Abstract
Breast cancer patients’ perceived risk of recurrence has been associated with psychological distress. Little is known about the change of patients’ perceived risk of recurrence over time and factors associated with their recurrence-risk perceptions. We prospectively recruited 549 newly diagnosed early-stage breast cancer patients; patients completed interviews at 6 weeks, 6 months, 1 year, and 2 years after definitive surgical treatment. A random-effects regression model with repeated ordinal measurements was used to estimate the relationship between perceived risk of recurrence and demographic, medical, and psychosocial factors. We analyzed data from 535 patients [34% ductal carcinoma in situ (DCIS); 20% non-white] who reported their perceived risk at one or more interviews. At the first interview, 16% reported having no lifetime risk of recurrence, and another 16% reported ≥50% risk of recurrence, including 15% of DCIS patients. Patients who were white (OR = 5.88, 95% CI 3.39–10.19) and had greater state anxiety (OR = 1.04, 95% CI 1.02–1.07) were more likely, while patients who received radiotherapy (OR = 0.72, 95% CI 0.54–0.96) and had more social support (OR = 0.59, 95% CI 0.46–0.75) were less likely to report higher risk of recurrence. Cancer stage was not significantly associated with perceived risk of recurrence. Perceived risk of recurrence did not change significantly over time. Educating early-stage breast cancer patients about their actual risk could result in more realistic recurrence-risk perceptions, and increasing social support could help alleviate anxiety associated with exaggerated risk perceptions.
Keywords: Breast cancer, Ductal carcinoma in situ, Cancer recurrence-risk perception, Recurrence, Anxiety, Social support
Introduction
Effective adjuvant therapies have reduced the risk of recurrence after surgical resection of early-stage breast cancer. Ten to 18% of women with ductal carcinoma in situ (DCIS) will develop recurrence (including locoregional or distant recurrence or contralateral breast cancer) within the first 5 years after lumpectomy followed by radiation therapy [1-3], which is higher than the risk of recurrence after mastectomy (<7%) [3-5]. In patients 50–69 years old with early invasive breast cancer (EIBC), the 5-year cumulative risk of recurrence ranges from 14 to 43% after primary treatment [6]. Some patients may have inaccurate perceptions of their risk of recurrence. Higher perceived risk of recurrence has been associated with cancer-specific worries [7] and general anxiety [8]. Perceived risk of breast cancer, one of the four components of the Health Belief Model, is considered to be a key motivator for mammography screening [9, 10]. However, this is not always the case. A prospective study showed that women who believed their risk to be high were less likely to adhere to screening guidelines than women who believed their risk to be moderate [11]. Additionally, high perceived risk of breast cancer was associated with being hyper vigilant in breast self-examination [12]. Therefore, a better understanding of factors associated with patients’ perceived risk of recurrence and whether perceived risk changes over time could provide insights into interventions designed to improve health behaviors and alleviate psychological distress.
Although studies have reported factors associated with perceived risk of breast cancer in women without a personal history of breast cancer, little is known about factors associated with recurrence-risk perceptions in breast cancer survivors. Two cross-sectional studies found that despite their better prognosis, women with DCIS reported similar perceptions of their risk of recurrence and death from breast cancer as women with EIBC [13, 14]. Treatment-related factors might be associated with patients’ risk perceptions [15-17]. Factors associated with perceived risk of developing breast cancer, e.g., age, race, education, family history of breast cancer, and state anxiety [18-20], also may be associated with recurrence-risk perceptions in breast cancer patients. Since lack of social support has been associated with poor health outcomes in breast cancer patients [21, 22], availability of support also may influence recurrence-risk perceptions.
To date, few studies have assessed breast cancer survivors’ perceived risk of recurrence [7, 8, 13, 14, 23, 24]. These studies were limited, however, by their cross-sectional design and data analysis, a small proportion of non-white participants, and lack of comprehensive assessment of demographic, clinical, and psychosocial factors in relation to recurrence-risk perceptions. Thus, we conducted a prospective study to characterize the trend in patients’ perceived risk of recurrence and its relation to a variety of demographic, clinical, and psychosocial factors.
Methods
Patients and procedure
Between October 2003 and June 2007, patients with newly diagnosed, first primary breast cancers (stages 0–IIA) were recruited at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine and at Saint Louis University School of Medicine for a longitudinal quality-of-life study. The Institutional Review Boards at both institutions approved the study. Eligible participants were able to speak English, had completed definitive surgical treatment, and were ≥40 years old, as annual screening mammography is recommended for this age group [25]. Patients were excluded if they had a history of breast cancer, received neoadjuvant chemotherapy, or demonstrated cognitive impairment based on weighted scores >10 on the Orientation-Memory-Concentration Test [26], which was administered to patients ≥65 years old.
Measures
After obtaining consent, we abstracted patients’ breast cancer pathology and treatment data from their medical records. Patients provided information about demographics, comorbidities, and psychosocial variables during four computer-assisted telephone interviews (CaTI) 4–6 weeks, 6 months, 1 year, and 2 years following their definitive surgery. The CaTI system minimizes error in data collection; skip patterns were programmed to ensure that interview questions are followed appropriately [27]. Perceived availability of social support was measured using the Medical Outcomes Study Social Support Survey [28]. Anxiety was measured with the validated Beck Anxiety Inventory® [29]. The extent to which participants experienced depressive symptoms “during the past week” was measured using the validated Center for Epidemiologic Studies-Depression Scale (CES-D) [30]. Based on the literature [31] and surgeons’ anecdotal reports of patients’ complaints after surgery, we developed an 8-item measure of breast surgery-associated side effects with higher scores (range 1–5) indicating more severe side effects. A single-factor solution was obtained in the factor analysis of the eight surgical side effect items, including limited arm mobility/frozen shoulder, tightness/tenderness in chest wall, tightness/tenderness/discomfort in the breast, arm weakness, lymphedema/swelling of the arm, swelling of the chest/breast/axilla, numbness/tingling or pins and needles, and tightness/pulling/stretching in the arm/axilla. The internal consistency was high (Cronbach’s alpha = 0.81, 6 and 12 months after surgery). Katz’s validated adaptation of the Charlson comorbidity index was used to measure the presence/history of several comorbid conditions [32, 33]. We asked patients whether they had a family history of breast cancer in their first-degree relatives.
