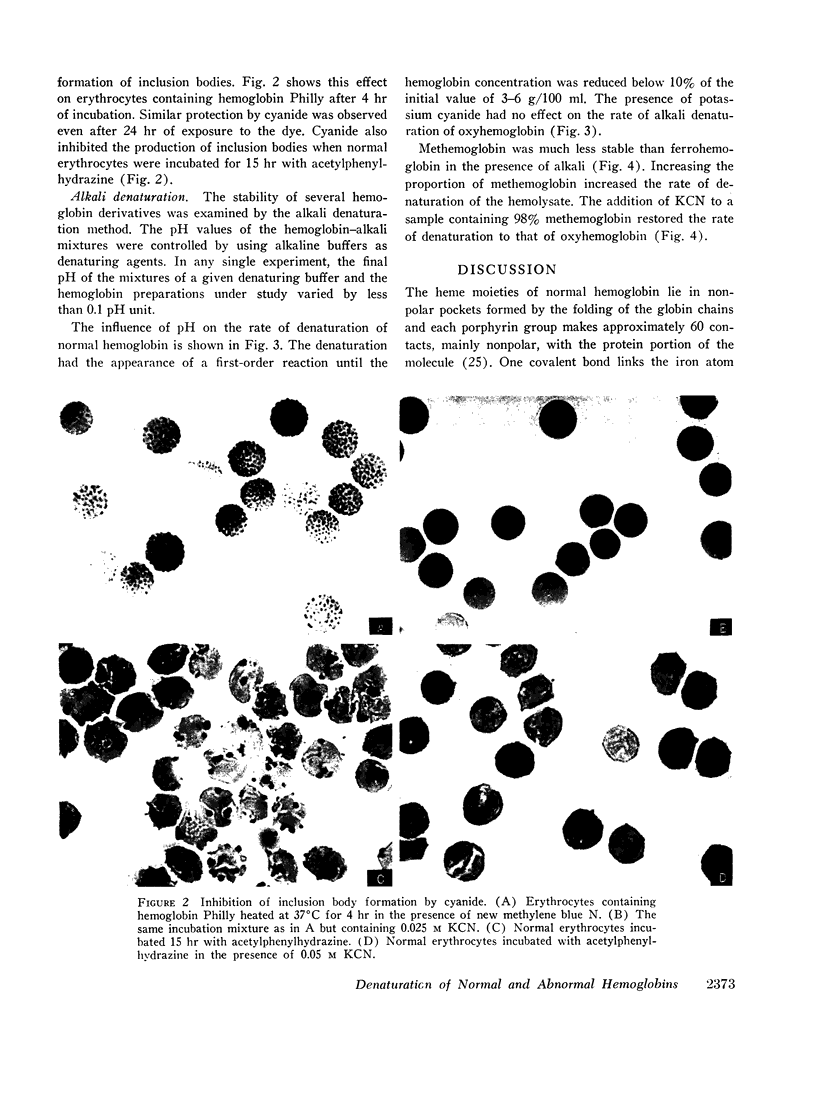
- SINGER K., CHERNOFF A. I., SINGER L. Studies on abnormal hemoglobins. I. Their demonstration in sickle cell anemia and other hematologic disorders by means of alkali denaturation. Blood. 1951 May;6(5):413–428. [PubMed] [Google Scholar]
- SINGER K., CHERNOFF A. I., SINGER L. Studies on abnormal hemoglobins. II. Their identification by means of the method of fractional denaturation. Blood. 1951 May;6(5):429–435. [PubMed] [Google Scholar]
- SMITHIES O. CHARACTERIZATION OF GENETIC VARIANTS OF BLOOD PROTEINS. Vox Sang. 1965 May-Jun;10:359–362. [PubMed] [Google Scholar]
- STEINHARDT J., ONA-PASCUAL R., BEYCHOK S., HO C. The stabilization of horse ferrihemoglobin to acid denaturation by combination with ligands. Biochemistry. 1963 Mar-Apr;2:256–266. doi: 10.1021/bi00902a009. [DOI] [PubMed] [Google Scholar]
- Schneider R. G., Ueda S., Alperin J. B., Brimhall B., Jones R. T. Hemoglobin sabine beta 91 (f 7) leu to pro. An unstable variant causing severe anemia with inclusion bodies. N Engl J Med. 1969 Apr 3;280(14):739–745. doi: 10.1056/NEJM196904032801402. [DOI] [PubMed] [Google Scholar]
- Steinhardt J., Polet H., Moezie F. Acid denaturation of horse carbonylhemoglobin in the absence of oxygen. J Biol Chem. 1966 Sep 10;241(17):3988–3996. [PubMed] [Google Scholar]
- VAN DEN OORD A., DANIELSSON H., RYHAGE R. ON THE STRUCTURE OF THE EMULSIFIERS IN GASTRIC JUICE FROM THE CRAB, CANCER PAGURUS L. J Biol Chem. 1965 May;240:2242–2247. [PubMed] [Google Scholar]
- VERNON L. P., KAMEN M. D. Hematin compounds in photosynthetic bacteria. J Biol Chem. 1954 Dec;211(2):643–662. [PubMed] [Google Scholar]