To determine their perceived risk of recurrence, we asked patients, “What do you think the chances are that you will have this disease again someday? Please answer using a percentage scale where 100 means that you will definitely get this disease again and 0 means there is no chance that you will get it again” [34]. We defined “recurrence” broadly as a recurrence in the same breast or in other organs or occurrence of a new breast cancer in the contralateral breast, presuming that the distinctions between locoregional and distant recurrence and contralateral breast cancer are likely lost on the patient who hears only that she has cancer again. Recognizing that patients probably do not know their actual risk of recurrence (because healthcare providers do not commonly give patients this type of numeric information), we categorized their responses into one of six groups corresponding to increasing levels of risk: 0, 1–9, 10–24, 25–49, ≥50%, and uncertain/don’t know, based on published work [34]. These categories were based on the lifetime risk of recurrence in patients with mutated BRCA1/2 gene (≥50%), patients who were not tested for BRCA1 or BRCA2 status but in whose family a mutation was found (10–50%), and patients at average (<10%) risk of recurrence (personal communication, Dr. Robyn Andersen, Fred Hutchinson Cancer Research Center, September, 2007). We used this 6-group ordinal variable in our analysis, assuming that patients’ assignment of a percentage value reflected their perceived “level of risk,” rather than their calculated risk of recurrence.
Statistical analysis
Independent predictors of the ordinal-scaled measure of perceived risk of recurrence were identified using an ordinal random-effects regression model that was based on the proportional odds assumption and considered the correlation between the repeated measurements of a subject across time [35, 36]. PROC NLMIXED in SAS was used to fit random-effects regression models with repeated ordinal measurements [37, 38].
Univariate random-effects regression models were developed to identify factors associated with patients’ perceived risk of recurrence that would be included in the multivariate model. Factors assessed included age at diagnosis, race, education, marital status, cancer stage, type of surgery, surgical side effects, receipt of chemotherapy, radiotherapy, and adjuvant hormone therapy, family history of breast cancer, time after definitive surgery, comorbidity, social support, anxiety, and elevated depressive symptoms. The multivariate random-effects regression model included all factors associated with perceived risk of recurrence in the univariate models at P < 0.20. Odds ratios (ORs) and their 95% confidence intervals (95% CI) were used to assess the magnitude of associations between explanatory variables and patients’ perceived risk of recurrence. An OR < 1.00 indicated that patients with exposure were less likely than patients in the comparison group to report higher categories of perceived risk of recurrence. In contrast, an OR > 1.00 indicated that patients with exposure were more likely than patients in the comparison group to report higher levels of perceived risk of recurrence. For example, we compared patients who received chemotherapy, the exposure, with patients who did not, the comparison group. All variables were entered into the model simultaneously.
Analyses were performed using SAS statistical software version 9.1 (SAS Institute, Cary, NC). Statistical tests were two-sided, and P < 0.05 was considered significant.
Results
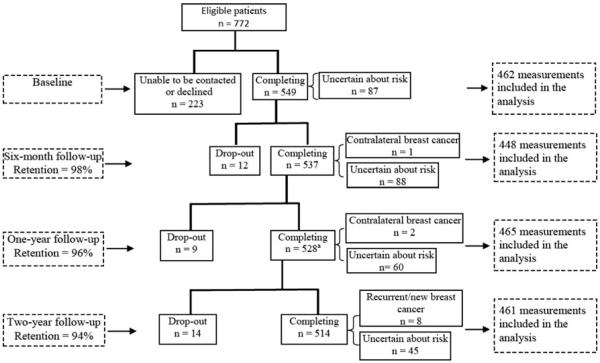
Overall, 549 of 772 eligible patients (71%) completed the first interview. A larger proportion of participants than non-participants were white (79 vs. 64%; P < 0.001). While there was no age difference between white participants and white non-participants, non-white participants were younger than non-white non-participants (mean age: 58 vs. 62 years; P = 0.020). There was no significant difference in cancer stage or type of breast surgery between participants and non-participants. Of the 549 participants, 537 (98%) completed the 6-month follow-up, 528 (96%) completed the one-year follow-up, and 514 (94%) completed the 2-year follow-up. Interviews were completed, on average, 1.4, 6.2, 12.3, and 24.5 months after definitive surgical treatment.
Nine patients developed recurrent or contralateral breast cancer during follow-up and were excluded from the analyses of data collected after these events. Additionally, patients who reported not knowing their risk of recurrence were excluded from the analyses of data collected at that interview (Fig. 1). The percentage of patients who reported being uncertain about their risk of recurrence varied at each interview, ranging from a high of 16.4% at the 6-month interview to a low of 8.8% at the 2-year follow-up. The random-effects regression model used in the following analyses included all available data for a patient, which limits bias associated with only including patients with complete data [35]. Therefore, data analysis included 462 patients who reported their perceived risk during the first interview, and 73 patients who were uncertain at the time of first interview but reported their risk in subsequent interviews (being uncertain was not always reported by the same patients). Descriptive statistics of these 535 patients at the time of first interview are shown in Table 1. Of 343 patients who received radiotherapy, 94.8% had undergone breast-conserving surgery (BCS); 88.0% of 192 patients without radiotherapy had undergone mastectomy. Married/partnered patients reported greater availability of social support compared with never-married patients (P < 0.0001).
Fig. 1.
Flow chart of study enrollment and completion of follow-up interviews. Superscript a one patient had missing data on her perceived risk of recurrence at her 1-year follow-up, and one patient developed recurrent breast cancer after the 1-year follow-up and died before her scheduled 2-year follow-up
Table 1.
Baseline characteristics of participants (n = 535)
| No. | % | |
|---|---|---|
| Age at definitive surgery (years) | ||
| 40–49 | 124 | 23.2 |
| 50–59 | 198 | 37.0 |
| ≥0 | 213 | 39.8 |
| Race | ||
| White | 430 | 80.4 |
| Black | 98 | 18.3 |
| Others | 7 | 1.3 |
| Marital status | ||
| Married/partnered | 328 | 61.3 |
| Widowed | 63 | 11.8 |
| Divorced/separated | 93 | 17.4 |
| Never married | 51 | 9.5 |
| Education | ||
| Less than high school | 42 | 7.9 |
| High school | 121 | 22.6 |
| Some college | 183 | 34.2 |
| College graduate or higher | 189 | 35.3 |
| Pathological stage | ||
| DCIS | 182 | 34.0 |
| Stage I | 274 | 51.2 |
| Stage IIA | 79 | 14.8 |
| Type of surgery | ||
| Breast-conserving surgery | 348 | 65.1 |
| Unilateral mastectomy | 157 | 29.4 |
| Bilateral mastectomy | 30 | 5.6 |
| Chemotherapya | 135 | 25.2 |
| Adjuvant hormone therapya | 335 | 62.6 |
| Radiation therapya | 343 | 64.1 |
| Family history of breast cancer | 122 | 22.8 |
| Elevated depressive symptoms | 92 | 17.2 |
| Mean | Standard | deviation |
|---|---|---|
| Surgical side effects | 1.71 | 0.72 |
| Social support | 4.47 | 0.66 |
| State anxiety | 6.34 | 7.09 |
| Charlson comorbidity index | 0.55 | 0.95 |
| Time since definitive surgery (months) | 1.41 | 0.56 |
Data represented the number of patients who received corresponding adjuvant therapy over 2 years after definitive surgery
Although patients with bilateral mastectomy were less likely to report higher perceived risk of recurrence at the first interview than patients with unilateral mastectomy (OR = 0.28, 95% CI 0.19–0.42; P = 0.0216), excluding patients with bilateral mastectomy from the “mastectomy” group did not influence the significance of predictors of perceived risk of recurrence. Therefore, we included patients with both unilateral and bilateral mastectomy in one group in the analyses.
Longitudinal course of perceived risk of recurrence
Figure 2 shows the observed frequency of perceived risk categories by cancer stage at the first interview. At the first interview, 16% of patients reported having no lifetime risk of recurrence (0%) and another 16% reported their risk to be ≥50%. A larger proportion of non-white patients reported their risk to be 0% compared with white patients (35 vs. 12%; P < 0.0001). Notably, 15% of DCIS patients reported their risk to be ≥50%. Perceived risk did not differ significantly by cancer stage at the first interview. Since patients’ perceived risk was positively skewed, we combined perceived risk categories as follows for subsequent analyses: 0, 1–9, 10–24, and 25–100%.
Fig. 2.
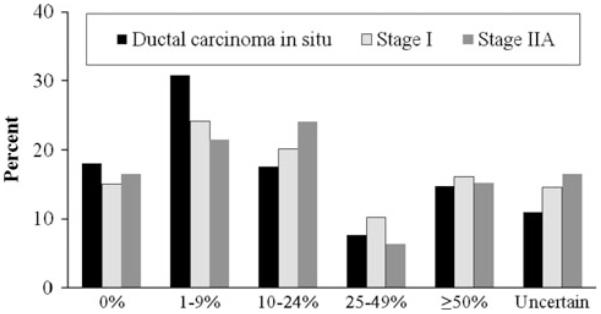
Distribution of each category of perceived risk of recurrence by breast cancer stage at the first interview, within 6 weeks after definitive surgical treatment. Overall chi-square test P = 0.6810
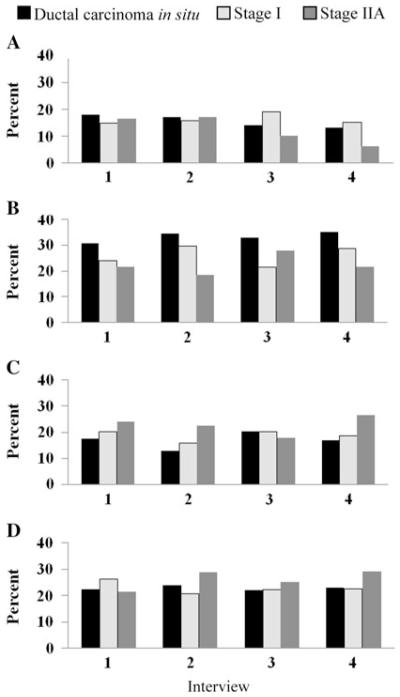
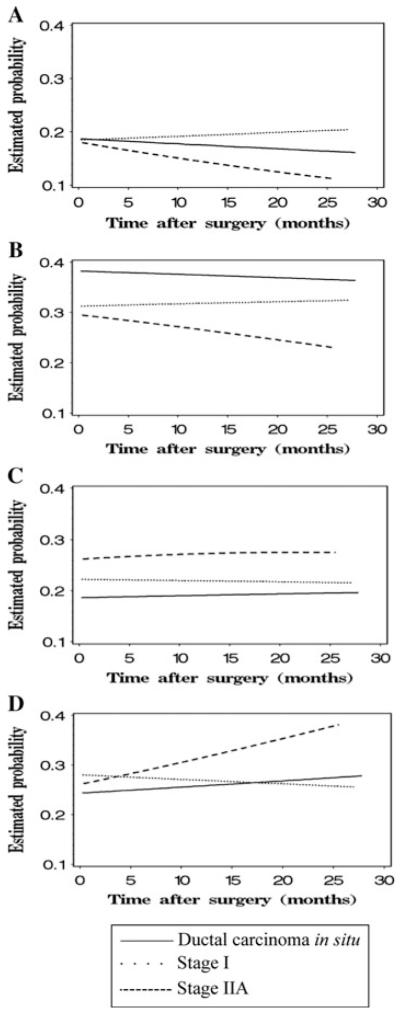
Figure 3 shows trends of the observed frequency of perceived risk categories by cancer stage. In Fig. 4, the marginal estimated probability of each perceived risk category by cancer stage was plotted against time after definitive surgery in months. As time after definitive surgery increased for DCIS and stage IIA patients, the probability of reporting risk to be 10–24 or ≥25% increased, and the probability of reporting risk to be 0 or 1–9% decreased; the probability of reporting each perceived risk category appeared stable for stage I patients. However, the change in perceived risk over time by cancer stage only approached significance (P = 0.0575). For the effect of stage at any given time, the marginal estimated probability of reporting each perceived risk category did not differ significantly between DCIS and stage I patients (P = 0.6381); compared with DCIS and stage I patients, stage IIA patients were somewhat more likely to report their risk to be ≥10% and less likely to report their risk to be <10% (P = 0.0633).
Fig. 3.
Observed percentages of patients reporting each of four categories of perceived risk of recurrence over time by breast cancer stage (Interviews 1, 2, 3, and 4 were conducted within 6 weeks, 6 months, 1 year, and 2 years after definitive breast surgery, respectively). a Percentage reporting risk of recurrence to be 0%. b Percentage reporting risk of recurrence to be 1–9%. c Percentage reporting risk of recurrence to be 10–24%. d Percentage reporting risk of recurrence to be 25–100%
Fig. 4.
The change of estimated probability of reporting different levels of risk of recurrence by cancer stage over time. a Probability of reporting risk of recurrence to be 0%. b Probability of reporting risk of recurrence to be 1–9%. c Probability of reporting risk of recurrence to be 10–24%. d Probability of reporting risk of recurrence to be 25–100%
Factors associated with perceived risk of recurrence
Table 2 shows OR estimates from the univariate and multivariate random-effects regression models. In the univariate models, patients who were older (OR = 0.97, 95% CI 0.95–0.99; P = 0.0161) and who reported greater availability of social support (OR = 0.55, 95% CI 0.44–0.70; P < 0.0001) were less likely to report higher recurrence-risk perceptions, whereas patients who were white (OR = 4.67, 95% CI 2.67–8.17; P < 0.0001), received chemotherapy (OR = 1.65, 95% CI 1.08–2.54; P = 0.0213) and reported higher levels of anxiety (OR = 1.06, 95% CI 1.03–1.08; P < 0.0001) and depressive symptoms (OR = 1.82, 95% CI 1.22–2.71; P = 0.0033) were more likely to report higher recurrence-risk perceptions.
Table 2.
Odds ratios (ORs) and their 95% confidence interval (CI) from random-effects regression analyses for the associations between ordinal-scaled perceived risk of recurrence and potential explanatory factors in women with ductal carcinoma in situ (DCIS) and early-stage invasive breast cancer
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age at diagnosisa | 0.97 | 0.95–0.99 | 0.0161 | 0.99 | 0.97–1.01 | 0.2332 |
| Race | ||||||
| Non-white | 1.00 | 1.00 | ||||
| White | 4.67 | 2.67–8.17 | <0.0001 | 5.88 | 3.39–10.19 | <0.0001 |
| Educationb | ||||||
| ≤High school graduate | 1.00 | – | ||||
| >High school graduate | 1.17 | 0.72–1.89 | 0.5352 | – | ||
| Marital statusb | ||||||
| Non-married/non-partnered | 1.00 | – | ||||
| Married/partnered | 1.28 | 0.81–2.03 | 0.2875 | – | ||
| Pathological stage | ||||||
| DCIS | 1.00 | 1.00 | ||||
| Stage I | 0.96 | 0.62–1.50 | 0.8567 | 1.07 | 0.66–1.72 | 0.7902 |
| Stage IIA | 1.73 | 0.92–3.23 | 0.0909 | 1.24 | 0.60–2.57 | 0.5635 |
| Type of surgeryb | ||||||
| Mastectomy | 1.00 | – | ||||
| Breast-conserving surgery | 0.86 | 0.54–1.38 | 0.5349 | – | ||
| Received chemotherapy | 1.65 | 1.08–2.54 | 0.0213 | 1.52 | 0.94–2.43 | 0.0856 |
| Received radiation therapy | 0.75 | 0.56–1.01 | 0.0554 | 0.72 | 0.54–0.96 | 0.0271 |
| Received adjuvant hormone therapyb | 1.00 | 0.76–1.32 | 0.9827 | – | ||
| Family history of breast cancerb | 1.03 | 0.66–1.62 | 0.8915 | – | ||
| Time after definitive surgery (months)a,b | 1.00 | 0.99–1.01 | 0.5531 | – | ||
| Surgical side effectsa | 1.21 | 0.96–1.52 | 0.1040 | 1.00 | 0.78–1.28 | 0.9923 |
| Charlson comorbidity indexa,b | 0.99 | 0.83–1.17 | 0.8730 | – | ||
| Social supporta | 0.55 | 0.44–0.70 | <0.0001 | 0.59 | 0.46–0.75 | <0.0001 |
| State anxietya | 1.06 | 1.03–1.08 | <0.0001 | 1.04 | 1.02–1.07 | 0.0016 |
| Elevated depressive symptoms | 1.82 | 1.22–2.71 | 0.0033 | 1.09 | 0.69–1.71 | 0.7106 |
Factors analyzed as continuous explanatory variables in which odds ratios correspond to a unit increase
Variable not included in the multivariate model, because not significantly associated with perceived risk of recurrence in univariate model
As we planned to include all factors associated with perceived risk of recurrence in the univariate models at P < 0.20, we retained stage, receipt of radiation therapy, and severity of surgical side effects as predictor variables in the multivariable model. As interactions between time after definitive surgery and each of these covariates were not significant, the multivariate model did not include interaction terms.
In the multivariate regression analysis (Table 2), patients who received radiotherapy (OR = 0.72, 95% CI 0.54–0.96; P = 0.0271) and reported greater availability of social support (OR = 0.59, 95% CI 0.46–0.75; P < 0.0001) were less likely to report higher recurrence-risk perceptions, whereas patients who were white (OR = 5.88, 95% CI 3.39–10.19; P < 0.0001) and reported higher levels of anxiety (OR = 1.04, 95% CI 1.02–1.07; P = 0.0016) were more likely to report higher recurrence-risk perceptions. Age, breast cancer stage, receipt of chemotherapy, surgical side effects, and depressive symptoms were not significantly associated with patients’ recurrence-risk perceptions.
Discussion
To our knowledge, this is the first longitudinal analysis of demographic, cancer-related, and psychosocial factors associated with perceived risk of recurrence among women with DCIS and EIBC. Although one other longitudinal study examined change in perceived risk over time [8], this study included only DCIS patients. Our longitudinal study confirmed the finding of no significant influence of cancer stage at diagnosis on patients’ perceived risk of recurrence that was reported previously in cross-sectional studies [13, 14]. In addition, our sample included a larger proportion of non-white patients (and representative of their proportion in the St. Louis metropolitan area) than the previous study reported [8], which allowed us to explore racial differences in patients’ perceived risk of recurrence. Moreover, we analyzed the contribution of adjuvant treatments to patients’ perceived risk of recurrence. Thus, our study makes important contributions to the literature about perceived risk of recurrence in both DCIS and EIBC survivors.
Surprisingly, 16% of patients within 6 weeks after surgery did not think they had any risk of recurrence during their lifetime, and 15% of DCIS patients, who generally have a relatively lower risk of recurrence after treatment compared to those with invasive disease [39], perceived their risk to be ≥50%. Patients’ perceived risk of recurrence did not change significantly over the course of the 2-year study, consistent with an earlier finding in DCIS patients alone [8].
Perceived risk of recurrence was higher in white than non-white patients (93% of whom were African American); and non-white patients were more likely than white patients to report their risk to be 0%. This finding is similar to other research reporting that more than half of African American breast cancer survivors with a 5–10% prior probability of having a BRCA1/2 mutation did not believe they were at increased risk for recurrent or new breast cancer [24]. Since African American breast cancer patients generally have higher risks of recurrence and death from breast cancer than white breast cancer patients [40, 41], non-white patients in our sample were overly optimistic in their recurrence-risk perceptions. Little is known about why African American breast cancer patients, in particular, have this optimistic bias about their risk of recurrence. Although family history of breast cancer and education have been found to be associated with cancer risk perceptions [24, 42], there were no significant racial differences in education or family history of breast cancer in our sample. Further research about factors modifying such racial difference in patients’ perceived risk of recurrence is warranted.
Racial differences in recurrence-risk perceptions may have clinical implications. For example, higher risk perception has been reported to be a motivator to screening mammography use in women without a personal history of breast cancer [9]. African American breast cancer survivors are less likely to receive post-treatment surveillance mammography and have shorter follow-up care compared with white survivors [43, 44], which may be due, in part, to optimistic bias in their recurrence-risk perceptions.
Radiotherapy is standard of care for patients who receive BCS to reduce the chance of local recurrence in the breast or chest wall. We found that patients who received adjuvant radiation therapy tended to perceive their risk of recurrence to be low, which is consistent with their lower “actual” risk of recurrence. The survival benefits of BCS with radiation are similar to the survival benefits of mastectomy in early-stage breast cancer patients with small tumors [45]. If surgeons discuss this information with their patients during the surgical treatment decision-making process, patients might understandably transfer this knowledge about survival benefits to their perceived risk of recurrence. Given that 94% of our patients who underwent BCS also received radiation therapy, the finding of the similarity in perceived risk of recurrence between patients who received BCS and patients who received mastectomy supports this hypothesis. Since we do not know what each patient’s physicians told her regarding her risk of recurrence (or of dying), we cannot discern the extent to which a patient’s knowledge of the survival benefits of radiation therapy influenced how she perceived her risk of recurrence, or if it did at all.
Consistent with the literature that social support works as a buffer against poorer psychological adjustment to stressful events [46, 47], we found that greater perceived social support was associated with lower perceived risk of recurrence. Given the observed association between being married/partnered and reporting more social support, it could be that patients without these intimate relationships might be less able to share their fears about recurrence with persons whom they trust [46], and therefore believe their risk of recurrence to be high. In addition, patients with more social support may be more likely to know (or to have been made aware of) long-term breast cancer survivors living without recurrence, and therefore base their own perceived risk of recurrence on the experiences of other survivors or on what their friends and family say to allay a patient’s concerns.
Patients with greater state anxiety were more likely to report higher risk of recurrence. This is consistent with the literature that heightened perceived cancer risk is associated with greater mood disturbance among women without a personal history of breast cancer [18] and among patients newly diagnosed with breast cancer [8, 13, 48]. However, due to the observational nature of this longitudinal study, we could not determine whether patients’ higher perceived risk of recurrence caused greater anxiety or greater levels of anxiety resulted in higher perceived risk. Greater anxiety has been related both to hyper vigilance in compliance with breast self-examinations [12] and to avoidance of screening [49], suggesting the potential impact of patients’ perceived risk of recurrence on adherence to follow-up care recommendations.
It is worth noting that prognostic factors, including age and cancer stage, were not significantly associated with patients’ perceived risk of recurrence. Although younger breast cancer survivors have a higher risk of recurrence [50, 51], we did not find a significant difference by age in recurrence-risk perceptions after controlling for other covariates. Our sample, however, did not include patients <40 years old, the age group at greatest risk for recurrence [50, 51], which may explain the lack of age difference in our patients’ risk perceptions. The similarity in recurrencerisk perceptions that we observed between DCIS and EIBC patients also was reported in two cross-sectional studies [13, 14]. It is possible that DCIS patients lack awareness about the relative prognosis of DCIS and EIBC, which may result in DCIS patients’ overestimation of their actual risk of recurrence. Alternatively, the similarity in treatment options for patients with DCIS and for most patients with stage I breast cancer may account for the lack of a significant association between cancer stage and recurrence-risk perceptions.
We found no significant association between having a family history of breast cancer and recurrence-risk perceptions, suggesting that patients did not think having a family history of breast cancer increased their chance of developing breast cancer again. Indeed, having a family history of breast cancer was found not to be associated with risk of recurrence in breast cancer survivors [52].
One limitation of this study is that we included only women ≥40 years with early-stage breast cancer, thus we cannot generalize our findings to younger women or women with more advanced breast cancer whose treatment options and prognosis differ from those for DCIS and EIBC patients. Second, a larger proportion of participants than non-participants were white, possibly resulting in selection bias. Non-white participants were younger than non-white non-participants, and younger age was associated with higher recurrence-risk perceptions in the univariate analysis. Therefore, the age difference between non-white participants and non-participants did not appear to bias our findings, since non-white participants reported lower risk than white participants.
The “best” or most accurate way to measure patients’ perceived risk of recurrence remains unknown. However, a 0–100% numerical measure has been demonstrated to be the better measure of perception of breast cancer risk among women without a personal history of breast cancer when compared with a verbal measure (e.g., “not at all likely” to “extremely likely”) and a measure asking respondents to compare their risk with that of the average person [53]. Like breast cancer risk perceptions, the 0–100% numerical measure of perceived risk of recurrence that we and others have used [8, 34] reflects patients’ subjective judgments of their risk of recurrence.
In conclusion, our research indicates that: (1) many early-stage breast cancer patients inaccurately perceived their risk of recurrence, (2) patients’ recurrence-risk perceptions did not change significantly during the first 2 years after surgery, and (3) patients’ recurrence-risk perceptions were associated with race, receipt of radiotherapy, and psychosocial variables (social support and anxiety) but not with cancer stage. These findings suggest the importance of effective physician–patient communication about a patient’s prognosis, especially for DCIS patients who have a relatively low risk of recurrence [39], and about the impact of potential treatments on patients’ risk of recurrence. Provision of social support could potentially alleviate anxiety associated with higher perceived risk of recurrence.
Acknowledgments
This study was supported by a grant from the National Cancer Institute and Breast Cancer Stamp Fund (R01CA102777), and by the National Cancer Institute Cancer Center Support Grant (P30 CA91842) to the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri. We thank our patient participants, the interviewers, and the Siteman Cancer Center’s Health Behavior and Outreach Core (Ms. Jennifer Tappenden and Dr. Yan Yan) and Biostatistics Core (Dr. Kenneth Schechtman) for data management and statistical services, and Pam Hunborg at Saint Louis University School of Medicine. We also greatly appreciate the many physicians who helped us recruit their patients for this study, including Drs. Barbara Monsees, Jill Dietz, Julie Margenthaler, Virginia Herrmann, Timothy Eberlein, Matthew Ellis, Imran Zoberi, Marie Taylor, Michael Naughton, Antonella Rastelli, Donald Lombardi, Cynthia Ma, Loren Michel, and Rama Suresh at Washington University School of Medicine, and Dr. Eddie Hsueh at Saint Louis University School of Medicine. The Beck Anxiety Inventory® and BAI® (copyright 1990, 1993 by Aaron T. Beck) are trademarks of The Psychological Corporation, a Harcourt Assessment Company. The BAI® was adapted and used by permission of the publisher, The Psychological Corporation. All rights reserved.
Contributor Information
Ying Liu, Division of Health Behavior Research, Department of Internal Medicine, Washington University School of Medicine, 4444 Forest Park, Suite 6700, St. Louis, MO 63108, USA.
Maria Pérez, Division of Health Behavior Research, Department of Internal Medicine, Washington University School of Medicine, 4444 Forest Park, Suite 6700, St. Louis, MO 63108, USA.
Mario Schootman, Division of Health Behavior Research, Department of Internal Medicine, Washington University School of Medicine, 4444 Forest Park, Suite 6700, St. Louis, MO 63108, USA; Alvin J. Cancer Center, Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO, USA.
Rebecca L. Aft, Alvin J. Cancer Center, Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO, USA; Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA; John Cochran Veterans Administration Hospital, St. Louis, MO, USA
William E. Gillanders, Alvin J. Cancer Center, Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO, USA; Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA
Matthew J. Ellis, Alvin J. Cancer Center, Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO, USA; Division of Medical Oncology, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, USA
Donna B. Jeffe, Division of Health Behavior Research, Department of Internal Medicine, Washington University School of Medicine, 4444 Forest Park, Suite 6700, St. Louis, MO 63108, USA; Alvin J. Cancer Center, Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO, USA
References
- 1.Bijker N, Peterse JL, Duchateau L, Julien JP, Fentiman IS, Duval C, Di Palma S, Simony-Lafontaine J, de Mascarel I, van de Vijver MJ. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263–2271. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino J, Redmond C, Fisher E, Margolese R, Dimitrov N, Wolmark N, Wickerham DL, Deutsch M, Ore L, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 3.Habel LA, Daling JR, Newcomb PA, Self SG, Porter PL, Stanford JL, Seidel K, Weiss NS. Risk of recurrence after ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 1998;7:689–696. [PubMed] [Google Scholar]
- 4.Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Harris JR, O’Malley F, Schnitt SJ, Singletary SE, Winchester DP. Standard for the management of ductal carcinoma in situ of the breast (DCIS) CA Cancer J Clin. 2002;52:256–276. doi: 10.3322/canjclin.52.5.256. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein MJ, Barth A, Poller DN, Gierson ED, Colburn WJ, Waisman JR, Gamagami P. Ten-year results comparing mastectomy to excision and radiation therapy for ductal carcinoma in situ of the breast. Eur J Cancer. 1995;31A:1425–1427. doi: 10.1016/0959-8049(95)00283-o. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Rothrock NE, Matthews AK, Sellergren SA, Fleming G, List M. State anxiety and cancer-specific anxiety in survivors of breast cancer. J Psychosoc Oncol. 2004;22:93–109. [Google Scholar]
- 8.Partridge A, Adloff K, Blood E, Dees EC, Kaelin C, Golshan M, Ligibel J, de Moor JS, Weeks J, Emmons K, Winer E. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100:243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 9.Moser RP, McCaul K, Peters E, Nelson W, Marcus SE. Associations of perceived risk and worry with cancer health-protective actions: data from the Health Information National Trends Survey (HINTS) J Health Psychol. 2007;12:53–65. doi: 10.1177/1359105307071735. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstock IM, Irwin M. The Health Belief Model: explaining health behavior through expectancies. In: Glanz K, Lewis FM, Rimer BK, editors. Health behavior and health education: theory, research, and practice. Jossey-Bassm; San Francisco: 1990. pp. 39–62. [Google Scholar]
- 11.Calvocoressi L, Kasl SV, Lee CH, Stolar M, Claus EB, Jones BA. A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and White women ages 40 to 79 years. Cancer Epidemiol Biomarkers Prev. 2004;13:2096–2105. [PubMed] [Google Scholar]
- 12.Brain K, Norman P, Gray J, Mansel R. Anxiety and adherence to breast self-examination in women with a family history of breast cancer. Psychosom Med. 1999;61:181–187. doi: 10.1097/00006842-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Rakovitch E, Franssen E, Kim J, Ackerman I, Pignol JP, Paszat L, Pritchard KI, Ho C, Redelmeier DA. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2003;77:285–293. doi: 10.1023/a:1021853302033. [DOI] [PubMed] [Google Scholar]
- 14.van Gestel YR, Voogd AC, Vingerhoets AJ, Mols F, Nieuwenhuijzen GA, van Driel OJ, van Berlo CL, van de Poll-Franse LV. A comparison of quality of life, disease impact and risk perception in women with invasive breast cancer and ductal carcinoma in situ. Eur J Cancer. 2006;43:549–556. doi: 10.1016/j.ejca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Kemeny MM, Wellisch DK, Schain WS. Psychosocial outcome in a randomized surgical trial for treatment of primary breast cancer. Cancer. 1988;62:1231–1237. doi: 10.1002/1097-0142(19880915)62:6<1231::aid-cncr2820620631>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Schover LR, Yetman RJ, Tuason LJ, Meisler E, Esselstyn CB, Hermann RE, Grundfest-Broniatowski S, Dowden RV. Partial mastectomy and breast reconstruction. A comparison of their effects on psychosocial adjustment, body image, and sexuality. Cancer. 1995;75:54–64. doi: 10.1002/1097-0142(19950101)75:1<54::aid-cncr2820750111>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Wade TD, Nehmy T, Koczwara B. Predicting worries about health after breast cancer surgery. Psychooncology. 2005;14:503–509. doi: 10.1002/pon.866. [DOI] [PubMed] [Google Scholar]
- 18.Bowen D, Hickman KM, Powers D. Importance of psychological variables in understanding risk perceptions and breast cancer screening of African American women. Womens Health. 1997;3:227–242. [PubMed] [Google Scholar]
- 19.Davis S, Stewart S, Bloom J. Increasing the accuracy of perceived breast cancer risk: results from a randomized trial with Cancer Information Service callers. Prev Med. 2004;39:64–73. doi: 10.1016/j.ypmed.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 20.Hughes C, Lerman C, Lustbader E. Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Res Treat. 1996;40:25–35. doi: 10.1007/BF01806000. [DOI] [PubMed] [Google Scholar]
- 21.Courtens AM, Stevens FC, Crebolder HF, Philipsen H. Longitudinal study on quality of life and social support in cancer patients. Cancer Nurs. 1996;19:162–169. doi: 10.1097/00002820-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds P, Boyd PT, Blacklow RS, Jackson JS, Greenberg RS, Austin DF, Chen VW, Edwards BK, National Cancer Institute Black/White Cancer Survival Study Group The relationship between social ties and survival among black and white breast cancer patients. Cancer Epidemiol Biomarkers Prev. 1994;3:253–259. [PubMed] [Google Scholar]
- 23.Bluman LG, Borstelmann NA, Rimer BK, Iglehart JD, Winer EP. Knowledge, satisfaction, and perceived cancer risk among women diagnosed with ductal carcinoma in situ. J Womens Health Gend Based Med. 2001;10:589–598. doi: 10.1089/15246090152543175. [DOI] [PubMed] [Google Scholar]
- 24.Brewster K, Wileyto EP, Kessler L, Collier A, Weathers B, Stopfer JE, Domchek S, Halbert CH. Sociocultural predictors of breast cancer risk perceptions in African American breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16:244–248. doi: 10.1158/1055-9965.EPI-06-0481. [DOI] [PubMed] [Google Scholar]
- 25.Leitch AM, Dodd GD, Costanza M, Linver M, Pressman P, McGinnis L, Smith RA. American Cancer Society guidelines for the early detection of breast cancer: update 1997. CA Cancer J Clin. 1997;47:150–153. doi: 10.3322/canjclin.47.3.150. [DOI] [PubMed] [Google Scholar]
- 26.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 27.Birkett NJ. Computer-aided personal interviewing. A new technique for data collection in epidemiologic surveys. Am J Epidemiol. 1988;127:684–690. doi: 10.1093/oxfordjournals.aje.a114843. [DOI] [PubMed] [Google Scholar]
- 28.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Schag CA, Ganz PA, Polinsky ML, Fred C, Hirji K, Petersen L. Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol. 1993;11:783–793. doi: 10.1200/JCO.1993.11.4.783. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Andersen MR, Urban N. Involvement in decision-making and breast cancer survivor quality of life. Ann Behav Med. 1999;21:201–209. doi: 10.1007/BF02884834. [DOI] [PubMed] [Google Scholar]
- 35.Hedeker D, Mermelstein RJ. Analysis of longitudinal substance use outcomes using ordinal random-effects regression models. Addiction. 2000;95(Suppl 3):S381–S394. doi: 10.1080/09652140020004296. [DOI] [PubMed] [Google Scholar]
- 36.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 37.Huang GH, Palta M, Allen C, LeCaire T, D’Alessio D. Self-rated health among young people with type 1 diabetes in relation to risk factors in a longitudinal study. Am J Epidemiol. 2004;159:364–372. doi: 10.1093/aje/kwh055. [DOI] [PubMed] [Google Scholar]
- 38.Sheu CF. Fitting mixed-effects models for repeated ordinal outcomes with the NLMIXED procedure. Behav Res Methods Instrum Comput. 2002;34:151–157. doi: 10.3758/bf03195436. [DOI] [PubMed] [Google Scholar]
- 39.Viani GA, Stefano EJ, Afonso SL, De Fendi LI, Soares FV, Leon PG, Guimaraes FS. Breast-conserving surgery with or without radiotherapy in women with ductal carcinoma in situ: a meta-analysis of randomized trials. Radiat Oncol. 2007;2:28. doi: 10.1186/1748-717X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran MS, Yang Q, Harris LN, Jones B, Tuck DP, Haffty BG. Long-term outcomes and clinicopathologic differences of African-American versus white patients treated with breast conservation therapy for early-stage breast cancer. Cancer. 2008;113:2565–2574. doi: 10.1002/cncr.23881. [DOI] [PubMed] [Google Scholar]
- 41.Deshpande AD, Jeffe DB, Gnerlich J, Iqbal AZ, Thummalakunta A, Margenthaler JA. Racial disparities in breast cancer survival: an analysis by age and stage. J Surg Res. 2009;153:105–113. doi: 10.1016/j.jss.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orom H, Kiviniemi MT, Underwood W, III, Ross L, Shavers VL. Perceived cancer risk: why is it lower among nonwhites than whites? Cancer Epidemiol Biomarkers Prev. 2010;19:746–754. doi: 10.1158/1055-9965.EPI-09-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connor CS, Touijer AK, Krishnan L, Mayo MS. Local recurrence following breast conservation therapy in African-American women with invasive breast cancer. Am J Surg. 2000;179:22–26. doi: 10.1016/s0002-9610(99)00258-5. [DOI] [PubMed] [Google Scholar]
- 44.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38:281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 46.Kornblith AB, Herndon JE, II, Zuckerman E, Viscoli CM, Horwitz RI, Cooper MR, Harris L, Tkaczuk KH, Perry MC, Budman D, Norton LC, Holland J. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91:443–454. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 47.Ringdal GI, Ringdal K, Jordhoy MS, Kaasa S. Does social support from family and friends work as a buffer against reactions to stressful life events such as terminal cancer? Palliat Support Care. 2007;5:61–69. doi: 10.1017/s1478951507070083. [DOI] [PubMed] [Google Scholar]
- 48.Carver CS, Meyer B, Antoni MH. Responsiveness to threats and incentives, expectancy of recurrence, and distress and disengagement: moderator effects in women with early stage breast cancer. J Consult Clin Psychol. 2000;68:965–975. doi: 10.1037//0022-006x.68.6.965. [DOI] [PubMed] [Google Scholar]
- 49.Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat. 1993;28:145–155. doi: 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- 50.Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, Fourquet A, Borger J, Jager J, Hoogenraad W, Collette L, Pierart M. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 51.Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, van Tienhoven G, Andersen KW, Sylvester RJ, van Dongen JA. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 52.Brewster AM, Do KA, Thompson PA, Hahn KM, Sahin AA, Cao Y, Stewart MM, Murray JL, Hortobagyi GN, Bondy ML. Relationship between epidemiologic risk factors and breast cancer recurrence. J Clin Oncol. 2007;25:4438–4444. doi: 10.1200/JCO.2007.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurmankin Levy A, Shea J, Williams SV, Quistberg A, Armstrong K. Measuring perceptions of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1893–1898. doi: 10.1158/1055-9965.EPI-05-0482. [DOI] [PubMed] [Google Scholar]